Closed-Loop Medication Management with an Electronic Health Record System in U.S. and Finnish Hospitals
Abstract
1. Introduction
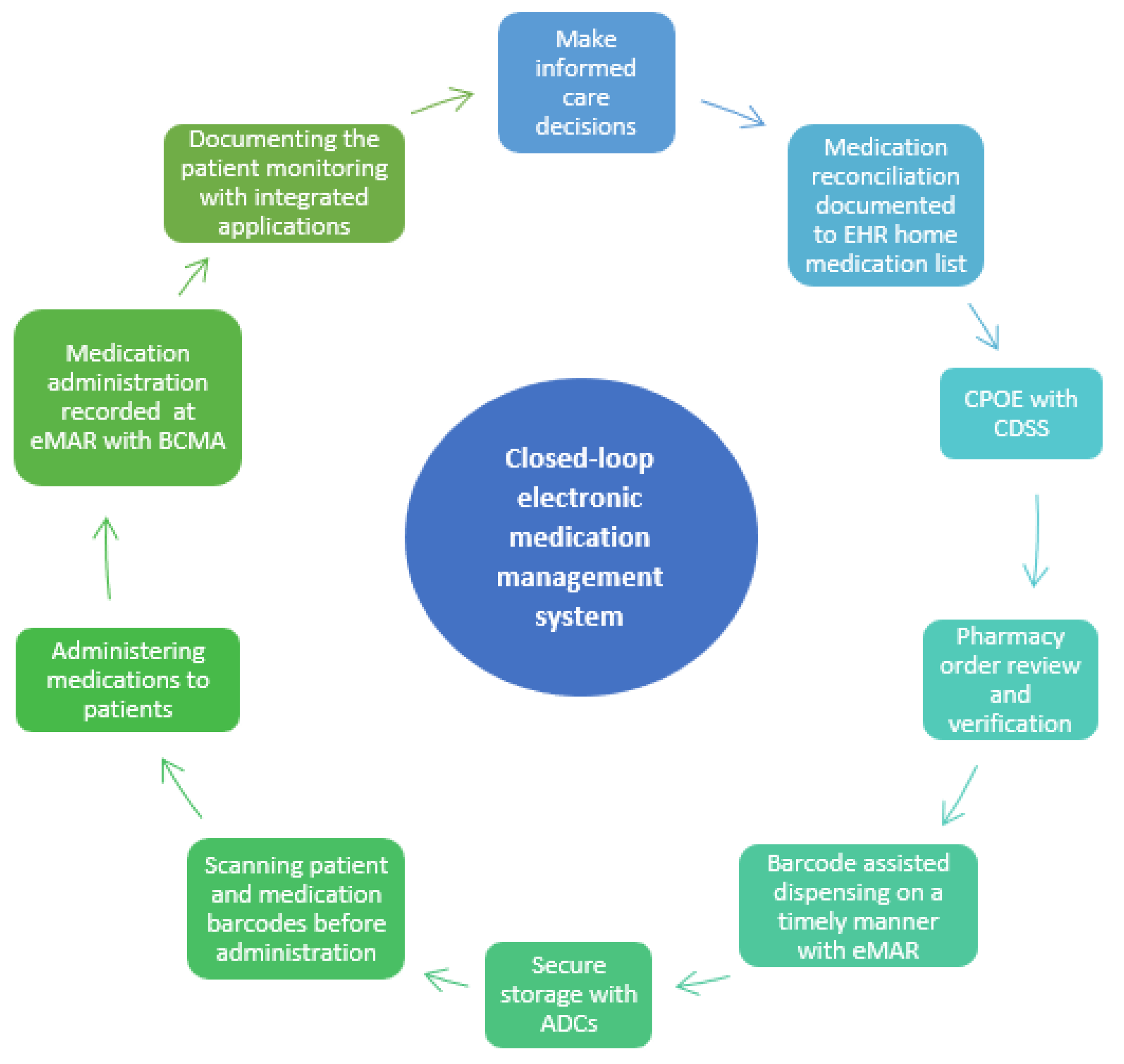
2. Closed-Loop EMMSs in U.S. and Finnish Hospitals
2.1. Development of Closed-Loop EMMSs in U.S. Hospitals
2.2. Helsinki University Hospital Introduced Closed-Loop EMMSs to Finland
| United States (Hospitals with 200 or More Beds) | Helsinki University Hospital, Finland [10] |
|---|---|
| Medication Reconciliation: home medication list obtained using two sources | |
| Medication reconciliation and nursing or pharmacy staff obtain the best-possible medication history (prior-to-admission medication lists) and compliance rates are monitored. External medication history information is pulled into the EHR from outside sources such as retail pharmacies. Pharmacists and pharmacy technicians are frequently involved [32,33,34]. | Medication reconciliation and nursing or pharmacy staff obtain the best-possible medication history (prior-to-admission medication lists) and compliance rates are monitored. Medication reconciliation and a structured home medication list are mandatory for in-patient medication. The EHR home medication list is integrated into the national Kanta system [35], which holds electronic prescriptions. Pharmacists are involved in many units. |
| Ordering/prescribing with computerized physician order entry (CPOE) | |
| Provides ordering support, through structured order and prescription forms, for most common doses/frequencies. Order panels and order sets developed for specific diagnoses or situations (e.g., admission) [36]. | |
| Clinical Decision Support System (CDSS) | |
| Sophisticated CDSS, e.g., with dose warnings (including dosing with older patients and renal impairment), duplicate medications, and electronic best practice advice (BPA) [37,38]. | |
| Dispensing and automated dispensing cabinets (ADCs) | |
| ADCs are widely used, integrated with EHR, and enable the dispensing of medicines according to verified electronic orders on many units. Medication removal by override is limited to urgently needed medications (e.g., antidotes, medications for intubation) [39]. ADC overrides display in EMR to be reconciled with prescriber order and allow BCMA. While some barcodes include lot number and expiration date, scanning technology in use is reading a medication’s National Drug Code (NDC) number. Starting in November 2023, barcodes must include lot numbers and expiration dates [40]. | ADCs are in use in many units and integrated with EHR, which enables the dispensing of medicines according to electronic orders. Medication removal by override is not yet limited. Nurses do the dispensing in a timely manner (max. 2 h before administration) by using the eMAR and scanning the barcodes of the medicine secondary packages (unit doses are not available yet). Barcodes include a lot number and expiration date [41]. A manual double-check is used when the barcode is not available and for HAMs. |
| Preparation outside of the pharmacy | |
