Maternal, Fetal and Neonatal Outcomes Related to Recreational Cannabis Use during Pregnancy: Analysis of a Real-World Clinical Data Warehouse between 2010 and 2019
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Source
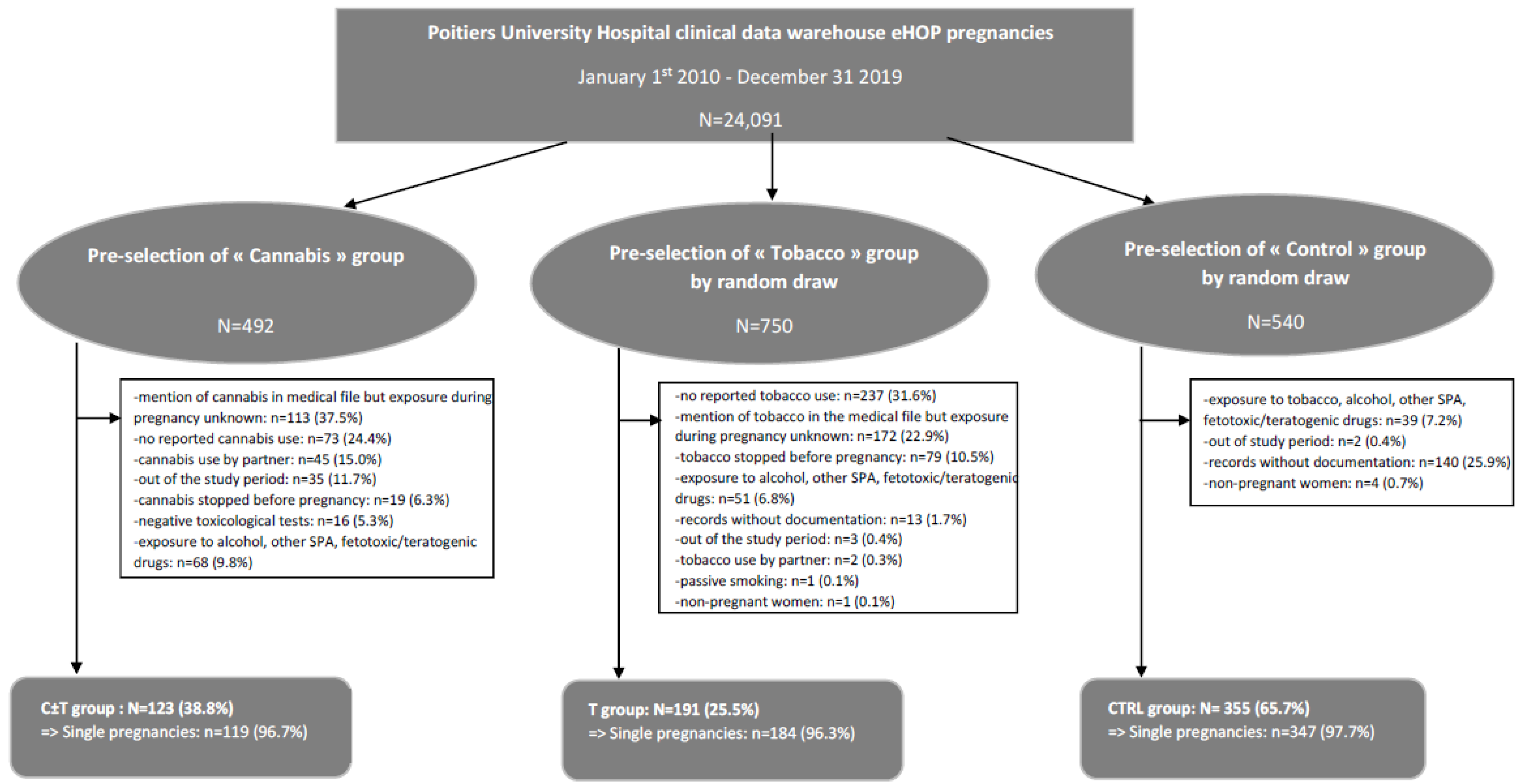
2.2. Study Population and Exposures
- -
- The subgroup “Cannabis” refers to those who were prenatally exposed to recreational cannabis, using diagnostic ICD-10 codes and the medical procedure CCAM corresponding to care of pregnant women and deliveries and either textual research in the records for different terms identifying cannabis exposure (cannabis, marijuana, etc.), results of toxicological tests revealing cannabis exposure or ICD-10 codes for hospital discharge diagnoses related to cannabis use.
