Locus Coeruleus Dysfunction and Trigeminal Mesencephalic Nucleus Degeneration: A Cue for Periodontal Infection Mediated Damage in Alzheimer’s Disease?
Abstract
1. Introduction
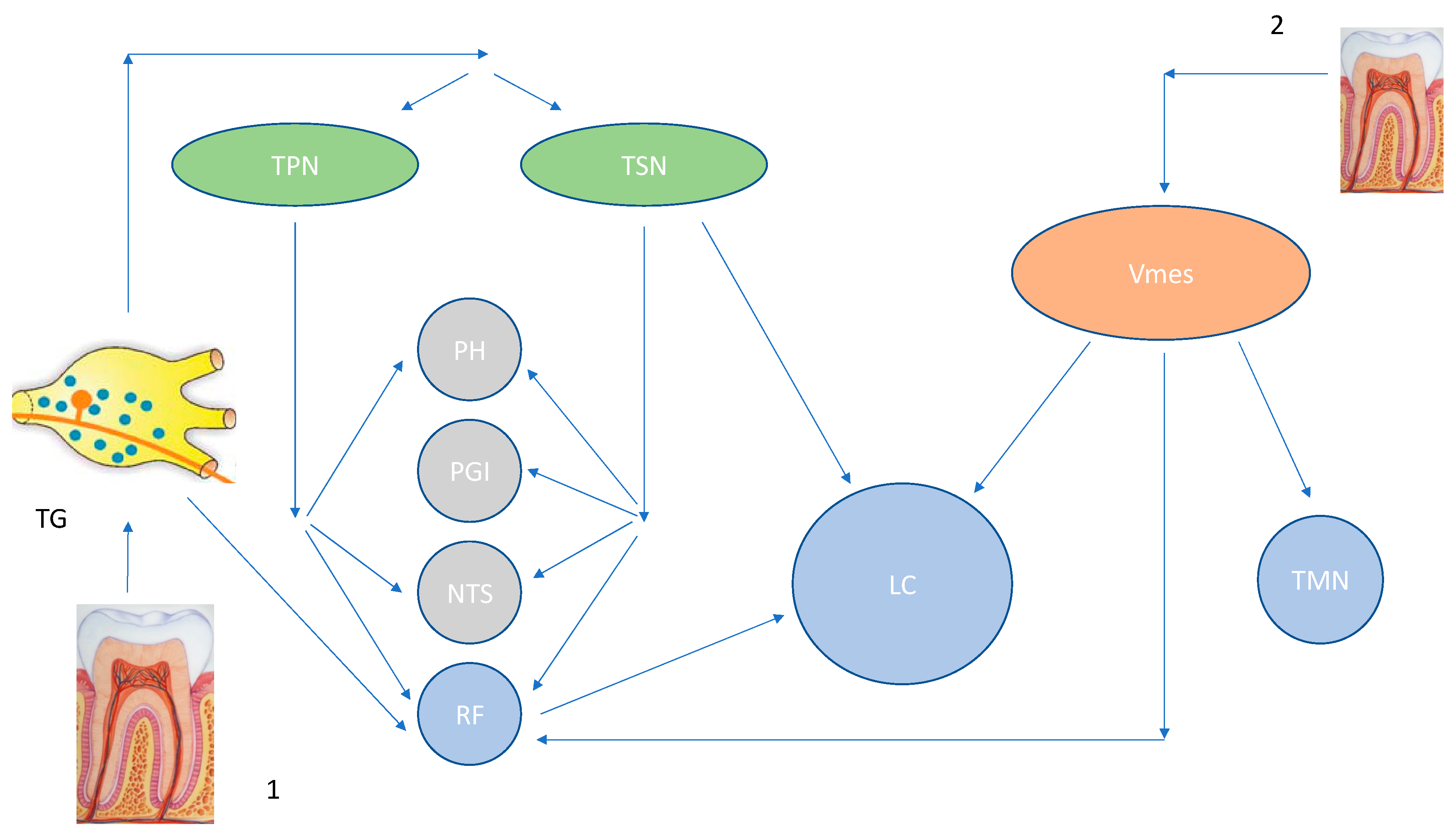
2. Locus Coeruleus and the Trigeminal Mesencephalic Nucleus
3. The Mesencephalic Nucleus (Vmes) and Its Connections with the Oral Structures
4. Tooth Loss Can Cause Central Neuroinflammation and Neurodegeneration
5. Locus Coeruleus Degeneration and the Blood-Brain Barrier Impairment
6. Treponema denticola as a Potential Cue for the Locus Coeruleus Impairment
7. Discussion
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hyman, B.T.; Phelps, C.H.; Beach, T.G.; Bigio, E.H.; Cairns, N.J.; Carrillo, M.C.; Dickson, D.W.; Duyckaerts, C.; Frosch, M.P.; Masliah, E.; et al. National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease. Alzheimers Dement. 2012, 8, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Braak, H.; Braak, E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991, 82, 239–259. [Google Scholar] [CrossRef] [PubMed]
- Braak, H.; Thal, D.R.; Ghebremedhin, E.; Del Tredici, K. Stages of the pathologic process in Alzheimer disease: Age categories from 1 to 100 years. J. Neuropathol. Exp. Neurol. 2011, 70, 960–969. [Google Scholar] [CrossRef] [PubMed]
- Giorgi, F.S.; Ryskalin, L.; Ruffoli, R.; Biagioni, F.; Limanaqi, F.; Ferrucci, M.; Busceti, C.L.; Bonuccelli, U.; Fornai, F. The neuroanatomy of the reticular nucleus locus coeruleus in Alzheimer’s disease. Front. Neuroanat. 2017, 11, 80. [Google Scholar] [CrossRef]
- Mather, M.; Harley, C.W. The locus coeruleus: Essential for maintaining cognitive function and the aging brain. Trends Cogn. Sci. 2016, 20, 214–226. [Google Scholar] [CrossRef]
- Ross, J.A.; McGonigle, P.; Van Bockstaele, E.J. Locus coeruleus, norepinephrine and Aβ peptides in Alzheimer’s disease. Neurobiol. Stress 2015, 2, 73–84. [Google Scholar] [CrossRef]
- Musiek, E.S.; Xiong, D.D.; Holtzman, D.M. Sleep, circadian rhythms, and the pathogenesis of Alzheimer disease. Exp. Mol. Med. 2015, 47, e148. [Google Scholar] [CrossRef]
- Aston-Jones, G.; Cohen, J.D. An integrative theory of locus coeruleus-norepinephrine function: Adaptive gain and optimal performance. Annu. Rev. Neurosci. 2005, 28, 403–450. [Google Scholar] [CrossRef]
- Uematsu, A.; Tan, B.Z.; Johansen, J.P. Projection specificity in heterogeneous locus coeruleus cell populations: Implications for learning and memory. Learn. Mem. 2015, 22, 444–451. [Google Scholar] [CrossRef]
- Berridge, C.W.; Waterhouse, B.D. The locus coeruleus–noradrenergic system: Modulation of behavioral state and state-dependent cognitive processes. Brain Res. Rev. 2003, 42, 33–84. [Google Scholar] [CrossRef]
- Schwarz, L.A.; Luo, L. Organization of the locus coeruleus-norepinephrine system. Curr. Biol. 2015, 25, R1051–R1056. [Google Scholar] [CrossRef] [PubMed]
- Luchsinger, J.A.; Tang, M.X.; Stern, Y.; Shea, S.; Mayeux, R. Diabetes Mellitus and Risk of Alzheimer’s Disease and Dementia with Stroke in a Multiethnic Cohort. Am. J. Epidemiol. 2001, 154, 635–641. [Google Scholar] [CrossRef]
- de la Torre, J.C. How do heart disease and stroke become risk factors for Alzheimer’s disease? Neurol. Res. 2006, 28, 637–644. [Google Scholar] [CrossRef]
- Stein, P.S.; Desrosiers, M.; Donegan, S.J.; Yepes, J.F.; Kryscio, R.J. Tooth loss, dementia and neuropathology in the Nun Study. J. Am. Dent. Assoc. 2007, 138, 1314–1322. [Google Scholar] [CrossRef]
- Chen, C.-K.; Wu, Y.-T.; Chang, Y.-C. Association between chronic periodontitis and the risk of Alzheimer’s disease: A retrospective, population-based, matched-cohort study. Alzheimers Res. Ther. 2017, 9, 56. [Google Scholar] [CrossRef]
- Li, L.; Cavuoto, M.; Biddiscombe, K.; Pike, K.E. Diabetes mellitus increases risk of incident dementia in APOEε4 carriers: A meta-analysis. J. Alzheimers Dis. 2020, 74, 1295–1308. [Google Scholar] [CrossRef]
- Hou, R.; Beardmore, R.; Holmes, C.; Osmond, C.; Darekar, A. A case-control study of the locus coeruleus degeneration in Alzheimer’s disease. Eur. Neuropsychopharmacol. 2021, 43, 153–159. [Google Scholar] [CrossRef]