| On units, intravenous preparation is limited to emergencies, drawing medications into syringes for IV Push or IM administration, or the use of vial and bag adaptor technology [42]. Efforts are made to dispense most medications as ready-to-use and unit-dosed by the hospital pharmacy | Ready-to-use medications are not widely available and preparing is commonly done by nurses or pharmacists. EHR provides the documentation with barcodes and instructions for preparation. The manual double-check is used when the barcode is not available and for HAMs. |
| Administration | |
| Medication administration is recorded promptly at the bedside using BCMA confirming the right patient, medication, dose, time, and route. | |
| Most hospitals use smart pumps, some hospitals utilize IV pump interoperability with EHR [39]. High-alert titrated infusion medications may include a MAR calculator to assist with titrations (e.g., heparin or insulin). Some HAMs may require a manual independent double-check process documented in EHR. | IV pump interoperability with EHR is not yet in use. |
| Patient Monitoring | |
| Interfaced when technology allows. Monitoring data included in dashboards; patient scoring tools, or machine learning used for early identification of diseases such as sepsis and acute kidney injury [43,44,45,46]. | Interfaced when technology allows. Monitoring data included in dashboards; patient scoring tools used for early identification of diseases such as sepsis. |
| Communication | |
| EMR allows for secure electronic instant communication between members of the healthcare team using secure instant messaging. Order communication between pharmacy and nurse. Follow-up communication between shifts. Epic users have the MyChart phone App for patients to read their charts and laboratory results, communicate with healthcare professionals, and report their home medications and allergies, for example. | |
| United States | Helsinki University Hospital, Finland [10] |
|---|---|
| Pharmacist Medication Order Verification | |
| Prospective pharmacist verification for all orders. Exceptions are emergent/urgent medication needs or medication in the presence of a physician [47]. | Retrospective pharmacist verification of specific orders (e.g., high-alert medications) in some units during weekdays and after the fact for weekends. |
| Purchasing, storing, and inventory | |
| Continuous inventory allows for as-needed purchasing and enhanced management of medication shortages facilitated by integration of her, ADCs, and in some hospitals, automated drug storage such as carousels or robots [39]. | There is integration between EHR and hospital pharmacy’s ERP regarding ADCs. Information on orders and patients comes from EHR to ERP and doses taken from ADC go to EHR. Storage automation and barcode scanning are in use with the hospital pharmacy’s ERP, which is in use for purchasing, storage, and inventory. |
| Dispensing | |
