The MothersBabies Study, an Australian Prospective Cohort Study Analyzing the Microbiome in the Preconception and Perinatal Period to Determine Risk of Adverse Pregnancy, Postpartum, and Child-Related Health Outcomes: Study Protocol
Abstract
:1. Introduction
- Establish the natural evolution of the maternal microbiome from preconception, through pregnancy, to the postpartum stage in multi-site microbiome communities (stool, vaginal, oral, skin, blood, and urine) in Australian women;
- Define the multi-site microbiome signatures (stool, vaginal, oral, skin, blood, and urine) that are associated with adverse outcomes in pregnancy (e.g., GDM, preeclampsia, perinatal mental health disorders, and obesity in pregnancy/EGWG).
2. Methods and Analysis
2.1. Study Design, Sample Selection, and Setting
2.2. Inclusion/Exclusion Criteria
2.3. Data Collection
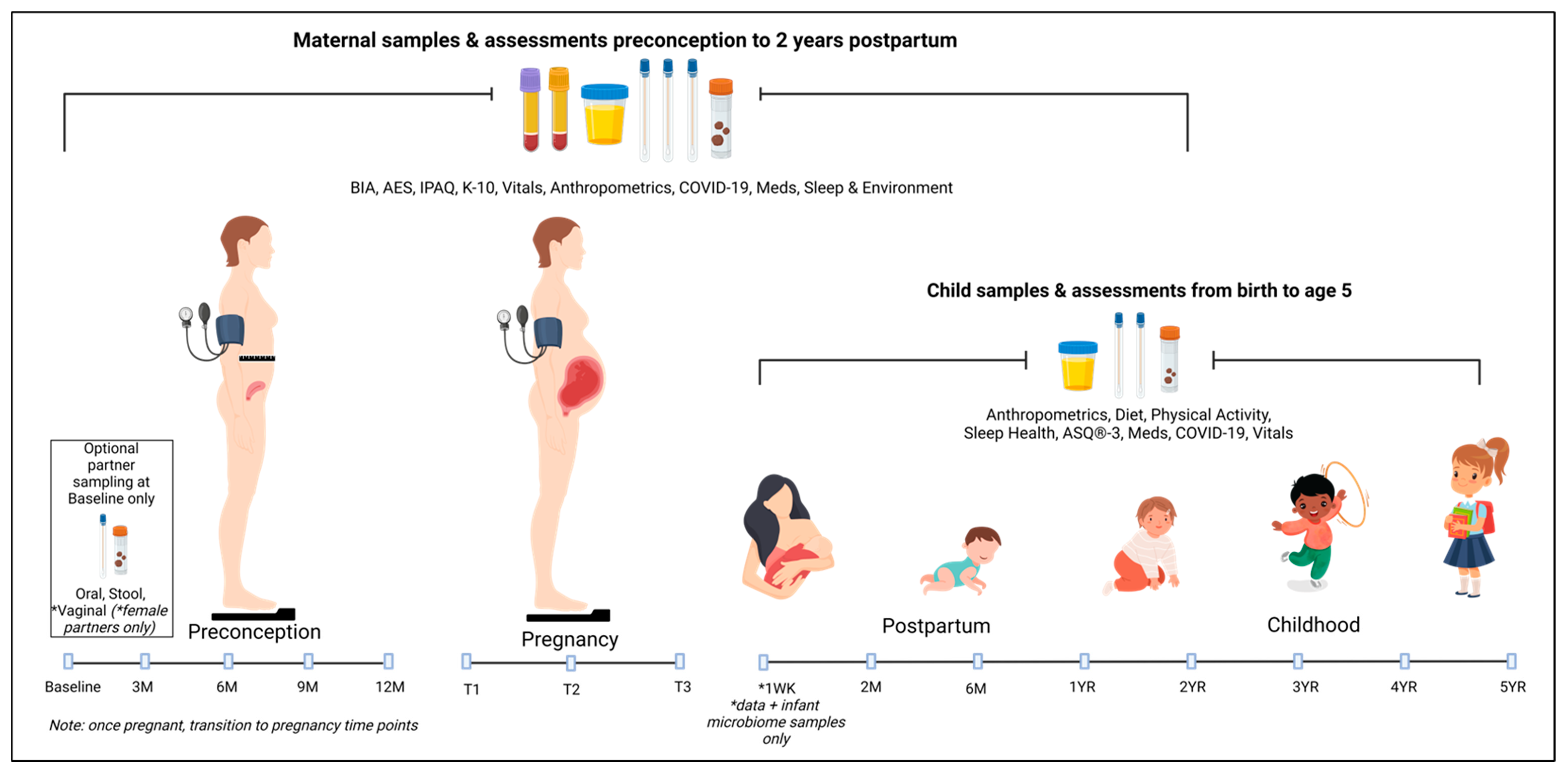
2.3.1. Timeline
2.3.2. Samples for Microbiome Analysis
2.3.3. Samples for Metabolomic Analysis
2.3.4. Clinical Data
2.3.5. Physical Measures
2.3.6. Questionnaires
- Maternal diet is recorded using the Australian Eating Survey [34] at Baseline and Trimester 3, and a 7-day food diary (Easy Diet Diary app (https://xyris.com.au/products/easy-diet-diary/ accessed on 17 November 2021)) at all other visits. Both of these tools are validated for Australian populations.
- Maternal physical activity is assessed using the International Physical Activity Questionnaire [35], a publicly available, open-access tool that is useful in evaluating physical activity in large-cohort studies. The long version will be used in this study, as it provides researchers with more detailed information for comparing estimates of physical activity.
- Maternal mood is assessed using the Kessler Psychological Distress Scale (K10) [36], a widely recommended simple measure of psychological distress, and as a measure of outcomes following treatment for common mental health disorders, including anxiety and depression in pregnancy [37]. Due to participants completing eSurveys, this was the preferred tool over the Edinburgh Depression Scale (EDS) [38], as researchers could not guarantee participant safety should they disclose a positive response to Q10 in the EDS.