- Tomlinson, B.; Irving, D.; Blessed, G. Cell loss in the locus coeruleus in senile dementia of Alzheimer type. J. Neurol. Sci. 1981, 49, 419–428. [Google Scholar] [CrossRef]
- Bondareff, W.; Mountjoy, C.Q.; Roth, M. Loss of neurons of origin of the adrenergic projection to cerebral cortex (nucleus locus ceruleus) in senile dementia. Neurology 1982, 32, 164–168. [Google Scholar] [CrossRef]
- German, D.C.; Manaye, K.F.; White, C.L.; Woodward, D.J.; McIntire, D.D.; Smith, W.K.; Kalaria, R.N.; Mann, D. Disease-specific patterns of locus coeruleus cell loss. Ann. Neurol. 1992, 32, 667–676. [Google Scholar] [CrossRef]
- Adolfsson, R.; Gottfries, C.; Roos, B.; Winblad, B. Changes in the brain catecholamines in patients with dementia of Alzheimer type. Br. J. Psychiatry 1979, 135, 216–223. [Google Scholar] [CrossRef]
- Kalinin, S.; Feinstein, D.L.; Xu, H.L.; Huesa, G.; Pellegrino, D.A.; Galea, E. Degeneration of noradrenergic fibres from the locus coeruleus causes tight-junction disorganisation in the rat brain. Eur. J. Neurosci. 2006, 24, 3393–3400. [Google Scholar] [CrossRef]
- Marien, M.R.; Colpaert, F.C.; Rosenquist, A.C. Noradrenergic mechanisms in neurodegenerative diseases: A theory. Brain Res. Rev. 2004, 45, 38–78. [Google Scholar] [CrossRef]
- Gyoneva, S.; Traynelis, S.F. Norepinephrine modulates the motility of resting and activated microglia via different adrenergic receptors. J. Biol. Chem. 2013, 288, 15291–15302. [Google Scholar] [CrossRef]
- Akiyama, H.; Barger, S.; Barnum, S.; Bradt, B.; Bauer, J.; Cole, G.M.; Cooper, N.R.; Eikelenboom, P.; Emmerling, M.; Fiebich, B.L.; et al. Inflammation and Alzheimer’s disease. Neurobiol. Aging 2000, 21, 383–421. [Google Scholar] [CrossRef]
- McGeer, P.L.; McGeer, E.G. Local neuroinflammation and the progression of Alzheimer’s disease. J. Neurovirol. 2002, 8, 529–538. [Google Scholar] [CrossRef] [PubMed]
- Heneka, M.T.; Carson, M.J.; El Khoury, J.; Landreth, G.E.; Brosseron, F.; Feinstein, D.L.; Jacobs, A.H.; Wyss-Coray, T.; Vitorica, J.; Ransohoff, R.M.; et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015, 14, 388–405. [Google Scholar] [CrossRef]
- Bondareff, W.; Mountjoy, C.Q.; Roth, M.; Rossor, M.N.; Iversen, L.L.; Reynolds, G.P. Age and histopathologic heterogeneity in Alzheimer’s disease: Evidence for subtypes. Arch. Gen. Psychiatry 1987, 44, 412–417. [Google Scholar] [CrossRef]
- Heneka, M.T.; Ramanathan, M.; Jacobs, A.H.; Dumitrescu-Ozimek, L.; Bilkei-Gorzo, A.; Debeir, T.; Sastre, M.; Galldiks, N.; Zimmer, A.; Hoehn, M.; et al. Locus ceruleus degeneration promotes Alzheimer pathogenesis in amyloid precursor protein 23 transgenic mice. J. Neurosci. 2006, 26, 1343–1354. [Google Scholar] [CrossRef]
- Marcyniuk, B.; Mann, D.M.; Yates, P.O. Loss of nerve cells from locus coeruleus iin Alzheimer’s disease is topographically arranged. Neurosci. Lett. 1986, 64, 247–252. [Google Scholar] [CrossRef]
- Jahan, K.; Pillai, K.K.; Vohora, D. DSP-4 induced depletion of brain noradrenaline and increased 6-hertz psychomotor seizure susceptibility in mice is prevented by sodium valproate. Brain Res. Bull. 2018, 142, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Kalinin, S.; Gavrilyuk, V.; Polak, P.E.; Vasser, R.; Zhao, J.; Heneka, M.T.; Feinstein, D.L. Noradrenaline deficiency in brain increases β-amyloid plaque burden in an animal model of Alzheimer’s disease. Neurobiol. Aging 2007, 28, 1206–1214. [Google Scholar] [CrossRef]
- Hammerschmidt, T.; Kummer, M.P.; Terwel, D.; Martinez, A.; Gorji, A.; Pape, H.C.; Rommelfanger, K.S.; Schroeder, J.P.; Stoll, M.; Schultze, J.; et al. Selective loss of noradrenaline exacerbates early cognitive dysfunction and synaptic deficits in APP/PS1 mice. Biol. Psychiatry 2013, 73, 454–463. [Google Scholar] [CrossRef]
- Ramón-Moliner, E.; Nauta, W.J. The isodendritic core of the brain stem. J. Comp. Neurol. 1966, 126, 311–335. [Google Scholar] [CrossRef] [PubMed]
- Theofilas, P.; Dunlop, S.; Heinsen, H.; Grinberg, L.T. Turning on the Light Within: Subcortical Nuclei of the Isodentritic Core and their Role in Alzheimer’s Disease Pathogenesis. J Alzheimers Dis. 2015, 46, 17–34. [Google Scholar] [CrossRef]
