Informing ASR Treatment Practices in a Florida Aquifer through a Human Health Risk Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Groundwater Conditions of the Study Area
2.1.1. Hydrogeology
2.1.2. Groundwater Chemistry
2.1.3. Inactivation Data for E. coli, Pseudomonas aeruginosa, Poliovirus Type 1 and Cryptosporidium parvum
2.2. Human Health Risk Assessment
2.2.1. Quantitative Microbial Risk Assessment
2.2.2. Hazard Identification
2.2.3. Exposure Assessment
| Model Parameters | Data Type | Model Values | References | ||
|---|---|---|---|---|---|
| N0 | Microorganisms and Concentrations at Time Zero | ||||
| E. coli | 3 CFU/mL | [30] | |||
| P. aeruginosa | 3 CFU/mL | Assumption | |||
| PV1 | 1.4 × 10−4 PFU/mL | [30] | |||
| C. parvum | 0.20 oocysts/mL | [30] | |||
| V | Volumes Consumed | ||||
| Drinking | 926 mL | [31] | |||
| Swimming | 32 mL | [32] | |||
| I | Microbial Inactivation | ||||
| Microorganism | Best Fit Model | Model Rate Constants | |||
| E. coli | Biphasic | k1 | −0.4878 log10/day | [15,16] | |
| k2 | −0.0118 log10/day | ||||
| P. aeruginosa | Biphasic | k1 | −0.4480 log10/day | [15,16] | |
| k2 | −0.0052 log10/day | ||||
| PV1 | Log-linear | kmax | −0.1900 log10/day | [15,16] | |
| C. parvum | Weibull | δ | 5.04 days for 1.0 log10 reduction | [15,16] | |
| p | 0.69 | ||||
2.2.4. Dose–Response Assessment
2.2.5. Risk of Infection Estimates
2.2.6. DALYs per Case Estimations
| Pathogen | DALYs per Case | Description | References |
|---|---|---|---|
| Pathogenic E. coli | 2.98 × 10−3 | 100% susceptible | [42,43] |
| P. aeruginosa | 9.00 × 10−4 | 100% susceptible Assuming only gastrointestinal infection and no death | [37,44] |
| Adenovirus (adapted from PV1) | 1.56 × 10−3 | 100% susceptible | [45] |
| Cryptosporidium spp. | 3.22 × 10−3 | 100% susceptible | [43,46] |
3. Results
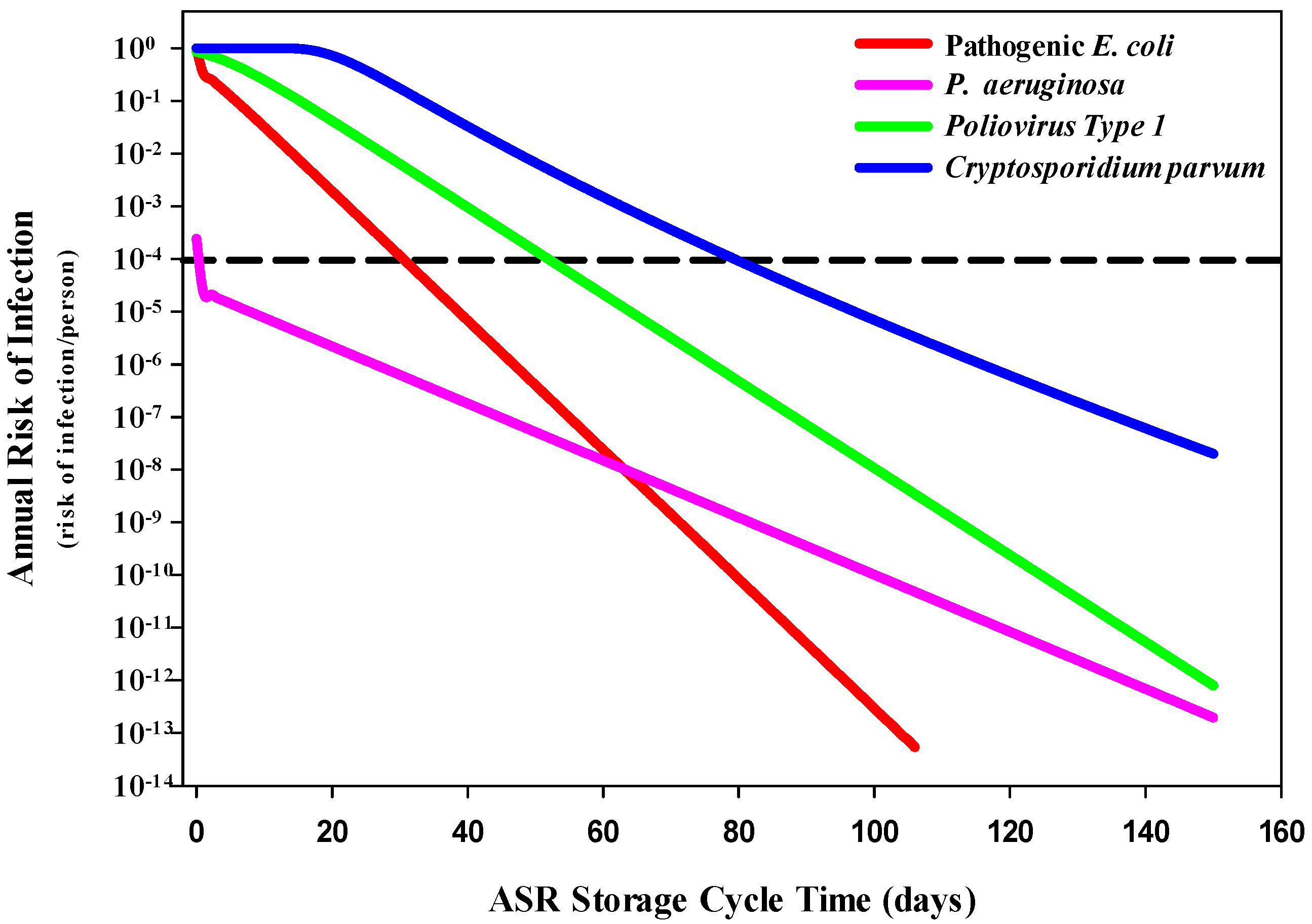
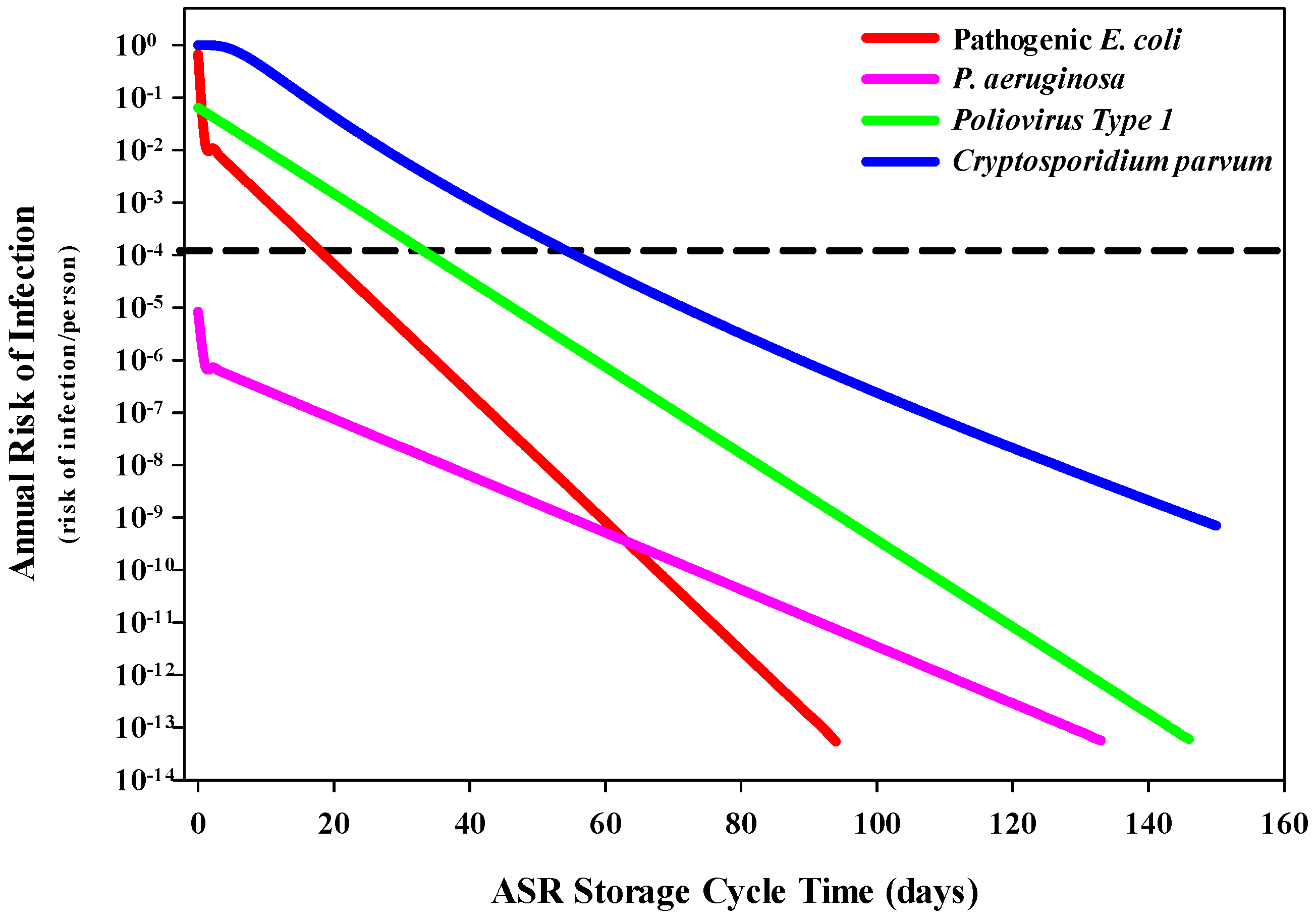