- -
- The subgroup “Tobacco” refers to those who were prenatally exposed to tobacco, using diagnostic ICD-10 codes and the medical procedure CCAM corresponding to care of pregnant women and deliveries and either textual research in the records for different terms identifying tobacco exposure (tobacco, cigarettes, etc.) or ICD-10 codes for hospital discharge diagnoses related to tobacco use.
- -
- The subgroup “Control” refers to those who were not prenatally exposed to cannabis, tobacco, alcohol or any other medical or non-medical psychoactive substance, using diagnostic ICD-10 codes and the medical procedure CCAM corresponding to care of pregnant women and deliveries and excluding terms and ICD-10 codes corresponding to an exposure to cannabis, tobacco, alcohol or any other medical or non-medical psychoactive substance.
- -
- The C ± T group, including pregnant women and their children exposed to cannabis alone or in association with tobacco.
- -
- The T group, including pregnant women and their children exposed to tobacco alone.
- -
- The CTRL group, including pregnant women and their children not exposed to either cannabis or tobacco.
- -
- No pregnancy, pregnancy out of the study period or records without documentation.
- -
- Exposure to psychoactive substances other than cannabis and tobacco such as alcohol, cocaine, etc., or exposure to fetotoxic, teratogenic or other drugs increasing the risk of maternal, fetal or neonatal complications.
- -
- Specifically for the C ± T group: no reported cannabis use, cannabis stopped before pregnancy, mention of cannabis in the medical file but exposure during pregnancy unknown, cannabis use by partner only, no reported use or negative toxicological tests (notably, in the absence of reported use).
- -
- Specifically for the T group: no reported tobacco use, tobacco stopped before pregnancy, mention of tobacco in the medical file but exposure during pregnancy unknown, tobacco use by the partner only or passive smoking.
- -
- Specifically for the CTRL group: exposure to any psychoactive substances (cannabis, tobacco, alcohol, cocaine, etc.) or to fetotoxic, teratogenic or other drugs increasing the risk of maternal, fetal or neonatal complications.
2.3. Outcomes
2.4. Covariates
2.5. Data Analysis
2.6. Ethical Statement
3. Results
3.1. Study Population
3.2. Exposure
3.3. Outcomes
3.3.1. Voluntary Interruption of Pregnancy
3.3.2. At Least One AE during Pregnancy
3.3.3. Composite Adverse Pregnancy Outcome
3.3.4. Gestational Diabetes
3.3.5. At Least One Neonatal AE
3.3.6. Prematurity
3.3.7. Small for Gestational Age
3.3.8. Congenital Malformations
3.3.9. Other Severe Adverse Outcomes
3.3.10. Sensitivity Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Spilka, S.; Richard, J.B.; Le Nezet, O.; Janssen, E.; Brissot, A.; Philipon, A.; Shah, J.; Chyderiotis, S.; Andler, R.; Cogordan, C. Les Niveaux d’usages des Drogues Illicites en France en 2017—Tendances 128. Observatoire Français des Drogues et Toxicomanies et Santé Publique France. 2018. Available online: https://www.ofdt.fr/publications/collections/periodiques/lettre-tendances/les-niveaux-dusages-des-drogues-illicites-en-france-en-2017-tendances-128-novembre-2018/ (accessed on 11 December 2022).
- INSERM—DREES—Santé Publique France. Enquête Nationale Périnatale. Rapport 2021. Les Naissances, Le Suivi à Deux Mois et les établissements. Situation et évolution Depuis 2016. October 2022. Available online: https://www.santepubliquefrance.fr/les-actualites/2022/sante-publique-france-partenaire-de-la-6e-edition-de-l-enquete-nationale-perinatale (accessed on 12 December 2022).