- Theofilas, P.; Ehrenberg, A.J.; Dunlop, S.; Di Lorenzo Alho, A.T.; Nguy, A.; Leite, R.E.P.; Rodriguez, R.D.; Mejia, M.B.; Suemoto, C.K.; Ferretti-Rebustini, R.E.L.; et al. Locus coeruleus volume and cell population changes during Alzheimer’s disease progression: A stereological study in human postmortem brains with potential implication for early-stage biomarker discovery. Alzheimers Dement. 2017, 13, 236–246. [Google Scholar] [CrossRef]
- Swanson, L.W. The locus coeruleus: A cytoarchitectonic, Golgi and immunohistochemical study in the albino rat. Brain Res. 1976, 110, 39–56. [Google Scholar] [CrossRef] [PubMed]
- Pickel, V.M.; Joh, T.H.; Reis, D.J. Ultrastructural localization of tyrosine hydroxylase in noradrenergic neurons of brain. Proc. Natl. Acad. Sci. USA 1975, 72, 659–663. [Google Scholar] [CrossRef]
- Matchett, B.J.; Grinberg, L.T.; Theofilas, P.; Murray, M.E. The mechanistic link between selective vulnerability of the locus coeruleus and neurodegeneration in Alzheimer’s disease. Acta Neuropathol. 2021, 141, 631–650. [Google Scholar] [CrossRef]
- Copray, J.C.; Ter Horst, G.J.; Liem, R.S.; van Willigen, J.D. Neurotransmitters and neuropeptides within the mesencephalic trigeminal nucleus of the rat: An immunohistochemical analysis. Neuroscience 1990, 37, 399–411. [Google Scholar] [CrossRef] [PubMed]
- Alvarado-Mallart, M.R.; Batini, C.; Buisseret-Delmas, C.; Corvisier, J. Trigeminal representations of the masticatory and extraocular proprioceptors as revealed by horseradish peroxidase retrograde transport. Exp. Brain Res. 1975, 23, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Jacquin, M.F.; Zeigler, H.P. Trigeminal orosensation and ingestive behavior in the rat. Behav. Neurosci. 1983, 97, 62–97. [Google Scholar] [CrossRef] [PubMed]
- Appenteng, K.; O’Donovan, M.J.; Somjen, G.; Stephens, J.A.; Taylor, A. The projection of jaw elevator muscle spindle afferents to fifth nerve motoneurones in the cat. J. Physiol. 1978, 279, 409–423. [Google Scholar] [CrossRef] [PubMed]
- Shigenaga, Y.; Sera, M.; Nishimori, T.; Suemune, S.; Nishimura, M.; Yoshida, A.; Tsuru, K. The central projection of masticatory afferent fibers to the trigeminal sensory nuclear complex and upper cervical spinal cord. J. Comp. Neurol. 1988, 268, 489–507. [Google Scholar] [CrossRef]
- Shigenaga, Y.; Mitsuhiro, Y.; Yoshida, A.; Cao, C.Q.; Tsuru, H. Morphology of single mesencephalic trigeminal neurons innervating masseter muscle of the cat. Brain Res. 1988, 445, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Lidsky, T.I.; Labuszewski, T.; Avitable, M.J.; Robinson, J.H. The effects of stimulation of trigeminal sensory afferents upon caudate units in cats. Brain Res. Bull. 1979, 4, 9–14. [Google Scholar] [CrossRef]
- Gottlieb, S.; Taylor, A.; Bosley, M.A. The distribution of afferent neurones in the mesencephalic nucleus of the fifth nerve in the cat. J. Comp. Neurol. 1984, 228, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Hassanali, J. Quantitative and somatotopic mapping of neurons in the trigeminal mesencephalic nucleus and ganglion innervating teeth in monkey and baboon. Arch. Oral Biol. 1997, 42, 673–682. [Google Scholar] [CrossRef]
- Muramoto, T.; Takano, Y.; Soma, K. Time-related changes in periodontal mechanoreceptors in rat molars after the loss of occlusal stimuli. Arch. Histol. Cytol. 2000, 63, 369–380. [Google Scholar] [CrossRef] [PubMed]
- Goto, T.; Kuramoto, E.; Dhar, A.; Wang, R.P.; Seki, H.; Iwai, H. Neurodegeneration of trigeminal mesencephalic neurons by the tooth loss triggers the progression of Alzheimer’s disease in 3× Tg-AD model mice. J. Alzheimers Dis. 2020, 76, 1443–1459. [Google Scholar] [CrossRef]
- Raappana, P.; Arvidsson, J. The reaction of mesencephalic trigeminal neurons to peripheral nerve transection in the adult rat. Exp. Brain Res. 1992, 90, 567–571. [Google Scholar] [CrossRef] [PubMed]
- Linden, R.W.; Scott, B.J. Distribution of mesencephalic nucleus and trigeminal ganglion mechanoreceptors in the periodontal ligament of the cat. J. Physiol. 1989, 410, 35–44. [Google Scholar] [CrossRef]