3.1. QMRA Risk of Infection Estimates
3.1.1. Human Health Risks Associated with Drinking Water
3.1.2. Human Health Risks Associated with Ingestion of Recreational Water While Swimming
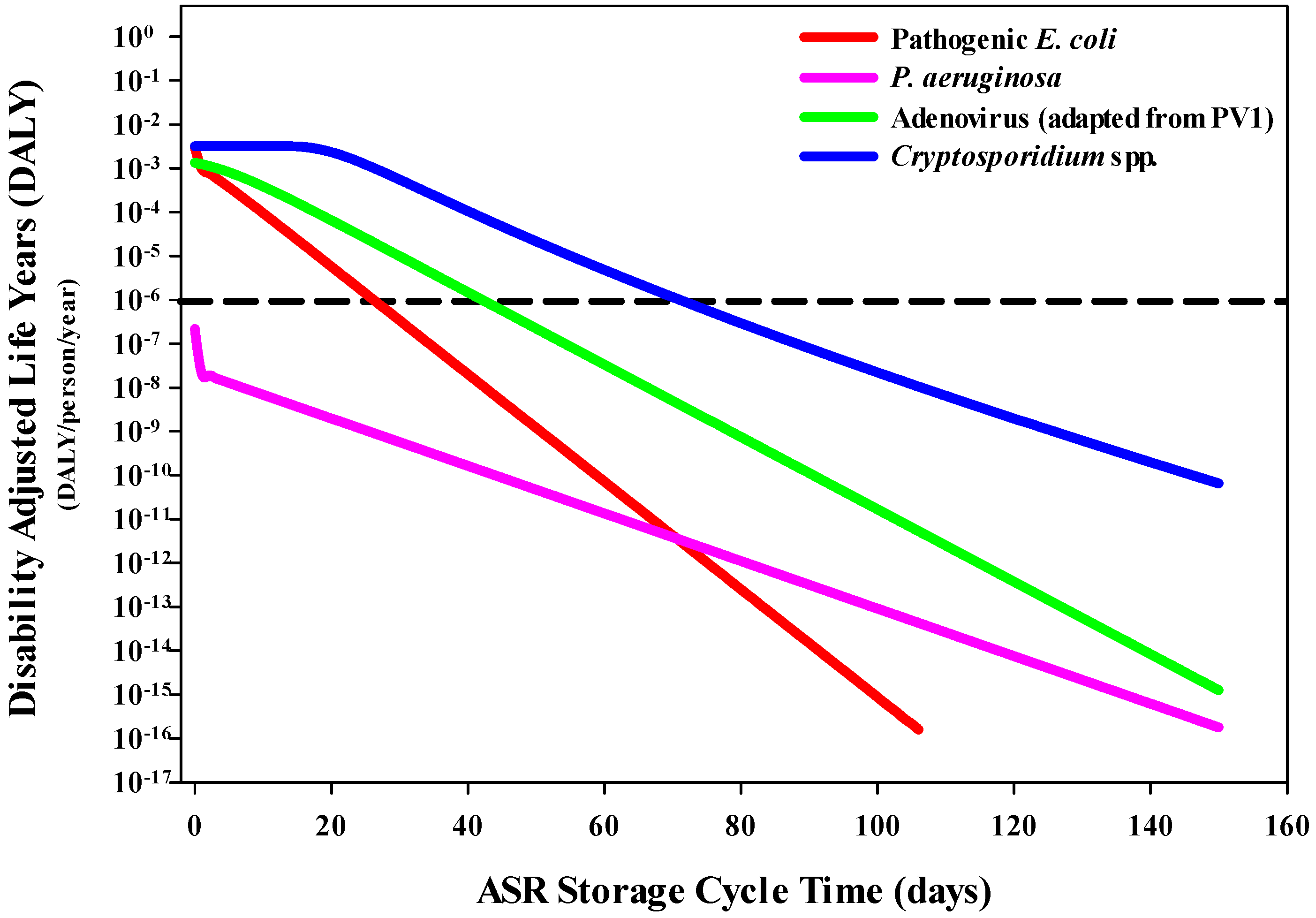
3.2. Disease Burden per Person per Year (through DALY Estimations)
3.2.1. DALYs Associated with Drinking Water Exposure
3.2.2. DALYs Associated with Recreational Water Exposure
4. Discussion
4.1. Pathogen Inactivation in MAR/ASR Systems
4.2. Microbial Health Risks Associated with ASR
4.3. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pyne, D. Aquifer Storage Recovery: A Guide to Groundwater Recharge through Wells, 2nd ed.; ASR Systems: Gainesville, FL, USA, 2005. [Google Scholar]
- National Park Service. Comprehensive Everglades Restoration Plan (CERP). 2023. Available online: https://www.evergladesrestoration.gov (accessed on 10 July 2023).
- South Florida Water Management District. Everglades. 2023. Available online: https://www.sfwmd.gov/our-work/cerp-project-planning (accessed on 10 July 2023).
- USEPA. USEPA Ground Water Rule; USEPA: Washington, DC, USA, 2006; Volume 65574–65660.
- Florida Department of Environmental Protection. Drinking Water Standards, Monitoring, and Reporting; Florida Department of Environmental Protection: Tallahassee, FL, USA, 2013; Volume Rules 102–828.
- Florida Department of Environmental Protection. Underground Injection Control; Florida Department of Environmental Protection: Tallahassee, FL, USA, 2013; Volume 100–900.
- USEPA. United States Safe Drinking Water Act; USEPA: Washington, DC, USA, 1974; Volume § 300f.
- USEPA. United States Safe Drinking Water Act Amendments of 1996; USEPA: Washington, DC, USA, 1996.
- F.A.C. 62-528.615; Underground Injection Control: Monitoring Requirements for Class V (Group 7) Wells. Florida Department of Environmental Protection: Tallahassee, FL, USA, 2002.