- Blayac, L.; Ponte, C.; Lavaud, M.; Micallef, J.; Lapeyre-Mestre, M. Increase of cannabis and cocaine use by pregnant women in France from 2005 to 2018: Insights of the annual cross sectional OPPIDUM survey. Therapie 2022, 78, 201–211. [Google Scholar] [CrossRef]
- Bouquet, E.; Eiden, C.; Fauconneau, B.; Pion, C.; French Addictovigilance Network (FAN); Pain, S.; Perault-Pochat, M.C. Adverse events of recreational cannabis use during pregnancy reported to the French Addictovigilance Network between 2011 and 2020. Sci. Rep. 2022, 12, 16509. [Google Scholar] [CrossRef]
- Sa, S.; Fonseca, B.M. Cannabis consumption in reproductive function and teratogenicity. In Cannabis Use, Neurobiology, Psychology and Treatment; Martin, C.R., Patel, V.B., Preedy, V.R., Eds.; Elsevier Inc.: London, UK; New York, NY, USA, 2023; pp. 541–553. [Google Scholar]
- Maia, J.; Almada, M.; Midão, L.; Fonseca, B.M.; Braga, J.; Gonçalves, D.; Teixeira, N.; Correia-da-Silva, G. The Cannabinoid Delta-tetrahydrocannabinol Disrupts Estrogen Signaling in Human Placenta. Toxicol. Sci. 2020, 177, 420–430. [Google Scholar] [CrossRef]
- Ayonrinde, O.T.; Ayonrinde, O.A.; Van Rooyen, D.V.; Tait, R.; Dunn, M.; Mehta, S.; White, S.; Ayonrinde, O.K. Association between gestational cannabis exposure and maternal, perinatal, placental, and childhood outcomes. J. Dev. Orig. Health Dis. 2021, 12, 694–703. [Google Scholar] [CrossRef] [PubMed]
- Cherki, S. Le Point SINTES N°8. Observatoire Français des Drogues et Toxicomanies. 2022. Available online: https://www.ofdt.fr/BDD/sintes/LePointSINTES08.pdf (accessed on 12 January 2023).
- Dujourdy, L.; Besacier, F. A study of cannabis potency in France over a 25 years period (1992–2016). Forensic Sci. Int. 2017, 272, 72–80. [Google Scholar] [CrossRef]
- Madec, J.; Bouzillé, G.; Riou, C.; Van Hille, P.; Merour, C.; Artigny, M.L.; Delamarre, D.; Raimbert, V.; Lemordant, P.; Cuggia, M. eHOP clinical data warehouse: From a prototype to the creation of an inter-regional clinical data centers network. Stud. Health Technol. Inform. 2019, 264, 1536–1537. [Google Scholar] [PubMed]
- Douchet, M.A. Tabagisme et arrêt du Tabac en 2022. Observatoire Français des Drogues et des Tendances Addictives. 2023. Available online: http://www.ofdt.fr/ofdt/fr/tt_23bil.pdf (accessed on 28 August 2023).