- The EPOCH Toddler Dietary Questionnaire (EPOCH-TDQ) [39] is a 13-item questionnaire, developed to assess aspects of dietary intake that are related to an increased risk of overweight or obesity in children aged from 1 to 2.9 years. The core questions provide data on vegetable intake, discretionary food and drink intake, and the type of grains and milk consumed. An additional set of questions (Questions 6–7) can be included, which provide data on feeding practices that may be associated with an increased obesity risk (milk intake and infant formula intake).
- The Ages & Stages (ASQ3) [40] developmental questionnaire, is used, and screens infant and child development in the areas of communication, gross motor, fine motor, problem-solving, and personal–social skills.
- The Movement Behaviour Questionnaire–Child (MBQ-C) [41] is a validated “fit-for-purpose” rapid assessment tool for measuring movement behaviours in children aged 0–5 years. The MBQ-C will be administered from the time the parent reports that the child has started walking. The MBQ-C assesses physical activity and screen time separately for weekdays and weekend days, and calculates a weighted daily average for the daily total active play, energetic play, passive screen time, and interactive screen time.
- Sleep health will be assessed using the MBQ-C, which assesses sleep routine in addition to sleep duration. Additionally, the Patient-Reported Outcomes Measurement Information System (PROMIS) EC Parent-Report SF v1.0-Sleep Health 8 a [42] asks eight short questions of parents to assess the quality of sleep of their children. PROMIS tools were developed in the English language, using extensive qualitative methods to ensure conceptual and semantic clarity. They have been tested for reliability and comparability against more established measures of these same content areas.
2.4. Impact of COVID-19 and Adaptation of Study
2.5. Primary Outcome and Covariate Assessment
- Examining how the pregnancy microbiome differs in women with pregnancy complications;
- Exploring whether the maternal microbiome is associated with excessive weight gain in pregnancy;
- Examining how COVID-19 infection/exposure has affected health outcomes for mothers and children;
- Examining how the developing paediatric microbiome in the first 5 years of life relates to the mode of birth, maternal microbiome transmission, mode of feeding, and non-communicable childhood diseases.