| Automated drug storage and retrieval (e.g., carousels or robots) may be used that coordinate patient orders with medication dispensing through an interface [39]. Dispensing and stocking are verified with barcode scanning. Unit-dose dispensing prioritized for medications (exceptions: bulk medications such as creams, ointments, ophthalmic/otic drops, and insulin pens). If not stocked in ADC, first doses are prioritized and sent to units regularly from the main pharmacy. Ongoing scheduled medications are dispensed to units at specified times during the day based on upcoming administration times. | Dispensing is integrated with EHR only regarding ADCs and multidose dispensing, which is in use in primary and social care, where the HUS Pharmacy also dispenses medications. Information on orders and patients comes from EHR to ERP and information on prepared doses (including lot numbers and expiration dates) goes back to EHR. Unit-dose dispensing is not yet in use, but HUS is planning and preparing it for its next new hospital. |
| Sterile Medication Preparation | |
| Use of barcode scanning of medication and diluent during preparation in sterile preparation facilities connected to order in EHR. Photo documentation and gravimetric confirmation are possible at many hospitals [39]. The final product is provided with a scannable barcode for BCMA. | Integrated into EHR system. Hospital pharmacy prepares patient-specific ready-to-use cytotoxic and biological medications, botulin toxin solutions, and total parenteral nutrition. Information on orders and patients comes from the EHR to the hospital pharmacy’s ERP and information on prepared doses (including lot numbers and expiration dates) goes back to the EHR. Preparation robots including gravimetric confirmation and barcode scanning are in use for cytotoxic medications. |
| Communication | |
| EHR allows for secure electronic instant communication between members of the healthcare team using secure instant messaging. Order communication between pharmacy and nurse. Follow-up communication between shifts. | |
2.3. Comparing Closed-Loop EMMSs in U.S. and Finnish Hospitals
3. Functionality of Closed-Loop EMMSs across the Medication Use System
3.1. Medication Reconciliation
3.2. Ordering/Prescribing with Computerized Physician Order Entry (CPOE) with Clinical Decision Support System (CDSS)
3.3. Order Verification
3.4. Dispensing and Preparing of Medicines
3.5. Barcoded Medication Administration (BCMA), Electronic Medication Administration Records (eMAR), and Integrated Smart Pumps
3.6. Patient Monitoring
3.7. Inventory and Stockpiling
3.8. Communication with Healthcare Colleagues and Patients
4. Future Directions of EMMSs in the U.S. and Finland
4.1. Existing Challenges
4.2. Opportunities and Application Prospects
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- National Coordinating Council for Medication Error Reporting and Prevention (NCC MERP): What Is a Medication Error? Available online: https://www.nccmerp.org/about-medication-errors (accessed on 4 May 2023).
- Medication without Harm—WHO Global Patient Safety Challenge on Medication Safety; WHO: Geneva, Switzerland, 2017; Available online: https://www.who.int/publications/i/item/WHO-HIS-SDS-2017.6 (accessed on 22 June 2023).