- Saurel-Cubizolles, M.J.; Prunet, C.; Blondel, B. Cannabis use during pregnancy in France in 2010. BJOG 2014, 121, 971–977. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, C.E.; Sheeder, J.; Allshouse, A.A.; Scott, S.; Wymore, E.; Hopfer, A.; Hermesch, A.; Metz, T.D. Marijuana use in young mothers and adverse outcomes: A retrospective cohort study. BJOG 2019, 126, 1491–1497. [Google Scholar] [CrossRef]
- Heude, B.; Thiebaugeorges, O.; Goua, V.; Forhan, A.; Kaminski, M.; Foliguet, B.; Scheitzer, M.; Magnin, G.; Charles, M.A.; EDEN Mother-Child Cohort Study Group. Pre-pregnancy body mass index and weight gain during pregnancy: Relations with gestational diabetes and hypertension, and birth outcomes. Matern. Child Health J. 2012, 16, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Block, S.R.; Watkins, S.M.; Salemi, J.L.; Rutkowski, R.; Tanner, J.P.; Correia, J.A.; Kirby, R.S. Maternal pre-pregnancy body mass index and risk of selected birth defects: Evidence of a dose-response relationship. Paediatr. Perinat. Epidemiol. 2013, 27, 521–531. [Google Scholar] [CrossRef] [PubMed]
- Corsi, D.J. Epidemiological challenges to measuring prenatal cannabis use and its potential harms. BJOG 2020, 127, 17. [Google Scholar] [CrossRef]
- Corsi, D.J.; Hsu, H.; Weiss, D.; Fell, D.B.; Walker, M. Trends and correlates of cannabis use in pregnancy: A population based study in Ontario, Canada from 2012 to 2017. Can. J. Public Health 2019, 110, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Michalski, C.A.; Hung, R.J.; Seeto, R.A.; Dennis, C.L.; Brooks, J.D.; Henderson, J.; Levitan, R.; Lye, S.J.; Mathews, S.G.; Knight, J.A. Association between maternal cannabis use and birth outcomes: An observational study. BMC Pregnancy Childbirth 2020, 20, 771. [Google Scholar] [CrossRef] [PubMed]
- Embersin-Kyprianou, C.; Yermachenko, A.; Massari, V.; El-Khoury-Lesueur, F.; Melchior, M. Unexpected pregnancy, experience of sexual violence and contraception among women who use cannabis or other illegal substance in the Great Paris Region: Data from the 2016 Health Barometer. Rev. Epidemiol. Sante Publique 2020, 68, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Filion, K.B.; Abenhaim, H.A.; Eisenberg, M.J. Prevalence and outcomes of prenatal recreational cannabis use in high-income countries: A scoping review. BJOG 2020, 127, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Bandoli, G.; Jelliffe-Pawlowski, L.; Schumacher, B.; Baer, R.J.; Felder, J.N.; Fuchs, J.D.; Oltman, S.P.; Steurer, M.A.; Marienfeld, C. Cannabis-related diagnosis in pregnancy and adverse maternal and infant outcomes. Drug Alcohol Depend. 2021, 225, 108757. [Google Scholar] [CrossRef]
- Luke, S.; Hobbs, A.J.; Smith, M.; Riddell, C.; Murphy, P.; Agborsangaya, C.; Cantin, C.; Fahey, J.; Der, K.; Pederson, A.; et al. Cannabis use in pregnancy and maternal and infant outcomes: A Canadian cross-jurisdictional population-based cohort study. PLoS ONE 2022, 17, e0276824. [Google Scholar] [CrossRef] [PubMed]
- Prewitt, K.C.; Hayer, S.; Garg, B.; Benson, A.; Hedges, M.; Caughey, A.; Lo, J.O. Impact of Prenatal Cannabis Use Disorder on Perinatal Outcomes. J. Addict. Med. 2023, 17, e192–e198. [Google Scholar] [CrossRef]