- Nakamura, T.K.; Nakamura, M.; Yoshikawa, M.; Kiyomura, H.; Hanai, H. Fine structure of probable sensory nerve endings in human periodontal ligaments. Okajimas Folia Anat. Jpn. 1986, 63, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Cauna, N. Fine Structure of the Receptor Organs and its Probable Functional Significance. In Ciba Foundation Symposium—Hormonal Factors in Carbohydrate Metabolism (Colloquia on Endocrinology); de Reuck, A.V.S., Knight, J., Eds.; 1966. [Google Scholar] [CrossRef]
- Cash, R.M.; Linden, R.W. The distribution of mechanoreceptors in the periodontal ligament of the mandibular canine tooth of the cat. J. Physiol. 1982, 330, 439–447. [Google Scholar] [CrossRef]
- Catton, W.T. Mechanoreceptor function. Physiol. Rev. 1970, 50, 297–318. [Google Scholar] [CrossRef]
- Delwel, S.; Binnekade, T.T.; Perez, R.S.; Hertogh, C.M.; Scherder, E.J.; Lobbezoo, F. Oral hygiene and oral health in older people with dementia: A comprehensive review with focus on oral soft tissues. Clin. Oral Investig. 2018, 22, 93–108. [Google Scholar] [CrossRef]
- Fereshtehnejad, S.M.; Lökk, J.; Wimo, A.; Eriksdotter, M. No Significant Difference in Cognitive Decline and Mortality between Parkinson’s Disease Dementia and Dementia with Lewy Bodies: Naturalistic Longitudinal Data from the Swedish Dementia Registry. J. Parkinsons Dis. 2018, 8, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Kamer, A.R.; Dasanayake, A.P.; Craig, R.G.; Glodzik-Sobanska, L.; Bry, M.; de Leon, M.J. Alzheimer’s disease and peripheral infections: The possible contribution from periodontal infections, model and hypothesis. J. Alzheimers Dis. 2008, 13, 437–449. [Google Scholar] [CrossRef] [PubMed]
- Hopper, A.T.; Campbell, B.M.; Kao, H.; Pintchovski, S.A.; Staal, R.G. Chapter Four—Recent Developments in Targeting Neuroinflammation in Disease. In Annual Reports in Medicinal Chemistry; Desai, M.C., Ed.; Academic Press: Cambridge, MA, USA, 2012; Volume 47, pp. 37–53. [Google Scholar] [CrossRef]
- Dominy, S.S.; Lynch, C.; Ermini, F.; Benedyk, M.; Marczyk, A.; Konradi, A.; Nguyen, M.; Haditsch, U.; Raha, D.; Griffin, C.; et al. Porphyromonas gingivalis in Alzheimer’s disease brains: Evidence for disease causation and treatment with small-molecule inhibitors. Sci. Adv. 2019, 5, eaau3333. [Google Scholar] [CrossRef]
- Oue, H.; Miyamoto, Y.; Okada, S.; Koretake, K.; Jung, C.G.; Michikawa, M.; Akagawa, Y. Tooth loss induces memory impairment and neuronal cell loss in APP transgenic mice. Behav. Brain Res. 2013, 252, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Lexomboon, D.; Trulsson, M.; Wårdh, I.; Parker, M.G. Chewing ability and tooth loss: Association with cognitive impairment in an elderly population study. J. Am. Geriatr. Soc. 2012, 60, 1951–1956. [Google Scholar] [CrossRef] [PubMed]
- Weijenberg, R.A.; Scherder, E.J.; Lobbezoo, F. Mastication for the mind—The relationship between mastication and cognition in ageing and dementia. Neurosci. Biobehav. Rev. 2011, 35, 483–497. [Google Scholar] [CrossRef] [PubMed]
- Dhar, A.; Kuramoto, E.; Fukushima, M.; Iwai, H.; Yamanaka, A.; Goto, T. The Periodontium Damage Induces Neuronal Cell Death in the Trigeminal Mesencephalic Nucleus and Neurodegeneration in the Trigeminal Motor Nucleus in C57BL/6J Mice. Acta Histochem. Cytochem. 2021, 54, 11–19. [Google Scholar] [CrossRef]
- Amano, N.; Yoshino, K.; Andoh, S.; Kawagishi, S. Representation of tooth pulp in the mesencephalic trigeminal nucleus and the trigeminal ganglion in the cat, as revealed by retrogradely transported horseradish peroxidase. Neurosci. Lett. 1987, 82, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Byers, M.R.; Taylor, P.E.; Khayat, B.G.; Kimberly, C.L. Effects of injury and inflammation on pulpal and periapical nerves. J. Endod. 1990, 16, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Wang, X.; Kong, W.; Jiang, Q. Tooth Loss Suppresses Hippocampal Neurogenesis and Leads to Cognitive Dysfunction in Juvenile Sprague-Dawley Rats. Front. Neurosci. 2022, 16, 839622. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.S.; Wu, C.Y.; Wu, S.Y.; Lin, H.H.; Cheng, D.H.; Lo, W.L. Age-Related Difference in Functional Brain Connectivity of Mastication. Front. Aging Neurosci. 2017, 9, 82. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.S.; Liang, Z.L.; Wu, M.J.; Feng, L.; Liu, L.L.; Zhang, J.J. Reduced brain-derived neurotrophic factor expression in cortex and hippocampus involved in the learning and memory deficit in molarless SAMP8 mice. Chin. Med. J. 2011, 124, 1540–1544. [Google Scholar] [PubMed]