- Pre-pregnancy BMI;
- Diet and physical activity;
- Mode and location of birth;
- Medication and supplements (including, but not limited to, antibiotics, prescription medication, over the counter medication, dietary supplements, pre/probiotics, vaccinations);
- Parity;
- Maternal age;
- Type of conception.
2.6. Microbiota DNA Extraction, Quantification, and Sequencing
2.7. Metabolomic Analysis
2.8. Sample Size Calculation
Power Calculation
2.9. Data Analysis Plan
2.10. Data Management, Ethical Procedures, and Confidentiality
2.11. Patient and Public Involvement Statement
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Neri, C.; Serafino, E.; Morlando, M.; Familiari, A. Microbiome and Gestational Diabetes: Interactions with Pregnancy Outcome and Long-Term Infant Health. J. Diabetes Res. 2021, 2021, 9994734. [Google Scholar] [CrossRef]
- Dominguez-Bello, M.G.; Godoy-Vitorino, F.; Knight, R.; Blaser, M.J. Role of the microbiome in human development. Gut 2019, 68, 1108–1114. [Google Scholar] [CrossRef] [PubMed]
- Stiemsma, L.T.; Michels, K.B. The Role of the Microbiome in the Developmental Origins of Health and Disease. Pediatrics 2018, 141, e20172437. [Google Scholar] [CrossRef] [PubMed]
- Nuriel-Ohayon, M.; Neuman, H.; Koren, O. Microbial Changes during Pregnancy, Birth, and Infancy. Front. Microbiol. 2016, 7, 1031. [Google Scholar] [CrossRef] [PubMed]
- Dunlop, A.L.; Mulle, J.G.; Ferranti, E.P.; Edwards, S.; Dunn, A.B.; Corwin, E.J. Maternal Microbiome and Pregnancy Outcomes That Impact Infant Health: A Review. Adv. Neonatal Care 2015, 15, 377–385. [Google Scholar] [CrossRef]
- Schoenmakers, S.; Steegers-Theunissen, R.; Faas, M. The matter of the reproductive microbiome. Obstet. Med. 2018, 12, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, J.; Heslehurst, N.; Hall, J.; Schoenaker, D.A.J.M.; Hutchinson, J.; Cade, J.E.; Poston, L.; Barrett, G.; Crozier, S.R.; Barker, M.; et al. Before the beginning: Nutrition and lifestyle in the preconception period and its importance for future health. Lancet 2018, 391, 1830–1841. [Google Scholar] [CrossRef]
- Genuis, S.J.; Genuis, R.A. Preconception Care: A New Standard of Care within Maternal Health Services. BioMed Res. Int. 2016, 2016, 30. [Google Scholar] [CrossRef]
- Calatayud, M.; Koren, O.; Collado, M.C. Maternal Microbiome and Metabolic Health Program Microbiome Development and Health of the Offspring. Trends Endocrinol. Metab. 2019, 30, 735–744. [Google Scholar] [CrossRef]
- Taddei, C.R.; Cortez, R.V.; Mattar, R.; Torloni, M.R.; Daher, S. Microbiome in normal and pathological pregnancies: A literature overview. Am. J. Reprod. Immunol. 2018, 80, e12993. [Google Scholar] [CrossRef]
- Neuman, H.; Koren, O. The Pregnancy Microbiome. In Intestinal Microbiome: Functional Aspects in Health and Disease; Nestlé Nutrition Institute Workshop Series; Vevey/S. Karger AG.: Basel, Switzerland, 2017; pp. 1–9. [Google Scholar] [CrossRef]