- El Marroun, H.; Tiemeier, H.; Steegers, E.A.P.; Jaddoe, V.W.V.; Hofman, A.; Verhulst, F.C.; van den Brink, W.; Huizink, A.C. Intrauterine cannabis exposure affects fetal growth trajectories: The Generation R Study. J. Am. Acad. Child. Adolesc. Psychiatry 2009, 48, 1173–1181. [Google Scholar] [CrossRef]
- Metz, T.D.; Borgelt, L.M. Marijuana use in pregnancy and while breastfeeding. Obstet. Gynecol. 2018, 132, 1198–1210. [Google Scholar] [CrossRef] [PubMed]
- Risidzé, M.; Gargaros, A.; Fébrissy, C.; Dubucs, C.; Weyl, A.; Ousselin, J.; Aziza, J.; Arnal, J.F.; Lenfant, F. Estrogen actions in placental vascular morphogenesis and spiral artery remodeling: A comparative view between Humans and Mice. Cells 2023, 12, 620. [Google Scholar] [CrossRef] [PubMed]
- Gong, H.; Chen, Y.; Xu, J.; Xie, X.; Yu, D.; Yang, B.; Kuang, H. The regulation of ovary and conceptus on the uterine natural killer cells during early pregnancy. Reprod. Biol. Endocrinol. 2017, 15, 73. [Google Scholar] [CrossRef]
- Wuu, J.; Hellerstein, S.; Lipworth, L.; Wide, L.; Xu, B.; Yu, G.P.; Kuper, H.; Lagiou, P.; Hankinson, S.E.; Ekbom, A.; et al. Correlates of pregnancy oestrogen, progesterone and sex-hormon-binding globulin in the USA and China. Eur. J. Cancer Prev. 2002, 11, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Bemanian, M.; Vold, J.H.; Chowdhury, R.; Aas, C.F.; Gjestad, R.; Johansson, K.A.; Fadnes, L.T. Folate Status as a Nutritional Indicator among People with Substance Use Disorder; A Prospective Cohort Study in Norway. Int. J. Environ. Res. Public Health 2022, 19, 5754. [Google Scholar] [CrossRef] [PubMed]
- Williams, P.J.; Bulmer, J.N.; Innes, B.A.; Broughton Pipkin, F. Possible roles for folic acid in the regulation of trophoblast invasion and placental development in normal early human pregnancy. Biol. Reprod. 2011, 84, 1148–1153. [Google Scholar] [CrossRef]
- Stone, R. Pregnant women and substance use: Fear, stigma, and barriers to care. Health Justice 2015, 3, 2. [Google Scholar] [CrossRef]
- Bouquet, E.; Pain, S.; Eiden, C.; Jouanjus, E.; Richard, N.; Fauconneau, B.; Pérault-Pochat, M.C.; French Addictovigilance Nework. Adverse events of recreational cannabis use reported to the French addictovigilance network (2012–2017). Br. J. Clin. Pharmacol. 2021, 87, 3925–3937. [Google Scholar] [CrossRef] [PubMed]

| Group | p | |||
|---|---|---|---|---|
| C ± T (N = 123) | T (N = 191) | CTRL (N = 355) | ||
| Maternal age at birth (years) | N = 123 | N = 191 | N = 354 | <0.0001 |
| Mean: 25.5 | Mean: 28.4 | Mean: 29.5 | ||
| SD: 5.7 | SD: 5.8 | SD: 6.0 | ||
| Range: 14–40 | Range: 18–44 | Range: 15–45 | ||
| Pre-pregnancy BMI (kg/m2) | N = 70 | N = 151 | N = 206 | 0.004 |
| Mean: 22.8 | Mean: 25.9 | Mean: 24.7 | ||
| SD: 5.5 | SD: 7.4 | SD: 6.0 | ||
| Range: 16–39 | Range: 15–60 | Range: 16–54 | ||
| Pre-pregnancy BMI (kg/m2) | N = 70 | N = 151 | N = 354 | 0.05 |
| <30 kg/m2 | 59 (84.3%) | 113 (74.8%) | 174 (84.5%) | |
| ≥30 kg/m2 | 11 (15.7%) | 38 (25.2%) | 32 (15.5%) | |
| Social protection | N = 110 | N = 185 | N = 344 | <0.0001 |
| No mutual insurance | 65 (52.8%) | 69 (36.1%) | 115 (32.4%) | |
| Mutual insurance | 25 (20.3%) | 98 (51.3%) | 201 (56.7%) | |
| CMUc/AME | 20 (16.3%) | 18 (9.4%) | 28 (8.0%) | |
| Psychiatric history | N = 103 | N = 159 | N = 251 | <0.0001 |
| yes | 18 (14.6%) | 6 (3.1%) | 4 (1.1%) | |