- Mulac, A.; Taxis, K.; Hagesaether, E.; Granas, A.G. Severe and fatal medication errors in hospitals: Findings from the Norwegian Incident Reporting System. Eur. J. Hosp. Pharm. 2020, 28, e56–e61. [Google Scholar] [CrossRef] [PubMed]
- Linden-Lahti, C.; Takala, A.; Holmström, A.-R.; Airaksinen, M. What Severe Medication Errors Reported to Health Care Supervisory Authority Tell About Medication Safety? J. Patient Saf. 2021, 17, e1179–e1185. [Google Scholar] [CrossRef] [PubMed]
- Franklin, B.D.; O’Grady, K.; Donyai, P.; Jacklin, A.; Barber, N. The impact of a closed-loop electronic prescribing and administration system on prescribing errors, administration errors and staff time: A before-and-after study. Qual. Saf. Health Care 2007, 16, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Austin, J.A.; Smith, I.R.; Tariq, A. The impact of closed-loop electronic medication management on time to first dose: A comparative study between paper and digital hospital environments. Int. J. Pharm. Pr. 2018, 26, 526–533. [Google Scholar] [CrossRef]
- Zheng, W.Y.; Lichtner, V.; Van Dort, B.A.; Baysari, M.T. The impact of introducing automated dispensing cabinets, barcode medication administration, and closed-loop electronic medication management systems on work processes and safety of controlled medications in hospitals: A systematic review. Res. Soc. Adm. Pharm. 2020, 17, 832–841. [Google Scholar] [CrossRef]
- Ciapponi, A.; Fernandez Nievas, S.E.; Seijo, M.; Rodríguez, M.B.; Vietto, V.; García-Perdomo, H.A.; Virgilio, S.; Fajreldines, A.V.; Tost, J.; Rose, C.J.; et al. Reducing medication errors for adults in hospital settings. Cochrane Database Syst. Rev. 2021, 25, CD009985. [Google Scholar]
- Pearce, R.; Whyte, I. Electronic medication management: Is it a silver bullet? Aust. Prescr. 2018, 41, 32–33. [Google Scholar] [CrossRef]
- Lindén-Lahti, C.; Kivivuori, S.-M.; Lehtonen, L.; Schepel, L. Implementing a New Electronic Health Record System in a University Hospital: The Effect on Reported Medication Errors. Healthcare 2022, 10, 1020. [Google Scholar] [CrossRef]
- The Clinical & Systems Transformation (CST) Project 2014. Available online: https://cstproject.ca/closedloop (accessed on 8 March 2023).
- Buchanan, C. A Brief History of Unit-Dose Drug Distribution. J. Pharm. Technol. 1985, 1, 127–129. [Google Scholar] [CrossRef]
- CDC—Background—EHRs—NIOSH Workplace Safety and Health Topic. Available online: https://www.cdc.gov/niosh/topics/ehr/background.html (accessed on 30 April 2023).
- Blumenthal, D. Launching HITECH. NEJM 2010, 362, 382–385. [Google Scholar] [CrossRef]
- Williams, C.; Mostashari, F.; Mertz, K.; Hogin, E.; Atwal, P. From The Office of The National Coordinator: The Strategy for Advancing the Exchange of Health Information. Health Aff. 2012, 31, 527–536. [Google Scholar] [CrossRef] [PubMed]
- Black, J.R.; Hulkower, R.L.; Ramanathan, T. Health Information Blocking. Public Health Rep. 2018, 133, 610–613. [Google Scholar] [CrossRef]
- The Commonwealth Fund: United States. Available online: https://www.commonwealthfund.org/international-health-policy-center/countries/united-statess (accessed on 2 May 2023).
- Leapfrog Group: About Us. Available online: https://www.leapfroggroup.org/about (accessed on 2 May 2023).
- Victoroff, M.S.; Drury, B.M.; Campagna, E.J.; Morrato, E.H. Impact of Electronic Health Records on Malpractice Claims in a Sample of Physician Offices in Colorado: A Retrospective Cohort Study. J. Gen. Intern. Med. 2013, 28, 637–644. [Google Scholar] [CrossRef][Green Version]
- The Joint Commission Federal Deemed Status Fact Sheet. Available online: https://www.jointcommission.org/resources/news-and-multimedia/fact-sheets/facts-about-federal-deemed-status/ (accessed on 30 April 2023).