- Kobayashi, T.; Nagai, M.; Da Silva, J.D.; Galaburda, A.M.; Rosenberg, S.H.; Hatakeyama, W.; Kuwajima, Y.; Kondo, H.; Ishikawa-Nagai, S. Retrograde transport of masseter muscle-derived neprilysin to hippocampus. Neurosci. Lett. 2019, 698, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Singhrao, S.K.; Olsen, I. Are Porphyromonas gingivalis outer membrane vesicles, microbullets for sporadic Alzheimer’s disease manifestation? J. Alzheimers Dis. Rep. 2018, 20, 219–228. [Google Scholar] [CrossRef]
- Kadry, H.; Noorani, B.; Cucullo, L. A blood-brain barrier overview on structure, function, impairment, and biomarkers of integrity. Fluids Barriers CNS 2020, 17, 69. [Google Scholar] [CrossRef] [PubMed]
- Abbott, N.J.; Rönnbäck, L.; Hansson, E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat. Rev. Neurosci. 2006, 7, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Balda, M.S.; Matter, K. Tight junctions at a glance. J. Cell Sci. 2008, 121 Pt 22, 3677–3682. [Google Scholar] [CrossRef] [PubMed]
- Bazzoni, G.; Dejana, E. Endothelial cell-to-cell junctions: Molecular organization and role in vascular homeostasis. Physiol. Rev. 2004, 84, 869–901. [Google Scholar] [CrossRef] [PubMed]
- Montagne, A.; Nation, D.A.; Sagare, A.P.; Barisano, G.; Sweeney, M.D.; Chakhoyan, A.; Pachicano, M.; Joe, E.; Nelson, A.R.; D’Orazio, L.M.; et al. APOE4 leads to blood-brain barrier dysfunction predicting cognitive decline. Nature 2020, 581, 71–76. [Google Scholar] [CrossRef]
- Van de Haar, H.J.; Burgmans, S.; Jansen, J.F.; van Osch, M.J.; van Buchem, M.A.; Muller, M.; Hofman, P.A.; Verhey, F.R.; Backes, W.H. Blood-Brain Barrier Leakage in Patients with Early Alzheimer Disease. Radiology 2016, 281, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Cirelli, C.; Tononi, G. Differential expression of plasticity-related genes in waking and sleep and their regulation by the noradrenergic system. J. Neurosci. 2000, 20, 9187–9194. [Google Scholar] [CrossRef]
- Rubin, L.L.; Staddon, J.M. The cell biology of the blood-brain barrier. Annu. Rev. Neurosci. 1999, 22, 11–28. [Google Scholar] [CrossRef] [PubMed]
- Troadec, J.-D.; Marien, M.; Darios, F.; Hartmann, A.; Ruberg, M.; Colpaert, F.; Michel, P.P. Noradrenaline provides long-term protection to dopaminergic neurons by reducing oxidative stress. J. Neurochem. 2001, 79, 200–210. [Google Scholar] [CrossRef]
- Alttoa, A.; Kõiv, K.; Eller, M.; Uustare, A.; Rinken, A.; Harro, J. Effects of low dose N-(2-chloroethyl)-N-ethyl-2-bromobenzylamine administration on exploratory and amphetamine-induced behavior and dopamine D2 receptor function in rats with high or low exploratory activity. Neuroscience 2005, 132, 979–990. [Google Scholar] [CrossRef] [PubMed]
- Devoto, P.; Flore, G.; Saba, P.; Fà, M.; Gessa, G.L. Co-release of noradrenaline and dopamine in the cerebral cortex elicited by single train and repeated train stimulation of the locus coeruleus. BMC Neurosci. 2005, 6, 31. [Google Scholar] [CrossRef] [PubMed]
- Teismann, P.; Schulz, J.B. Cellular pathology of Parkinson’s disease: Astrocytes, microglia, and inflammation. Cell Tissue Res. 2004, 318, 149–161. [Google Scholar] [CrossRef] [PubMed]
- Breidert, T.; Callebert, J.; Heneka, M.T.; Landreth, G.; Launay, J.M.; Hirsch, E.C. Protective action of the peroxisome proliferator-activated receptor-gamma agonist pioglitazone in a mouse model of Parkinson’s disease. J. Neurochem. 2002, 82, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Sedghi, L.; DiMassa, V.; Harrington, A.; Lynch, S.V.; Kapila, Y.L. The oral microbiome: Role of key organisms and complex networks in oral health and disease. Periodontol. 2000 2021, 87, 107–131. [Google Scholar] [CrossRef] [PubMed]