- Page, D.; Dillon, P.; Toze, S.; Sidhu, J.P.S. Characterizing Aquifer Treatment for Pathogens in Managed Aquifer Recharge. Water Sci. Technol. 2010, 62, 2009–2015. [Google Scholar] [CrossRef] [PubMed]
- Toze, S.; Bekele, E.; Page, D.; Sidhu, J.; Shackleton, M. Use of Static Quantitative Microbial Risk Assessment to Determine Pathogen Risks in an Unconfined Carbonate Aquifer Used for Managed Aquifer Recharge. Water Res. 2010, 44, 1038–1049. [Google Scholar] [CrossRef] [PubMed]
- Page, D.; Dillon, P.; Vanderzalm, J.; Toze, S.; Sidhu, J.; Barry, K.; Levett, K.; Kremer, S.; Regel, R. Risk Assessment of Aquifer Storage Transfer and Recovery with Urban Stormwater for Producing Water of a Potable Quality. J. Environ. Qual. 2010, 39, 2029–2039. [Google Scholar] [CrossRef] [PubMed]
- Sidhu, J.; Toze, S.; Hodgers, L.; Shackleton, M.; Barry, K.; Page, D.; Dillon, P. Pathogen Inactivation during Passage of Stormwater through a Constructed Reedbed and Aquifer Transfer, Storage and Recovery. Water Sci. Technol. 2010, 62, 1191–1197. [Google Scholar] [CrossRef]
- Page, D.W.; Vanderzalm, J.L.; Barry, K.E.; Torkzaban, S.; Gonzalez, D.; Dillon, P.J. E. coli and Turbidity Attenuation during Urban Stormwater Recycling via Aquifer Storage and Recovery in a Brackish Limestone Aquifer. Ecol. Eng. 2015, 84, 427–434. [Google Scholar] [CrossRef]
- Lisle, J.; Lukasik, G. Natural Inactivation of MS2, Poliovirus Type 1 and Cryptosporidium Parvum in An Anaerobic and Reduced Aquifer. J. Appl. Microbiol. 2021, 132, 2464–2474. [Google Scholar] [CrossRef]
- Lisle, J.T. Natural Inactivation of Escherichia Coli in Anaerobic and Reduced Groundwater. J. Appl. Microbiol. 2016, 120, 1739–1750. [Google Scholar] [CrossRef]
- Soller, J.A.; Eftim, S.; Wade, T.J.; Ichida, A.M.; Clancy, J.L.; Johnson, T.B.; Schwab, K.; Ramirez-Toro, G.; Nappier, S.; Ravenscroft, J.E. Use of Quantitative Microbial Risk Assessment to Improve Interpretation of a Recreational Water Epidemiological Study. Microb. Risk Anal. 2016, 1, 2–11. [Google Scholar] [CrossRef]
- Boehm, A.B.; Soller, J.A. Refined Ambient Water Quality Thresholds for Human-Associated Fecal Indicator HF183 for Recreational Waters with and without Co-Occurring Gull Fecal Contamination. Microb. Risk Anal. 2020, 16, 100139. [Google Scholar] [CrossRef]
- Abuzerr, S.; Hadi, M.; Zinszer, K.; Nasseri, S.; Yunesian, M.; Hossein Mahvi, A.; Nabizadeh, R. Quantitative Microbial Risk Assessment to Estimate Annual Infection Risk and Disease Burden Attributable to Escherichia Coli O157:H7 in Drinking Water in the Gaza Strip: A Prospective Study. Lancet 2022, 399, S4. [Google Scholar] [CrossRef]
- Haas, C.N.; Rose, J.B.; Gerba, C.P. Quantitative Microbial Risk Assessment, 1st ed.; Wiley, Inc.: Hoboken, NJ, USA, 1999. [Google Scholar]
- Haas, C.N.; Rose, J.B.; Gerba, C.P. Quantitative Microbial Risk Assessment, 2nd ed.; Wiley Blackwell: Hoboken, NJ, USA, 2014. [Google Scholar]
- USGS. Survival of Bacterial Indicators and the Functional Diversity of Native Microbial Communities in Teh Floridan Aquifer, South Florida; United States Geological Survey: Menlo Park, CA, USA, 2014; p. 72.
- Reese, R.; Richardson, E. Synthesis of the Hydrogeological Framework of the Floridan Aquifer System Adn Delineation of a Major Avon Park Permeable Zone in Central and Southern Florida; USGS: Menlo Park, CA, USA, 2007.
- Scott, T. Florida Geological Survey Bulletin Series; USGS: Menlo Park, CA, USA, 1988; pp. 1–152.