- Murray, M.D. Chapter 11. Automated Medication Dispensing Devices. In Making Health Care Safer: A Critical Analysis of Patient Safety Practices. Evidence Report/Technology Assessment No. 43 (Prepared by the University of California at San Francisco–Stanford Evidence-Based Practice Center under Contract No. 290-97-0013); AHRQ Publication No. 01-E058; Agency for Healthcare Research and Quality: Rockville, MD, USA, 2001; pp. 111–116. [Google Scholar]
- Wideman, M.V.; Whittler, M.E.; Anderson, T.M. In Barcode Medication Administration: Lessons Learned from an Intensive Care Unit Implementation; Henriksen, K., Battles, J.B., Marks, E.S., Lewin, D.I., Eds.; Advances in Patient Safety: From Research to Implementation (Volume 3, Implementation Issues); Agency for Healthcare Research and Quality: Rockville, MD, USA, 2005. [Google Scholar]
- Ash, J.S.; Gorman, P.N.; Lavelle, M.; Lyman, J. Multiple perspectives on physician order entry. In Proceedings of the AMIA Symposium, Los Angeles, CA, USA, 4–8 November 2000; pp. 27–31. [Google Scholar]
- Institute of Medicine; America, Committee on Quality of Health Care in America; Donaldson, M.S.; Corrigan, J.M.; Kohn, L.T. To Err Is Human; National Academies Press: Washington, DC, USA, 2000. [Google Scholar]
- Vaghasiya, M.R.; Penm, J.; Kuan, K.K.Y.; Gunja, N.; Liu, Y.; Kim, E.D.; Petrina, N.; Poon, S. Implementation of an Electronic Medication Management System in a large tertiary hospital: A case of qualitative inquiry. BMC Med. Inf. Decis. Mak. 2021, 21, 226. [Google Scholar] [CrossRef]
- Medication Safety Self Assessment® for Oncology|Institute for Safe Medication Practices. Available online: https://www.ismp.org/assessments/international-oncology (accessed on 24 June 2023).
- Assessing Medication Safety in Settings Not Designated Solely for Pediatric Patients|Institute for Safe Medication Practices. Available online: https://www.ismp.org/resources/assessing-medication-safety-settings-not-designated-solely-pediatric-patients (accessed on 24 June 2023).
- Guidelines for Safe Medication Use in Perioperative and Procedural Settings|Institute for Safe Medication Practices. Available online: https://www.ismp.org/resources/guidelines-safe-medication-use-perioperative-and-procedural-settings (accessed on 24 June 2023).
- High-Alert Medications in Acute Care Settings|Institute for Safe Medication Practices. Available online: https://www.ismp.org/recommendations/high-alert-medications-acute-list (accessed on 24 June 2023).
- The Ministry of Social Affairs and Health: Closed-Loop Medication Management. Working Group Memorandum on Practices Used in Hospitals. Available online: http://urn.fi/URN:ISBN:978-952-00-5433-5 (accessed on 22 June 2023).
- ISMP List of High-Alert Medications in Acute Care Settings. Available online: https://www.ismp.org/sites/default/files/attachments/2018-08/highAlert2018-Acute-Final.pdf (accessed on 7 July 2023).
- Factsheet: Medication Reconciliation. Available online: https://ratings.leapfroggroup.org/sites/default/files/inline-files/2022%20Medication%20Reconciliation%20Fact%20Sheet.pdf (accessed on 30 April 2023).
- Schnipper, J.L.; Mixon, A.; Stein, J.; Wetterneck, T.B.; Kaboli, P.J.; Mueller, S.; Labonville, S.; Minahan, J.A.; Burdick, E.; Orav, E.J.; et al. Effects of a multifaceted medication reconciliation quality improvement intervention on patient safety: Final results of the MARQUIS study. BMJ Qual. Saf. 2018, 27, 954–964. [Google Scholar] [CrossRef] [PubMed]
- Mixon, A.S.; Kripalani, S.; Stein, J.; Wetterneck, T.B.; Kaboli, P.; Mueller, S.; Burdick, E.; Nolido, N.V.; Labonville, S.; Minahan, J.A.; et al. An On-Treatment Analysis of the MARQUIS Study: Interventions to Improve Inpatient Medication Reconciliation. J. Hosp. Med. 2019, 14, 614–617. [Google Scholar] [CrossRef] [PubMed]
- Kanta 2023, What Are Kanta Services? Available online: https://www.kanta.fi/en/professionals/what-are-kanta-services (accessed on 8 March 2023).