- Holt, S.C.; Ebersole, J.L. Porphyromonas gingivalis, Treponema denticola, and Tannerella forsythia: The “red complex”, a prototype polybacterial pathogenic consortium in periodontitis. Periodontol. 2000 2005, 38, 72–122. [Google Scholar] [CrossRef] [PubMed]
- Grenier, D. Demonstration of a bimodal coaggregation reaction between Porphyromonas gingivalis and Treponema denticola. Oral Microbiol. Immunol. 1992, 7, 280–284. [Google Scholar] [CrossRef]
- Nilius, A.M.; Spencer, S.C.; Simonson, L.G. Stimulation of in vitro growth of Treponema denticola by extracellular growth factors produced by Porphyromonas gingivalis. J. Dent. Res. 1993, 72, 1027–1031. [Google Scholar] [CrossRef] [PubMed]
- Kigure, T.; Saito, A.; Seida, K.; Yamada, S.; Ishihara, K.; Okuda, K. Distribution of Porphyromonas gingivalis and Treponema denticola in human subgingival plaque at different periodontal pocket depths examined by immunohistochemical methods. J. Periodontal Res. 1995, 30, 332–341. [Google Scholar] [CrossRef]
- Hollmann, R.; Van der Hoeven, H.J. Inability of intact cells of Treponema denticola to degrade human serum proteins IgA, IgG and albumin. J. Clin. Periodontol. 1999, 26, 477–479. [Google Scholar] [CrossRef] [PubMed]
- Grenier, D.; Goulet, V.; Mayrand, D. The Capacity of Porphyromonas gingivalis to Multiply Under Iron-limiting Conditions Correlates with its Pathogenicity in an Animal Model. J. Dent. Res. 2001, 80, 1678–1682. [Google Scholar] [CrossRef]
- Yoneda, M.; Yoshikane, T.; Motooka, N.; Yamada, K.; Hisama, K.; Naito, T.; Okada, I.; Yoshinaga, M.; Hidaka, K.; Imaizumi, K.; et al. Stimulation of growth of Porphyromonas gingivalis by cell extracts from Tannerella forsythia. J. Periodontal. Res. 2005, 40, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Ng, H.M.; Slakeski, N.; Butler, C.A.; Veith, P.D.; Chen, Y.Y.; Liu, S.W.; Hoffmann, B.; 35 Dashper, S.G.; Reynolds, E.C. The Role of Treponema denticola Motility in Synergistic Biofilm Formation with Porphyromonas gingivalis. Front. Cell. Infect. Microbiol. 2019, 9, 432. [Google Scholar] [CrossRef] [PubMed]
- Dale, B.A. Periodontal epithelium: A newly recognized role in health and disease. Periodontol. 2000 2002, 30, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Weinberg, A. Role of bacteria in health and disease of periodontal tissues. Periodontol. 2000 2006, 40, 50–76. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.; Choi, Y. The fate of Treponema denticola within human gingival epithelial cells. Mol. Oral Microbiol. 2012, 27, 471–482. [Google Scholar] [CrossRef]
- Yilmaz, O.; Verbeke, P.; Lamont, R.J.; Ojcius, D.M. Intercellular spreading of Porphyromonas gingivalis infection in primary gingival epithelial cells. Infect. Immun. 2006, 74, 703–710. [Google Scholar] [CrossRef]
- Kim, Y.; Jo, A.R.; Jang, D.H.; Cho, Y.J.; Chun, J.; Min, B.M.; Choi, Y. Toll-like receptor 9 mediates oral bacteria-induced, I.L.;-8 expression in gingival epithelial cells. Immunol. Cell Biol. 2012, 90, 655–663. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.; Kim, Y.S.; Min, B.-M.; Han, S.H.; Choi, Y. Innate immune responses of gingival epithelial cells to non-periodontopathic and periodontopathic bacteria. J. Periodontal Res. 2007, 42, 503–510. [Google Scholar] [CrossRef]
- Shin, J.E.; Kim, Y.S.; Oh, J.E.; Min, B.M.; Choi, Y. Treponema denticola suppresses expression of human beta-defensin-3 in gingival epithelial cells through inhibition of TLR2 activation axis. Infect. Immun. 2010, 78, 672–679. [Google Scholar] [CrossRef]
- Bearer, E.L. HSV, axonal transport and Alzheimer’s disease: In vitro and in vivo evidence for causal relationships. Future Virol. 2012, 7, 885–899. [Google Scholar] [CrossRef]
- Satpute-Krishnan, P.; DeGiorgis, J.A.; Bearer, E.L. Fast anterograde transport of herpes simplex virus: Role for the amyloid precursor protein of Alzheimer’s disease. Aging Cell 2003, 2, 305–318. [Google Scholar] [CrossRef] [PubMed]