- Koren, O.; Goodrich, J.K.; Cullender, T.C.; Spor, A.; Laitinen, K.; Backhed, H.K.; Gonzalez, A.; Werner, J.J.; Angenent, L.T.; Knight, R.; et al. Host remodeling of the gut microbiome and metabolic changes during pregnancy. Cell 2012, 150, 470–480. [Google Scholar] [CrossRef]
- Turjeman, S.; Collado, M.C.; Koren, O. The gut microbiome in pregnancy and pregnancy complications. Curr. Opin. Endocr. Metab. Res. 2021, 18, 133–138. [Google Scholar] [CrossRef]
- Jiang, G.; Zhou, Z.; Li, X.; Qian, Y.; Wang, K. The Gut Microbiome During Pregnancy. Matern.-Fetal Med. 2023, 5, 36–43. [Google Scholar] [CrossRef]
- Susic, D.; Davis, G.; O’Sullivan, A.J.; McGovern, E.; Harris, K.; Roberts, L.M.; Craig, M.E.; Mangos, G.; Hold, G.L.; El-Omar, E.M.; et al. Microbiome Understanding in Maternity Study (MUMS), an Australian prospective longitudinal cohort study of maternal and infant microbiota: Study protocol. BMJ Open 2020, 10, e040189. [Google Scholar] [PubMed]
- Aagaard, K.; Ma, J.; Antony, K.M.; Ganu, R.; Petrosino, J.; Versalovic, J. The Placenta Harbors a Unique Microbiome. Sci. Transl. Med. 2014, 6, 237ra265. [Google Scholar] [CrossRef]
- Aagaard, K.; Riehle, K.; Ma, J.; Segata, N.; Mistretta, T.-A.; Coarfa, C.; Raza, S.; Rosenbaum, S.; Van den Veyver, I.; Milosavljevic, A.; et al. A Metagenomic Approach to Characterization of the Vaginal Microbiome Signature in Pregnancy. PLoS ONE 2012, 7, e36466. [Google Scholar] [CrossRef]
- Fettweis, J.M.; Serrano, M.G.; Brooks, J.P.; Edwards, D.J.; Girerd, P.H.; Parikh, H.I.; Huang, B.; Arodz, T.J.; Edupuganti, L.; Glascock, A.L.; et al. The vaginal microbiome and preterm birth. Nat. Med. 2019, 25, 1012–1021. [Google Scholar] [CrossRef]
- Serrano, M.G.; Parikh, H.I.; Brooks, J.P.; Edwards, D.J.; Arodz, T.J.; Edupuganti, L.; Huang, B.; Girerd, P.H.; Bokhari, Y.A.; Bradley, S.P.; et al. Racioethnic diversity in the dynamics of the vaginal microbiome during pregnancy. Nat. Med. 2019, 25, 1001–1011. [Google Scholar] [CrossRef] [PubMed]
- Kunasegaran, T.; Balasubramaniam, V.; Arasoo, V.J.T.; Palanisamy, U.D.; Ramadas, A. The Modulation of Gut Microbiota Composition in the Pathophysiology of Gestational Diabetes Mellitus: A Systematic Review. Biology 2021, 10, 1027. [Google Scholar] [CrossRef]
- Rold, L.S.; Bundgaard-Nielsen, C.; Niemann Holm-Jacobsen, J.; Glud Ovesen, P.; Leutscher, P.; Hagstrom, S.; Sorensen, S. Characteristics of the gut microbiome in women with gestational diabetes mellitus: A systematic review. PLoS ONE 2022, 17, e0262618. [Google Scholar] [CrossRef]
- Tsarna, E.; Christopoulos, P. The role of gut microbiome in prevention, diagnosis and treatment of gestational diabetes mellitus. J. Obstet. Gynaecol. 2022, 42, 719–725. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Xie, X.; Yuan, T.; Wang, Y.; Zhao, F.; Zhou, Z.; Zhang, H. Epidemiological trends of maternal hypertensive disorders of pregnancy at the global, regional, and national levels: A population-based study. BMC Pregnancy Childbirth 2021, 21, 364. [Google Scholar] [CrossRef]