- Radley, D.C.; Wasserman, M.R.; Olsho, L.E.; Shoemaker, S.J.; Spranca, M.D.; Bradshaw, B. Reduction in medication errors in hospitals due to adoption of computerized provider order entry systems. JAMIA 2013, 20, 470–476. [Google Scholar] [CrossRef]
- Moss, J.; Berner, E.S. Evaluating clinical decision support tools for medication administration safety in a simulated environment. Int. J. Med. Inform. 2015, 84, 308–318. [Google Scholar] [CrossRef]
- Clinical Decision Support|HealthIT.gov. Available online: https://www.healthit.gov/topic/safety/clinical-decision-support (accessed on 30 April 2023).
- Halvorsen, D. State of pharmacy automation 2022. Pharm. Purch. Prod. 2022, 19, 1–68. [Google Scholar]
- AmerisourceBergen DSCSA: Are You Ready for November 2023? Available online: https://www.amerisourcebergen.com/insights/dscsa-are-you-ready-for-november-2023 (accessed on 30 June 2023).
- The European Commission: Commission Delegated Regulation (EU) 2016/161. Available online: https://health.ec.europa.eu/system/files/2016-11/reg_2016_161_en_0.pdf (accessed on 22 June 2023).
- ISMP Survey Provides Insights into Preparation and Admixture Practices OUTSIDE the Pharmacy|Institute for Safe Medication Practices. Available online: https://www.ismp.org/resources/ismp-survey-provides-insights-preparation-and-admixture-practices-outside-pharmacy (accessed on 25 June 2023).
- Amland, R.C.; Hahn-Cover, K.E. Clinical Decision Support for Early Recognition of Sepsis. Am. J. Med. Qual. 2019, 34, 494–501. [Google Scholar] [CrossRef]
- Malhotra, R.; Kashani, K.B.; Macedo, E.; Kim, J.; Bouchard, J.; Wynn, S.; Li, G.; Ohno-Machado, L.; Mehta, R. A risk prediction score for acute kidney injury in the intensive care unit. Nephrol. Dial. Transplant. 2017, 32, 814–822. [Google Scholar] [CrossRef]
- Shawwa, K.; Ghosh, E.; Lanius, S.; Schwager, E.; Eshelman, L.; Kashani, K.B. Predicting acute kidney injury in critically ill patients using comorbid conditions utilizing machine learning. Clin. Kidney J. 2021, 14, 1428–1435. [Google Scholar] [CrossRef] [PubMed]
- Gale, B.; Hall, K. The Use of Patient Monitoring Systems to Improve Sepsis Recognition and Outcomes: A Systematic Review. J. Patient Saf. 2020, 16, S8–S11. [Google Scholar] [CrossRef] [PubMed]
- Mansur, J. Top Joint Commission Compliance Challenges. Pharm. Purch. Prod. 2015, 12, 30–36. [Google Scholar]
- Kvarnström, K.; Linden-Lahti, C. Lääkemääräysten kliininen kaksoistarkistus—Uusi toimintatapa sairaalassa. Suom. Lääkäril. 2020, 75, 2386–2388. (In Finnish) [Google Scholar]
- The Finnish Medicines Agency. Administrative Regulations: 6/2011. Apteekkien lääkevalmistus (Manufacturing Medicinal Products in Pharmacies, Hospital Pharmacies and Dispensaries, Only in Finnish). Available online: https://www.fimea.fi/documents/160140/764653/20675_FINAL_Apteekkien_laakevalmistus_maarays_SUOMI_2011-12-16.pdf (accessed on 9 July 2023).
- The Finnish Medicines Agency. Administrative Regulations: 6/2022 Good Manufacturing Practice for Medicinal Products and the Requirements for the Manufacturing of Investigational Medicinal Products (Unofficial Translation). Available online: https://www.fimea.fi/documents/542809/842303/Administrative+regulation+6+2022+unofficial+EN+translation.pdf/dd28e9f0-92fc-bf45-7845-e181e3b5447c?t=1658302955829 (accessed on 22 June 2023).
- Education Is “Predictably Disappointing” and Should Never Be Relied Upon Alone to Improve Safety|Institute for Safe Medication Practices. Available online: https://www.ismp.org/resources/education-predictably-disappointing-and-should-never-be-relied-upon-alone-improve-safety (accessed on 25 June 2023).