- Rother, M.; Böck, A.; Wyss, C. Selenium-dependent growth of Treponema denticola: Evidence for a clostridial-type glycine reductase. Arch. Microbiol. 2001, 177, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Seshadri, R.; Myers, G.S.; Tettelin, H.; Eisen, J.A.; Heidelberg, J.F.; Dodson, R.J.; Davidsen, T.M.; DeBoy, R.T.; Fouts, D.E.; Haft, D.H.; et al. Comparison of the genome of the oral pathogen Treponema denticola with other spirochete genomes. Proc. Natl. Acad. Sci. USA 2004, 101, 5646–5651. [Google Scholar] [CrossRef] [PubMed]
- Chu, L.; Lai, Y.; Xu, X.; Eddy, S.; Yang, S.; Song, L.; Kolodrubetz, D. A 52-kDa leucyl aminopeptidase from treponema denticola is a cysteinylglycinase that mediates the second step of glutathione metabolism. J. Biol. Chem. 2008, 283, 19351–19358. [Google Scholar] [CrossRef]
- Chi, B.; Qi, M.; Kuramitsu, H.K. Role of dentilisin in Treponema denticola epithelial cell layer penetration. Res. Microbiol. 2003, 154, 637–643. [Google Scholar] [CrossRef] [PubMed]
- Fenno, J.C.; Wong, G.W.; Hannam, P.M.; McBride, B.C. Mutagenesis of outer membrane virulence determinants of the oral spirochete Treponema denticola. FEMS Microbiol. Lett. 1998, 163, 209–215. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ellen, R.P. Virulence determinants of oral treponemes. In Pathogenic Treponema; Radolf, J.D., Lukehart, S.A., Eds.; Caister Academic Press: Norwich, UK, 2006; pp. 357–386. [Google Scholar]
- Setubal, J.C.; Reis, M.; Matsunaga, J.; Haake, D.A. Lipoprotein computational prediction in spirochaetal genomes. Microbiology 2006, 152 Pt 1, 113–121. [Google Scholar] [CrossRef]
- Grenier, D.; Mayrand, D. Functional characterization of extracellular vesicles produced by Bacteroides gingivalis. Infect. Immun. 1987, 55, 111–117. [Google Scholar] [CrossRef]
- Dashper, S.G.; Seers, C.A.; Tan, K.H.; Reynolds, E.C. Virulence factors of the oral spirochete Treponema denticola. J. Dent. Res. 2011, 90, 691–703. [Google Scholar] [CrossRef]
- Perlson, E.; Maday, S.; Fu, M.M.; Moughamian, A.J.; Holzbaur, E.L. Retrograde axonal transport: Pathways to cell death? Trends Neurosci. 2010, 33, 335–344. [Google Scholar] [CrossRef]
- Oakes, S.G.; Repesh, L.A.; Pozos, R.S.; Fitzgerald, T.J. Electrophysiological dysfunction and cellular disruption of sensory neurones during incubation with Treponema Pallidum. Br. J. Vener. Dis. 1982, 58, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Riviere, G.R.; Riviere, K.H.; Smith, K.S. Molecular and immunological evidence of oral Treponema in the human brain and their association with Alzheimer’s disease. Oral Microbiol. Immunol. 2002, 17, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Secher, L.; Weismann, K.; Kobayasi, T. Treponema Pallidum in peripheral nerve tissue of syphilitic chancres. Acta Dermatovener. 1982, 62, 407–411. [Google Scholar]
- Su, X.; Tang, Z.; Lu, Z.; Liu, Y.; He, W.; Jiang, J.; Zhang, Y.; Wu, H. Oral Treponema denticola Infection Induces Aβ1-40 and Aβ1-42 Accumulation in the Hippocampus of C57BL/6 Mice. J. Mol. Neurosci. 2021, 71, 1506–1514. [Google Scholar] [CrossRef] [PubMed]
- Emery, D.C.; Shoemark, D.K.; Batstone, T.E.; Waterfall, C.M.; Coghill, J.A.; Cerajewska, T.L.; Davies, M.; West, N.X.; Allen, S.J. 16S rRNA next generation sequencing analysis shows bacteria in Alzheimer’s post-mortem brain. Front. Aging Neurosci. 2017, 20, 195. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, H.; Eribe, E.R.K.; Singhrao, S.K.; Olsen, I. High throughput sequencing detects gingivitis and periodontal oral bacteria in Alzheimer’s disease autopsy brains. J. Neurosci. Res. 2019, 1, 3. [Google Scholar] [CrossRef]
- Pisani, F.; Pisani, V.; Arcangeli, F.; Harding, A.; Singhrao, S.K. The Mechanistic Pathways of Periodontal Pathogens Entering the Brain: The Potential Role of Treponema denticola in Tracing Alzheimer’s Disease Pathology. Int. J. Environ. Res. Public Health 2022, 19, 9386. [Google Scholar] [CrossRef]
- Takahashi, K.; Kayama, Y.; Lin, J.S.; Sakai, K. Locus coeruleus neuronal activity during the sleep-waking cycle in mice. Neuroscience 2010, 169, 1115–1126. [Google Scholar] [CrossRef] [PubMed]
- Aston-Jones, G.; Rajkowski, J.; Cohen, J. Locus coeruleus and regulation of behavioral flexibility and attention. Prog. Brain Res. 2000, 126, 165–182. [Google Scholar] [CrossRef] [PubMed]