- Chen, X.; Li, P.; Liu, M.; Zheng, H.; He, Y.; Chen, M.X.; Tang, W.; Yue, X.; Huang, Y.; Zhuang, L.; et al. Gut dysbiosis induces the development of pre-eclampsia through bacterial translocation. Gut 2020, 69, 513–522. [Google Scholar] [CrossRef] [PubMed]
- Pinto, Y.; Frishman, S.; Turjeman, S.; Eshel, A.; Nuriel-Ohayon, M.; Shrossel, O.; Ziv, O.; Walters, W.; Parsonnet, J.; Ley, C.; et al. Gestational diabetes is driven by microbiota-induced inflammation months before diagnosis. Gut 2023, 72, 918–928. [Google Scholar] [CrossRef] [PubMed]
- Backhed, F.; Roswall, J.; Peng, Y.; Feng, Q.; Jia, H.; Kovatcheva-Datchary, P.; Li, Y.; Xia, Y.; Xie, H.; Zhong, H.; et al. Dynamics and Stabilization of the Human Gut Microbiome during the First Year of Life. Cell Host Microbe 2015, 17, 690–703. [Google Scholar] [CrossRef]
- Sordillo, J.E.; Korrick, S.; Laranjo, N.; Carey, V.; Weinstock, G.M.; Gold, D.R.; O’Connor, G.; Sandel, M.; Bacharier, L.B.; Beigelman, A.; et al. Association of the Infant Gut Microbiome With Early Childhood Neurodevelopmental Outcomes: An Ancillary Study to the VDAART Randomized Clinical Trial. JAMA Netw. Open 2019, 2, e190905. [Google Scholar] [CrossRef]
- Neuman, H.; Forsythe, P.; Uzan, A.; Avni, O.; Koren, O. Antibiotics in early life: Dysbiosis and the damage done. FEMS Microbiol. Rev. 2018, 42, 489–499. [Google Scholar] [CrossRef]
- Mohammadkhah, A.I.; Simpson, E.B.; Patterson, S.G.; Ferguson, J.F. Development of the Gut Microbiome in Children, and Lifetime Implications for Obesity and Cardiometabolic Disease. Children 2018, 5, 160. [Google Scholar] [CrossRef]
- Stanislawski, M.A.; Dabelea, D.; Wagner, B.D.; Iszatt, N.; Dahl, C.; Sontag, M.K.; Knight, R.; Lozupone, C.A.; Eggesbø, M. Gut Microbiota in the First 2 Years of Life and the Association with Body Mass Index at Age 12 in a Norwegian Birth Cohort. MBio 2018, 9. [Google Scholar] [CrossRef]
- Kummeling, I.; Stelma, F.F.; Dagnelie, P.C.; Snijders, B.E.; Penders, J.; Huber, M.; van Ree, R.; van den Brandt, P.A.; Thijs, C. Early life exposure to antibiotics and the subsequent development of eczema, wheeze, and allergic sensitization in the first 2 years of life: The KOALA Birth Cohort Study. Pediatrics 2007, 119, e225–e231. [Google Scholar] [CrossRef]
- Invitek Molecular. Invitek Diagnostics: Microbiome Sample Management. Available online: https://www.chemie-brunschwig.ch/documents/suppliers-information/invitek-molecular/Gut-Microbiome-2022.pdf (accessed on 1 June 2022).
- Copan. eNAT® Nucleic Acid Collection and Preservation Medium. Available online: https://www.copangroup.com/product-ranges/enat/ (accessed on 1 June 2022).
- Collins, C.E.; Boggess, M.M.; Watson, J.F.; Guest, M.; Duncanson, K.; Pezdirc, K.; Rollo, M.; Hutchesson, M.J.; Burrows, T.L. Reproducibility and comparative validity of a food frequency questionnaire for Australian adults. Clin. Nutr. 2014, 33, 906–914. [Google Scholar] [CrossRef]
- International Physical Activity Questionnaire (IPAQ). IPAQ Long Last 7 Days Self-Administered Format. Available online: https://sites.google.com/site/theipaq/ (accessed on 10 December 2018).