- Lin, S.C.; Jha, A.K.; Adler-Milstein, J. Electronic Health Records Associated with Lower Hospital Mortality after Systems Have Time to Mature. Health Aff. 2018, 37, 1128–1135. [Google Scholar] [CrossRef]
- Schepel, L.; Lehtonen, L.; Airaksinen, M.; Lapatto-Reiniluoto, O. How to Identify Organizational High-Alert Medications. J. Patient Saf. 2018, 17, e1358–e1363. [Google Scholar] [CrossRef]
- Kvarnström, K.; Niittynen, I.; Kallio, S.; Lindén-Lahti, C.; Airaksinen, M.; Schepel, L. Developing an In-House Comprehensive Medication Review Training Program for Clinical Pharmacists in a Finnish Hospital Pharmacy. Int. J. Environ. Res. Public Health 2023, 20, 6158. [Google Scholar] [CrossRef]
- Landex, N. The Epic healthcare system in Denmark. Ugeskr Laeger. 2017, 179, V69572. (In Danish) [Google Scholar]
- Ellingsen, G.; Hertzum, M.; Christensen, B.; Wynn, R. National Integration Components Challenge the Epic Implementation in Central Norway. Stud. Health Technol. Inform. 2022, 25, 500–504. [Google Scholar]
- Hertzum, M.; Ellingsen, G.; Cajander, Å. Implementing Large-Scale Electronic Health Records: Experiences from implementations of Epic in Denmark and Finland. Int. J. Med. Inform. 2022, 167, 104868. [Google Scholar] [CrossRef]
- Partnership for Health IT Patient Safety; Safe Practices to Reduce CPOE Alert Fatigue through Monitoring, Analysis, and Optimization. 2021. Available online: Hitsafety@ecri.org (accessed on 27 June 2023).
- McGreevey, J.D.; Mallozzi, C.P.; Perkins, R.M.; Shelov, E.; Schreiber, R. Reducing Alert Burden in Electronic Health Records: State of the Art Recommendations from Four Health Systems. Appl. Clin. Inform. 2020, 11, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Meyer, L.D.; Raymond, C.B.; Rodrigue, C.M.J. Development and Evaluation of a Checklist for Medication Order Review by Pharmacists. Can. J. Hosp. Pharm. 2011, 64, 199–206. [Google Scholar] [CrossRef]
- Dupree, L.H.; Schmittgen, J.; Taylor, T.H. Teaching pharmacy students a systematic approach to medication order verification. Curr. Pharm. Teach. Learn. 2022, 14, 1015–1020. [Google Scholar] [CrossRef] [PubMed]
- The Joint Commission Medication Dispensing—Use of Auto-Verification Technology. Available online: https://www.jointcommission.org/standards/standard-faqs/hospital-and-hospital-clinics/medication-management-mm/000002352/ (accessed on 25 June 2023).
- Hassink JJM, Jansen MMPM, Helmons PJEffects of bar code-assisted medication administration (BCMA) on frequency, type and severity of medication administration errors: A review of the literature. Eur. J. Hosp. Pharm. 2012, 19, 489–494. [CrossRef]
- Institute for Safe Medication Practices: The Differences Between Human Error, At-Risk Behavior, and Reckless Behavior Are Key to a Just Culture. Medication Safety Alert! Featured Articles 18 June 2020. Available online: https://www.ismp.org/resources/differences-between-human-error-risk-behavior-and-reckless-behavior-are-key-just-culture (accessed on 26 June 2023).
- Kulasa, K.; Serences, B.; Nies, M.; El-Kareh, R.; Kurashige, K.; Box, K. Insulin Infusion Computer Calculator Programmed Directly Into Electronic Health Record Medication Administration Record. J. Diabetes Sci. Technol. 2021, 15, 214–221. [Google Scholar] [CrossRef]
- Guidelines for Optimizing Safe Implementation and Use of Smart Infusion Pumps|Institute for Safe Medication Practices. Available online: https://www.ismp.org/guidelines/safe-implementation-and-use-smart-pumps (accessed on 30 April 2023).