- James, T.; Kula, B.; Choi, S.; Khan, S.S.; Bekar, L.K.; Smith, N.A. Locus coeruleus in memory formation and Alzheimer’s disease. Eur. J. Neurosci. 2021, 54, 6948–6959. [Google Scholar] [CrossRef]
- Borodovitsyna, O.; Duffy, B.C.; Pickering, A.E.; Chandler, D.J. Anatomically and functionally distinct locus coeruleus efferents mediate opposing effects on anxiety-like behavior. Neurobiol. Stress 2020, 13, 100284. [Google Scholar] [CrossRef] [PubMed]
- Beardmore, R.; Hou, R.; Darekar, A.; Holmes, C.; Boche, D. The Locus Coeruleus in Aging and Alzheimer’s Disease: A Postmortem and Brain Imaging Review. J. Alzheimers Dis. 2021, 83, 5–22. [Google Scholar] [CrossRef] [PubMed]
- Panzarella, V.; Mauceri, R.; Baschi, R.; Maniscalco, L.; Campisi, G.; Monastero, R. Oral Health Status in Subjects with Amnestic Mild Cognitive Impairment and Alzheimer’s Disease: Data from the Zabút Aging Project. J. Alzheimers Dis. 2022, 87, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Ide, M.; Harris, M.; Stevens, A.; Sussams, R.; Hopkins, V.; Culliford, D.; Fuller, J.; Ibbett, P.; Raybould, R.; Thomas, R.; et al. Periodontitis and Cognitive Decline in Alzheimer’s Disease. PLoS ONE 2016, 11, e0151081. [Google Scholar] [CrossRef]
- Kim, J.H.; Oh, J.K.; Wee, J.H.; Kim, Y.H.; Byun, S.H.; Choi, H.G. Association between Tooth Loss and Alzheimer’s Disease in a Nested Case-Control Study Based on a National Health Screening Cohort. J. Clin. Med. 2021, 10, 3763. [Google Scholar] [CrossRef]
- Leblhuber, F.; Huemer, J.; Steiner, K.; Gostner, J.M.; Fuchs, D. Knock-on effect of periodontitis to the pathogenesis of Alzheimer’s disease? Wien. Klin. Wochenschr. 2020, 132, 493–498. [Google Scholar] [CrossRef]
- Schwahn, C.; Frenzel, S.; Holtfreter, B.; Van der Auwera, S.; Pink, C.; Bulow, R.; Friedrich, N.; Volzke, H.; Biffar, R.; Kocher, T.; et al. Effect of periodontal treatment on preclinical Alzheimer’s disease—Results of a trial emulation approach. Alzheimer’s Dement. 2022, 18, 127–141. [Google Scholar] [CrossRef]
- De Cicco, V.; Tramonti Fantozzi, M.P.; Cataldo, E.; Barresi, M.; Bruschini, L.; Faraguna, U.; Manzoni, D. Trigeminal, Visceral and Vestibular Inputs May Improve Cognitive Functions by Acting through the Locus Coeruleus and the Ascending Reticular Activating System: A New Hypothesis. Front. Neuroanat. 2018, 11, 130. [Google Scholar] [CrossRef]
- David, M.C.B.; Del Giovane, M.; Liu, K.Y.; Gostick, B.; Benedict Rowe, J.; Oboh, I.; Howard, R.; Malhotra, P.A. Cognitive and neuropsychiatric effects of noradrenergic treatment in Alzheimer’s disease: Systematic review and meta-analysis. J. Neurol. Neurosurg. Psychiatry 2022, 93, 1080–1090. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pisani, F.; Pisani, V.; Arcangeli, F.; Harding, A.; Singhrao, S.K. Locus Coeruleus Dysfunction and Trigeminal Mesencephalic Nucleus Degeneration: A Cue for Periodontal Infection Mediated Damage in Alzheimer’s Disease? Int. J. Environ. Res. Public Health 2023, 20, 1007. https://doi.org/10.3390/ijerph20021007
Pisani F, Pisani V, Arcangeli F, Harding A, Singhrao SK. Locus Coeruleus Dysfunction and Trigeminal Mesencephalic Nucleus Degeneration: A Cue for Periodontal Infection Mediated Damage in Alzheimer’s Disease? International Journal of Environmental Research and Public Health. 2023; 20(2):1007. https://doi.org/10.3390/ijerph20021007
Chicago/Turabian StylePisani, Flavio, Valerio Pisani, Francesca Arcangeli, Alice Harding, and Sim K. Singhrao. 2023. "Locus Coeruleus Dysfunction and Trigeminal Mesencephalic Nucleus Degeneration: A Cue for Periodontal Infection Mediated Damage in Alzheimer’s Disease?" International Journal of Environmental Research and Public Health 20, no. 2: 1007. https://doi.org/10.3390/ijerph20021007
APA StylePisani, F., Pisani, V., Arcangeli, F., Harding, A., & Singhrao, S. K. (2023). Locus Coeruleus Dysfunction and Trigeminal Mesencephalic Nucleus Degeneration: A Cue for Periodontal Infection Mediated Damage in Alzheimer’s Disease? International Journal of Environmental Research and Public Health, 20(2), 1007. https://doi.org/10.3390/ijerph20021007