- Kessler, R.C.; Barker, P.R.; Colpe, L.J.; Epstein, J.F.; Gfroerer, J.C.; Hiripi, E.; Howes, M.J.; Normand, S.L.; Manderscheid, R.W.; Walters, E.E.; et al. Screening for serious mental illness in the general population. Arch. Gen. Psychiatry 2003, 60, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Spies, G.; Stein, D.J.; Roos, A.; Faure, S.C.; Mostert, J.; Seedat, S.; Vythilingum, B. Validity of the Kessler 10 (K-10) in detecting DSM-IV defined mood and anxiety disorders among pregnant women. Arch. Women’s Ment. Health 2009, 12, 69–74. [Google Scholar] [CrossRef]
- Cox, J.L.; Holden, J.M.; Sagovsky, R. Detection of postnatal depression: Development of the 10-item Edinburgh Postnatal Depression Scale. Br. J. Psychiatry 1987, 150, 782–786. [Google Scholar] [CrossRef] [PubMed]
- Zarnowiecki, D.; Byrne, R. New rapid assessment tools to measure obesity related behaviours in 0–5 years old, oral presentation in ‘EPOCH Special Symposia’. In Proceedings of the Australia and New Zealand Obesity Society Annual Scientific Meeting, Brisbane, Australia, 20–22 July 2021. [Google Scholar]
- Squires, J.; Bricker, D. Ages & Stages Questionnaires®, Third Edition (ASQ-3™): A Parent-Completed Child Monitoring System, 3rd ed.; Brooks Publishing Company: Baltimore, MD, USA, 2009; ISBN 978-1-59857-027-4. [Google Scholar]
- Trost, S.G.; Terranova, C.; Brookes, D.; Chai, L.K.; Taylor, R.; Byrne, T. Psychometric properties of the Movement Behaviour Questionnaire for Babies (MBQ-B) and Young Children (MBQ-C). In Proceedings of the International Society for Behavioral Nutrition and Physical Activity (ISBNPA) Annual Meeting, Phoenix, AZ, USA, 18–21 May 2022. [Google Scholar]
- Bevans, K.B.; Meltzer, L.J.; De La Motte, A.; Kratchman, A.; Viel, D.; Forrest, C.B. Qualitative Development and Content Validation of the PROMIS Pediatric Sleep Health Items. Behav. Sleep. Med. 2019, 17, 657–671. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.N.; Boyle, J.A.; Lang, A.Y.; Harrison, C.L. Preconception Health Attitudes and Behaviours of Women: A Qualitative Investigation. Nutrients 2019, 11, 1490. [Google Scholar] [CrossRef] [PubMed]
- Butera, A.; Maiorani, C.; Morandini, A.; Simonini, M.; Colnaghi, A.; Morittu, S.; Barbieri, S.; Ricci, M.; Guerrisi, G.; Piloni, D.; et al. Assessment of Oral Microbiome Changes in Healthy and COVID-19-Affected Pregnant Women: A Narrative Review. Microorganisms 2021, 9, 2385. [Google Scholar] [CrossRef]
- Finlay, B.B.; Amato, K.R.; Azad, M.; Blaser, M.J.; Bosch, T.C.G.; Chu, H.; Dominguez-Bello, M.G.; Ehrlich, S.D.; Elinav, E.; Geva-Zatorsky, N.; et al. The hygiene hypothesis, the COVID pandemic, and consequences for the human microbiome. Proc. Natl. Acad. Sci. USA 2021, 118, e2010217118. [Google Scholar] [CrossRef] [PubMed]
- Yun Kit, Y.; Tao, Z.; Grace Chung-Yan, L.; Fen, Z.; Qin, L.; Amy, Y.L.L.; Arthur, C.K.C.; Chun Pan, C.; Eugene, Y.K.T.; Kitty, S.C.F.; et al. Gut microbiota composition reflects disease severity and dysfunctional immune responses in patients with COVID-19. Gut 2021, 70, 698. [Google Scholar] [CrossRef]
