Slow Sand Filters for the 21st Century: A Review
Abstract
:1. Introduction
2. Mechanisms of Pollutant Removal
3. Filter Characteristics
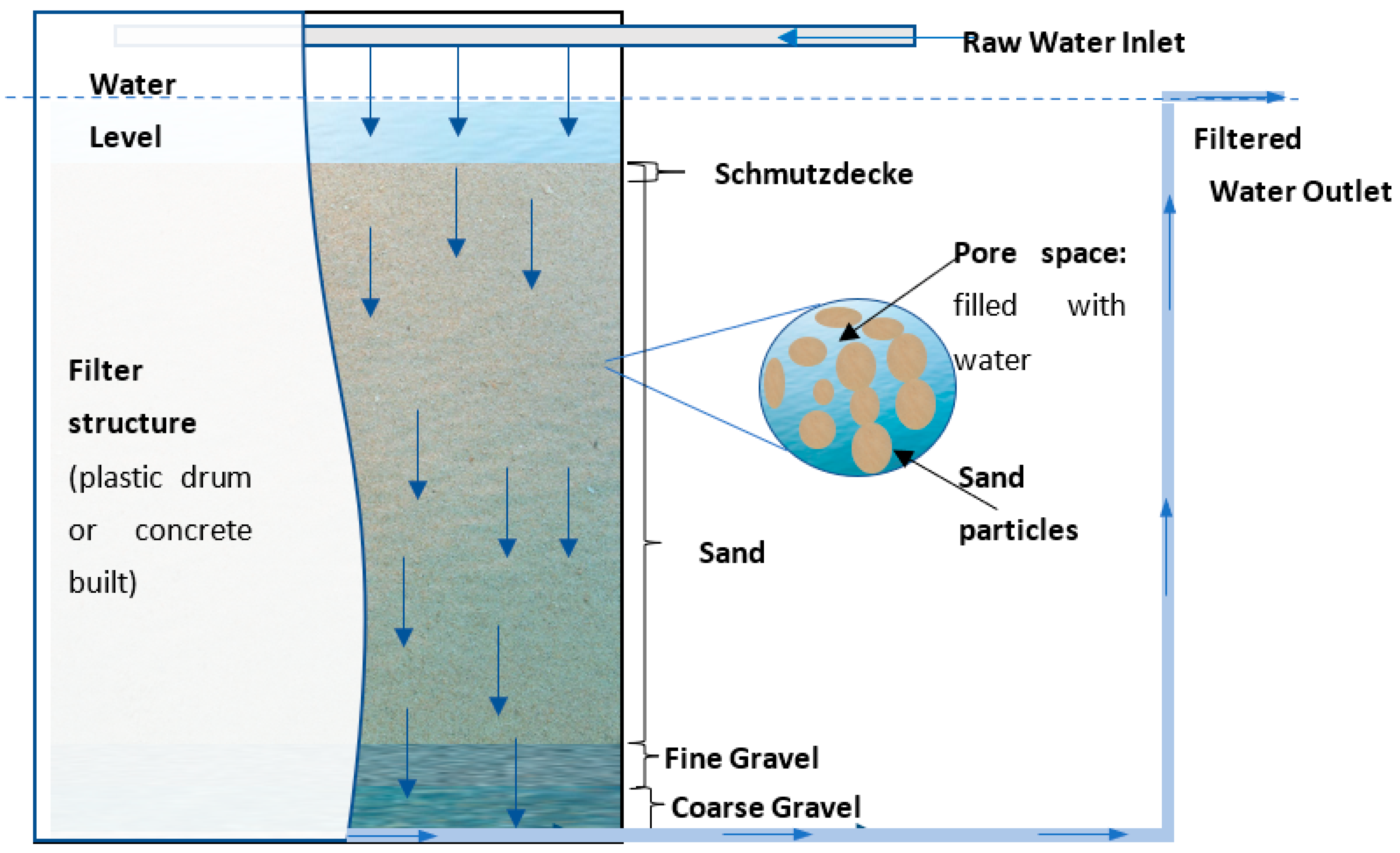
3.1. Filter Design
Filter Design Modifications
3.2. Filter Media
3.2.1. Media Amendments
3.2.2. Media Depth
3.3. Biological Maturity
4. Operating Conditions
4.1. Hydraulic Residence Time
4.2. Temperature
4.3. Mode of operation and O2 Requirements
4.4. Source Water Quality
5. Contaminant Removal
5.1. Protozoa and Larger Organisms
5.2. Bacteria
5.3. Virus
5.4. Turbidity
5.5. Organic Contaminants
5.6. Inorganic Contaminants
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. Progress on Drinking Water, Sanitation and Hygiene: 2017 Update and SDG Baselines; World Health Organization (WHO) and the United Nations Children’s Fund: Geneva, Switzerland, 2017.