- Owens, C.E.L.; Angles, M.L.; Cox, P.T.; Byleveld, P.M.; Osborne, N.J.; Rahman, M.B. Implementation of Quantitative Microbial Risk Assessment (QMRA) for Public Drinking Water Supplies: Systematic Review. Water Res. 2020, 174, 115614. [Google Scholar] [CrossRef] [PubMed]
- Mendez, K. Quantitative Microbial Risk Assessment in Water Reuse. Master’s Thesis, The University of Texas Health Science Center at Houston School of Public Health El Paso Campus, El Paso, TX, USA, 2022. [Google Scholar]
- Regli, S.; Rose, J.B.; Haas, C.N.; Gerba, C.P. Modeling the Risk From Giardia and Viruses in Drinking Water. J. AWWA 1991, 83, 76–84. [Google Scholar] [CrossRef]
- World Health Organization. Guidelines for Drinking-Water Quality [Electronic Resource]: Incorporating 1st and 2nd Addenda, Vol.1, Recommendations, 3rd ed.; World Health Organization: Geneva, Switzerland, 2008; ISBN 978-92-4-154761-1. [Google Scholar]
- Ford, T.E. Microbiological Safety of Drinking Water: United States and Global Perspectives. Environ. Health Perspect. 1999, 107, 191–206. [Google Scholar] [CrossRef] [PubMed]
- Mirecki, J. Central and Southern Florida Project, Comprehensive Everglades Restoration Plan: Aquifer Storage and Recovery Pilot Projects Technical Data Report, Kissimmee River ASR System, Hillsboro ASR System; US Army Corps of Engineers: Jacksonville, FL, USA; South Florida Water Management District: West Palm Beach, FL, USA, 2013; p. 370. [Google Scholar]
- Kahn, H.D.; Stralka, K. Estimated Daily Average per Capita Water Ingestion by Child and Adult Age Categories Based on USDA’s 1994–1996 and 1998 Continuing Survey of Food Intakes by Individuals. J. Expo. Sci. Environ. Epidemiol. 2009, 19, 396–404. [Google Scholar] [CrossRef] [PubMed]
- Dufour, A.P.; Behymer, T.D.; Cantú, R.; Magnuson, M.; Wymer, L.J. Ingestion of Swimming Pool Water by Recreational Swimmers. J. Water Health 2017, 15, 429–437. [Google Scholar] [CrossRef]
- Soller, J.A.; Eisenberg, J.N.S. An Evaluation of Parsimony for Microbial Risk Assessment Models. Environmetrics 2008, 19, 61–78. [Google Scholar] [CrossRef]
- Koprowski, H. Immunization against Poliomyelitis with Living Attenuated Virus. Am. J. Trop. Med. Hyg. 1956, 5, 440–452. [Google Scholar] [CrossRef]
- Teunis, P.F.M.; Ogden, I.D.; Strachan, N.J.C. Hierarchical Dose Response of E. Coli O157:H7 from Human Outbreaks Incorporating Heterogeneity in Exposure. Epidemiol. Infect. 2008, 136, 761–770. [Google Scholar] [CrossRef] [PubMed]
- U.S. EPA. National Primary Drinking Water Regulations: Long Term 2 Enhanced Surface Water Treatment Rule. Final Rule. Fed. Regist. 2006, 67, 1811–1844. [Google Scholar]
- Mena, K.D.; Gerba, C.P. Risk Assessment of Pseudomonas Aeruginosa in Water. In Reviews of Environmental Contamination and Toxicology; Springer Science Business Media, LLC.: Berlin/Heidelberg, Germany, 2009; Volume 201. [Google Scholar]
- Timm, C.; Luther, S.; Jurzik, L.; Hamza, I.A.; Kistemann, T. Applying QMRA and DALY to Assess Health Risks from River Bathing. Int. J. Hyg. Environ. Health 2016, 219, 681–692. [Google Scholar] [CrossRef]
- Havelaar, A.H.; De, H.A.E.; Teunis, P.F.; Evers, E.G.; Van, K.H.J.; Versteegh, J.F.; Van, K.J.E.; Slob, W. Balancing the Risks and Benefits of Drinking Water Disinfection: Disability Adjusted Life-Years on the Scale. Environ. Health Perspect. 2000, 108, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.J. Quantifying the Burden of Disease: The Technical Basis for Disability-Adjusted Life Years. Bull World Health Organ. 1994, 72, 429–445. [Google Scholar] [PubMed]
- Florida Department of Health Life Expectancy Report. Available online: https://www.flhealthcharts.gov/ChartsReports/rdPage.aspx?rdReport=ChartsProfiles.LifeExpectancyProfile&islYears=2020 (accessed on 20 May 2023).