| no | 85 (69.1%) | 153 (80.1%) | 247 (69.6%) | |
| Abuse/dependence history | N = 113 | N = 172 | N = 353 | <0.0001 |
| yes | 103 (83.7%) | 73 (38.2%) | 3 (0.8%) | |
| no | 10 (8.1%) | 99 (51.8%) | 350 (98.6%) | |
| Gyneco-obstetrical history | N = 62 | N = 191 | N = 309 | <0.001 |
| yes | 60 (48.8%) | 81 (42.4%) | 105 (29.6%) | |
| no | 62 (43.1%) | 110 (57.6%) | 204 (57.5%) | |
| Nulliparous | N = 105 | N = 181 | N = 301 | 0.0004 |
| yes | 62 (50.4%) | 87 (45.5%) | 129 (36.3%) | |
| no | 43 (35.0%) | 94 (49.2%) | 172 (48.5%) | |
| Exposures | C ± T Group | T Group | p |
|---|---|---|---|
| Cannabis use during pregnancy | |||
| In the first trimester | N = 123 | ||
| yes | 123 (100%) | ||
| no | 0 (0%) | ||
| daily | 31 (25.2%) | ||
| ≥10 times/day | 2 (1.6%) | ||
| In the second trimester | N = 76 | ||
| yes | 59 (77.6%) | ||
| no | 9 (11.8%) | ||
| unknown | 8 (10.6) | ||
| daily | 17 (28.8%) | ||
| ≥10 times/day | 0 (0.0%) | ||
| In the third trimester | N = 73 | ||
| yes | 50 (68.5%) | ||
| no | 15 (20.5%) | ||
| unknown | 8 (11.0%) | ||
| daily | 18 (36.0%) | ||
| ≥10 times/day | 0 (0.0%) | ||
| Tobacco use during pregnancy | |||
| In the first trimester | N = 123 | N = 191 | |
| yes | 107 (87.0%) | 191 (100.0%) | <0.00001 |
| no | 1 (0.8%) | 0 (0.0%) | |
| unknown | 15 (12.2%) | 0 (0.0%) | |
| daily | 53 (49.5%) | 128 (67.0%) | <0.00001 |
| ≥10 cigarettes/day | 26 (24.3%) | 54 (28.3%) | 0.16 |
| In the second trimester | N = 76 | N = 157 | |
| yes | 62 (81.6%) | 144 (91.7%) | 0.02 |
| no | 7 (9.2%) | 9 (5.7%) | |
| unknown | 7 (9.2%) | 4 (2.6%) | |
| daily | 28 (45.2%) | 91 (63.2%) | 0.002 |
| ≥10 cigarettes/day | 10 (16.1%) | 25 (17.4%) | 0.98 |
| In the third trimester | N = 73 | N = 146 | |
| yes | 56 (76.7%) | 125 (85.6%) | 0.10 |
| no | 10 (13.7%) | 11 (7.5%) | |
| unknown | 7 (9.6%) | 10 (6.9%) | |
| daily | 30 (53.6%) | 80 (64.0%) | 0.06 |
| ≥10 cigarettes/day | 12 (21.4%) | 21 (16.8%) | 0.69 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bouquet, E.; Blouin, P.; Pérault-Pochat, M.-C.; Carlier-Guérin, C.; Millot, F.; Ricco, J.-B.; De Keizer, J.; Pain, S.; Guétarni, F. Maternal, Fetal and Neonatal Outcomes Related to Recreational Cannabis Use during Pregnancy: Analysis of a Real-World Clinical Data Warehouse between 2010 and 2019. Int. J. Environ. Res. Public Health 2023, 20, 6686. https://doi.org/10.3390/ijerph20176686
Bouquet E, Blouin P, Pérault-Pochat M-C, Carlier-Guérin C, Millot F, Ricco J-B, De Keizer J, Pain S, Guétarni F. Maternal, Fetal and Neonatal Outcomes Related to Recreational Cannabis Use during Pregnancy: Analysis of a Real-World Clinical Data Warehouse between 2010 and 2019. International Journal of Environmental Research and Public Health. 2023; 20(17):6686. https://doi.org/10.3390/ijerph20176686
Chicago/Turabian StyleBouquet, Emilie, Pascal Blouin, Marie-Christine Pérault-Pochat, Caroline Carlier-Guérin, Frédéric Millot, Jean-Baptiste Ricco, Joe De Keizer, Stéphanie Pain, and Farid Guétarni. 2023. "Maternal, Fetal and Neonatal Outcomes Related to Recreational Cannabis Use during Pregnancy: Analysis of a Real-World Clinical Data Warehouse between 2010 and 2019" International Journal of Environmental Research and Public Health 20, no. 17: 6686. https://doi.org/10.3390/ijerph20176686