- Etti, M.; Alger, J.; Salas, S.P.; Saggers, R.; Ramdin, T.; Endler, M.; Gemzell-Danielsson, K.; Alfvén, T.; Ahmed, Y.; Callejas, A.; et al. Global research priorities for COVID-19 in maternal, reproductive and child health: Results of an international survey. PLoS ONE 2021, 16, e0257516. [Google Scholar] [CrossRef]
- Romano-Keeler, J.; Zhang, J.; Sun, J. COVID-19 and the neonatal microbiome: Will the pandemic cost infants their microbes? Gut Microbes 2021, 13, 1912562. [Google Scholar] [CrossRef]
- Du, M.; Yang, J.; Han, N.; Liu, M.; Liu, J. Association between the COVID-19 pandemic and the risk for adverse pregnancy outcomes: A cohort study. BMJ Open 2021, 11, e047900. [Google Scholar] [CrossRef]
- Zanardo, V.; Tortora, D.; Sandri, A.; Severino, L.; Mesirca, P.; Straface, G. COVID-19 pandemic: Impact on gestational diabetes mellitus prevalence. Diabetes Res. Clin. Pract. 2022, 183, 109149. [Google Scholar] [CrossRef]
- Dona, A.C.; Jiménez, B.; Schäfer, H.; Humpfer, E.; Spraul, M.; Lewis, M.R.; Pearce, J.T.M.; Holmes, E.; Lindon, J.C.; Nicholson, J.K. Precision High-Throughput Proton NMR Spectroscopy of Human Urine, Serum, and Plasma for Large-Scale Metabolic Phenotyping. Anal. Chem. 2014, 86, 9887–9894. [Google Scholar] [CrossRef]
- Gratton, J.; Phetcharaburanin, J.; Mullish, B.H.; Williams, H.R.T.; Thursz, M.; Nicholson, J.K.; Holmes, E.; Marchesi, J.R.; Li, J.V. Optimized Sample Handling Strategy for Metabolic Profiling of Human Feces. Anal. Chem. 2016, 88, 4661–4668. [Google Scholar] [CrossRef]
- Jiménez, B.; Holmes, E.; Heude, C.; Tolson, R.F.; Harvey, N.; Lodge, S.L.; Chetwynd, A.J.; Cannet, C.; Fang, F.; Pearce, J.T.M.; et al. Quantitative Lipoprotein Subclass and Low Molecular Weight Metabolite Analysis in Human Serum and Plasma by 1H NMR Spectroscopy in a Multilaboratory Trial. Anal. Chem. 2018, 90, 11962–11971. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef]
- Bushnell, B. BBMap. Available online: https://sourceforge.net/projects/bbmap/ (accessed on 4 July 2022).
- Li, H. Minimap2: Pairwise alignment for nucleotide sequences. Bioinformatics 2018, 34, 3094–3100. [Google Scholar] [CrossRef]
- Wood, D.E.; Lu, J.; Langmead, B. Improved metagenomic analysis with Kraken 2. Genome Biol. 2019, 20, 257. [Google Scholar] [CrossRef]
- Zhou, C.; Xu, Q.; He, S.; Ye, W.; Cao, R.; Wang, P.; Ling, Y.; Yan, X.; Wang, Q.; Zhang, G. GTDB: An integrated resource for glycosyltransferase sequences and annotations. Database 2020, 2020, baaa047. [Google Scholar] [CrossRef]
- Lu, J.; Breitwieser, F.; Thielen, P.; Salzberg, S. Bracken: Estimating species abundance in metagenomics data. PeerJ Comput. Sci. 2017, 3, e104. [Google Scholar] [CrossRef]
- Beghini, F.; McIver, L.J.; Blanco-Míguez, A.; Dubois, L.; Asnicar, F.; Maharjan, S.; Mailyan, A.; Manghi, P.; Scholz, M.; Thomas, A.M.; et al. Integrating taxonomic, functional, and strain-level profiling of diverse microbial communities with bioBakery 3. eLife 2021, 10, e65088. [Google Scholar] [CrossRef]
- Oksanen, J.; Simpson, G.L.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.B.; Solymos, P.; Stevens, M.H.H.; Szoecs, E.; et al. Vegan: Community Ecology Package. Available online: https://cran.r-project.org/web/packages/vegan/index.html (accessed on 4 July 2022).