- Katukiza, A.Y.; Ronteltap, M.; van der Steen, P.; Foppen, J.W.A.; Lens, P.N.L. Quantification of Microbial Risks to Human Health Caused by Waterborne Viruses and Bacteria in an Urban Slum. J. Appl. Microbiol. 2014, 116, 447–463. [Google Scholar] [CrossRef] [PubMed]
- Fuhrimann, S.; Nauta, M.; Pham-Duc, P.; Tram, N.T.; Nguyen-Viet, H.; Utzinger, J.; Cissé, G.; Winkler, M.S. Disease Burden Due to Gastrointestinal Infections among People Living along the Major Wastewater System in Hanoi, Vietnam. Adv. Water Resour. 2017, 108, 439–449. [Google Scholar] [CrossRef]
- Rasheduzzaman, M.; Singh, R.; Haas, C.N.; Tolofari, D.; Yassaghi, H.; Hamilton, K.A.; Yang, Z.; Gurian, P.L. Reverse QMRA as a Decision Support Tool: Setting Acceptable Concentration Limits for Pseudomonas Aeruginosa and Naegleria Fowleri. Water 2019, 11, 1850. [Google Scholar] [CrossRef]
- Byrne, D.M.; Hamilton, K.A.; Houser, S.A.; Mubasira, M.; Katende, D.; Lohman, H.A.C.; Trimmer, J.T.; Banadda, N.; Zerai, A.; Guest, J.S. Navigating Data Uncertainty and Modeling Assumptions in Quantitative Microbial Risk Assessment in an Informal Settlement in Kampala, Uganda. Environ. Sci. Technol. 2021, 55, 5463–5474. [Google Scholar] [CrossRef]
- Gibney, K.B.; O’Toole, J.; Sinclair, M.; Leder, K. Disease Burden of Selected Gastrointestinal Pathogens in Australia, 2010. Int. J. Infect. Dis. 2014, 28, 176–185. [Google Scholar] [CrossRef]
- Ayuso-Gabella, N.; Page, D.; Masciopinto, C.; Aharoni, A.; Salgot, M.; Wintgens, T. Quantifying the Effect of Managed Aquifer Recharge on the Microbiological Human Health Risks of Irrigating Crops with Recycled Water. Agric. Water Manag. 2011, 99, 93–102. [Google Scholar] [CrossRef]
- Australian Health Ministers’ Conference; Natural Resource Management Ministerial Council. National Guidelines for Water Recycling: Managing Health and Environmental Risks (Phase 1); Environment Protection and Heritage Council: Adelaide, Australia, 2006.