- Kuitunen, S.K.; Kärkkäinen, K.; Linden-Lahti, C.; Schepel, L.; Holmström, A.R. Dose error reduction software in medication safety risk management—Optimising the smart infusion pump dosing limits in neonatal intensive care unit prior to implementation. BMC Pediatr. 2022, 22, 118. [Google Scholar] [CrossRef]
- Misra, S.; Avari, P.; Lumb, A.; Flanagan, D.; Choudhary, P.; Rayman, G.; Dhatariya, K. How Can Point-of-Care Technologies Support In-Hospital Diabetes Care? J. Diabetes Sci. Technol. 2023, 17, 509–516. [Google Scholar] [CrossRef]
- Verma, P. Hospital Bosses love AI. Doctors and Nurses are Worried. The Washington Post [Internet]. 10 August 2023; Tech; [About 4 P.]. Available online: https://www.washingtonpost.com/technology/2023/08/10/ai-chatbots-hospital-technology/ (accessed on 12 August 2023).
- Harris, I.; Dowell, P.; Mossburg, S. Annual Perspective: Topics in Medication Safety. Available online: https://psnet.ahrq.gov/perspective/annual-perspective-topics-medication-safety (accessed on 12 August 2023).
- Tolley, C.L.; Watson, N.W.; Heed, A.; Einbeck, J.; Medows, S.; Wood, L.; Campbell, L.; Slight, S.P. The impact of a novel medication scanner on administration errors in the hospital setting: A before and after feasibility study. BMC Med. Inform. Decis. Mak. 2022, 22, 86. [Google Scholar] [CrossRef]
- Patel, L.; Shukla, T.; Huang, X.; Ussery, D.W.; Wang, S. Machine Learning Methods in Drug Discovery. Molecules 2020, 25, 5277. [Google Scholar] [CrossRef]
- Aronson, J.K. Artificial Intelligence in pharmacovigilance: An introduction to terms, concepts, applications, and limitations. Drug Saf. 2022, 45, 407–418. [Google Scholar] [CrossRef]
- Zhao, M.; Hoti, K.; Wang, H.; Raghu, A.; Katabi, D. Assessment of medication self-administration using artificial intelligence. Nat. Med. 2021, 27, 727–735. [Google Scholar] [CrossRef] [PubMed]
- Bates, D.W.; Levine, D.; Syrowatka, A.; Kuznetsova, M.; Craig, K.J.T.; Rui, A.; Jackson, G.P.; Rhee, K. The potential of artificial intelligence to improve patient safety: A scoping review. NPJ Digit. Med. 2021, 4, 54. [Google Scholar] [CrossRef] [PubMed]
- Rozenblum, R.; Rodriguez-Monguio, R.; Volk, L.Y.; Forsythe, K.J.; Myers, S.; McGurrin, M.; Williams, D.H.; Bates, D.W.; Schiff, G.; Seoane-Vazquez, E. Using a machine learning system to identify and prevent medication prescribing errors: A clinical and cost analysis evaluation. Jt. Comm. J. Qual. Patient Saf. 2020, 46, 3–10. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shermock, S.B.; Shermock, K.M.; Schepel, L.L. Closed-Loop Medication Management with an Electronic Health Record System in U.S. and Finnish Hospitals. Int. J. Environ. Res. Public Health 2023, 20, 6680. https://doi.org/10.3390/ijerph20176680
Shermock SB, Shermock KM, Schepel LL. Closed-Loop Medication Management with an Electronic Health Record System in U.S. and Finnish Hospitals. International Journal of Environmental Research and Public Health. 2023; 20(17):6680. https://doi.org/10.3390/ijerph20176680
Chicago/Turabian StyleShermock, Susan B., Kenneth M. Shermock, and Lotta L. Schepel. 2023. "Closed-Loop Medication Management with an Electronic Health Record System in U.S. and Finnish Hospitals" International Journal of Environmental Research and Public Health 20, no. 17: 6680. https://doi.org/10.3390/ijerph20176680
APA StyleShermock, S. B., Shermock, K. M., & Schepel, L. L. (2023). Closed-Loop Medication Management with an Electronic Health Record System in U.S. and Finnish Hospitals. International Journal of Environmental Research and Public Health, 20(17), 6680. https://doi.org/10.3390/ijerph20176680