- Yang, S. otuSummary: Summarizing OTU Table Regarding the Composition, Abundance and Beta Diversity of Abundant and Rare Biospheres. Available online: https://cran.r-project.org/web/packages/otuSummary/index.html (accessed on 4 July 2022).
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef] [PubMed]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2009. [Google Scholar]
- Harris, P.A.; Taylor, R.; Minor, B.L.; Elliott, V.; Fernandez, M.; O’Neal, L.; McLeod, L.; Delacqua, G.; Delacqua, F.; Kirby, J.; et al. The REDCap consortium: Building an international community of software platform partners. J. Biomed. Inform. 2019, 95, 103208. [Google Scholar] [CrossRef]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef]
| Maternal | Partner | Child | |
|---|---|---|---|
| Stool × 2 | X | X | X |
| Oral | X | X | X |
| Skin | X | X | |
| Urine * | X | X | |
| Blood * | X | ||
| Vaginal | X | X ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Strout, N.; Pasic, L.; Hicks, C.; Chua, X.-Y.; Tashvighi, N.; Butler, P.; Liu, Z.; El-Assaad, F.; Holmes, E.; Susic, D.; et al. The MothersBabies Study, an Australian Prospective Cohort Study Analyzing the Microbiome in the Preconception and Perinatal Period to Determine Risk of Adverse Pregnancy, Postpartum, and Child-Related Health Outcomes: Study Protocol. Int. J. Environ. Res. Public Health 2023, 20, 6736. https://doi.org/10.3390/ijerph20186736
Strout N, Pasic L, Hicks C, Chua X-Y, Tashvighi N, Butler P, Liu Z, El-Assaad F, Holmes E, Susic D, et al. The MothersBabies Study, an Australian Prospective Cohort Study Analyzing the Microbiome in the Preconception and Perinatal Period to Determine Risk of Adverse Pregnancy, Postpartum, and Child-Related Health Outcomes: Study Protocol. International Journal of Environmental Research and Public Health. 2023; 20(18):6736. https://doi.org/10.3390/ijerph20186736
Chicago/Turabian StyleStrout, Naomi, Lana Pasic, Chloe Hicks, Xin-Yi Chua, Niki Tashvighi, Phoebe Butler, Zhixin Liu, Fatima El-Assaad, Elaine Holmes, Daniella Susic, and et al. 2023. "The MothersBabies Study, an Australian Prospective Cohort Study Analyzing the Microbiome in the Preconception and Perinatal Period to Determine Risk of Adverse Pregnancy, Postpartum, and Child-Related Health Outcomes: Study Protocol" International Journal of Environmental Research and Public Health 20, no. 18: 6736. https://doi.org/10.3390/ijerph20186736
APA StyleStrout, N., Pasic, L., Hicks, C., Chua, X.-Y., Tashvighi, N., Butler, P., Liu, Z., El-Assaad, F., Holmes, E., Susic, D., Samaras, K., Craig, M. E., Davis, G. K., Henry, A., Ledger, W. L., & El-Omar, E. M. (2023). The MothersBabies Study, an Australian Prospective Cohort Study Analyzing the Microbiome in the Preconception and Perinatal Period to Determine Risk of Adverse Pregnancy, Postpartum, and Child-Related Health Outcomes: Study Protocol. International Journal of Environmental Research and Public Health, 20(18), 6736. https://doi.org/10.3390/ijerph20186736