- Gibbons, C.L.; Mangen, M.-J.J.; Plass, D.; Havelaar, A.H.; Brooke, R.J.; Kramarz, P.; Peterson, K.L.; Stuurman, A.L.; Cassini, A.; Fèvre, E.M.; et al. Measuring Underreporting and Under-Ascertainment in Infectious Disease Datasets: A Comparison of Methods. BMC Public Health 2014, 14, 147. [Google Scholar] [CrossRef]
- Mena, K.D.; Gerba, C.P. Risk Assessment of Pseudomonas aeruginosa in Water. Rev. Environ. Contam. Toxicol. 2009, 201, 71–115. [Google Scholar]
- Lew, J.F.; Glass, R.I.; Gangarosa, R.E.; Cohen, I.P.; Bern, C.; Moe, C.L. Diarrheal Deaths in the United States, 1979 through 1987. A Special Problem for the Elderly. JAMA 1991, 265, 3280–3284. [Google Scholar] [CrossRef] [PubMed]


| Well Designation | Location | Aquifer Zone | Production Interval | |
|---|---|---|---|---|
| Latitude | Longitude | (mbls) 3 | ||
| MZ1 | 26°45′11.42″ | −81°21′17.72″ | UF 1 | 204–255 |
| MZ3 | APPZ 2 | 503–536 | ||
| 42U | 27°13′11.16″ | −80°57′21.98″ | UF | 171–317 |
| 42L | APPZ | 399–469 | ||
| 15U | 26°44′16.08″ | −80°21′48.68″ | UF | 277–349 |
| 15M | APPZ | 427–483 | ||
| MW10 | 27°09′17.30″ | −80°52′27.40″ | UF | 174–268 |
| Well Designations | ||||||||
|---|---|---|---|---|---|---|---|---|
| Parameter | Units | MZ1 | MZ3 | 42U | 42L | 15U | 15M | MW10 |
| Temperature | °C | 28.7 | 27.8 | 28.2 | 28.5 | 27.9 | 28.0 | 25.6 |
| Specific conductance | mS/cm | 3.146 | 27.98 | 1.029 | 6.044 | 5.876 | 5.009 | 1.270 |
| Total dissolved solids | g/L | 2.045 | 18.19 | 0.669 | 3.928 | 3.819 | 3.255 | 0.728 |
| pH | 8.02 | 7.38 | 8.04 | 7.61 | 7.60 | 7.64 | 7.89 | |
| ORP | mV | −312 | −309 | −338 | −351 | −355 | −365 | −260 |
| Calcium | mg/L | 80.0 | 550.0 | 44.0 | 200.0 | 120.0 | 110.0 | 46.4 |
| Chloride | mg/L | 640.0 | 9700.0 | 160.0 | 1600.0 | 1600.0 | 1300.0 | 232.6 |
| Fluoride | mg/L | 0.78 | BDL 1 | 0.57 | 0.29 | 0.97 | 1.10 | 0.53 |
| Magnesium | mg/L | 75.0 | 650.0 | 33.0 | 140.0 | 130.0 | 120.0 | 36.5 |
| Potassium | mg/L | 24.0 | 230.0 | 5.5 | 40.0 | 36.0 | 29.0 | 7.3 |
| Silica | mg/L | 9.8 | 9.1 | 14.0 | 12.0 | 13.0 | 13.0 | 8.2 |
| Sodium | mg/L | 440.0 | 4700.0 | 98.0 | 800.0 | 890.0 | 740.0 | 137.4 |
| Manganese | mg/L | 0.013 | 0.035 | 0.007 | 0.006 | 0.011 | 0.010 | 0.045 |
| Iron (total) | mg/L | 0.17 | 0.22 | 0.12 | 0.20 | 0.34 | 0.40 | 0.09 |
| Ammonium | mg/L | 0.19 | 0.28 | 0.20 | 0.26 | 0.44 | 0.33 | 0.22 |
| Sulfate | mg/L | 380.0 | 1800.0 | 180.0 | 510.0 | 450.0 | 370.0 | 184.6 |
| Sulfide | mg/L | 2.1 | 1.6 | 1.4 | 1.6 | 3.7 | 4.2 | 1.1 |
| Dissolved organic carbon | mg/L | 1.2 | 1.1 | 1.1 | 1.2 | 1.7 | 1.9 | 1.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gitter, A.; Mena, K.D.; Lisle, J.T. Informing ASR Treatment Practices in a Florida Aquifer through a Human Health Risk Approach. Int. J. Environ. Res. Public Health 2023, 20, 6833. https://doi.org/10.3390/ijerph20196833
Gitter A, Mena KD, Lisle JT. Informing ASR Treatment Practices in a Florida Aquifer through a Human Health Risk Approach. International Journal of Environmental Research and Public Health. 2023; 20(19):6833. https://doi.org/10.3390/ijerph20196833
Chicago/Turabian StyleGitter, Anna, Kristina D. Mena, and John T. Lisle. 2023. "Informing ASR Treatment Practices in a Florida Aquifer through a Human Health Risk Approach" International Journal of Environmental Research and Public Health 20, no. 19: 6833. https://doi.org/10.3390/ijerph20196833