- WHO. Progress on Sanitation and Drinking Water–2015 Update and MDG Assessment; Audaz, A.G., Ed.; UNICEF and World Health Organization: Geneva, Switzerland, 2015.
- WWAP. United Nations World Water Development Report 2020: Water and Climate Change; WWAP (United Nations World Water Assessment Programme): Paris, France, 2020. [Google Scholar]
- WHO. Preventing Diarrhoea through Better Water, Sanitation and Hygiene: Exposures and Impacts in Low- and Middle-Income Countries; World Health Organization: Geneva, Switzerland, 2014.
- Pruss-Ustun, A.; World Health Organization. Safer Water, Better Health: Costs, Benefits and Sustainability of Interventions to Protect and Promote Health; World Health Organization: Geneva, Switzerland, 2008.
- WWAP. The United Nations World Water Development Report 4: Managing Water under Uncertainty and Risk; WWAP (United Nations World Water Assessment Programme): Paris, France, 2012. [Google Scholar]
- Agrawal, A.; Sharma, N.; Sharma, P. Designing an economical slow sand filter for households to improve water quality parameters. Mater. Today Proc. 2021, 43, 1582–1586. [Google Scholar] [CrossRef]
- Guchi, E. Review on Slow Sand Filtration in Removing Microbial Contamination and Particles from Drinking Water. Am. J. Food Nutr. 2015, 3, 47–55. [Google Scholar]
- Zhao, Y.; Wang, X.; Liu, C.; Wang, S.; Wang, X.; Hou, H.; Wang, J.; Li, H. Purification of harvested rainwater using slow sand filters with low-cost materials: Bacterial community structure and purifying effect. Sci. Total Environ. 2019, 674, 344–354. [Google Scholar] [CrossRef] [PubMed]
- Elliott, M.A.; Stauber, C.E.; Koksal, F.; DiGiano, F.A.; Sobsey, M.D. Reductions of E-coli, echovirus type 12 and bacteriophages in an intermittently operated household-scale slow sand filter. Water Res. 2008, 42, 2662–2670. [Google Scholar] [CrossRef]
- Baker, M.N. The Quest for Pure Water; The American Water Works Association Inc.: New York, NY, USA, 1948; pp. 7–76. [Google Scholar]
- Haig, S.J.; Collins, G.; Davies, R.L.; Dorea, C.C.; Quince, C. Biological aspects of slow sand filtration: Past, present and future. Water Sci. Technol. Water Supply 2011, 11, 468–472. [Google Scholar] [CrossRef]
- Barrett, J.M.; Hendricks, D.W. Manual of Design for Slow Sand Filtration; AWWA and AWWA Research Foundation: New York, NY, USA, 1991; pp. 21–57. [Google Scholar]
- Huisman, L.; Wood, W.E. Slow sand Filtration; World Health Organization: Geneva, Switzerland, 1974.
- Poynter, S.F.B.; Slade, J.S. The removal of viruses by slow sand filtration. Prog. Wat. Tech. 1977, 9, 75–88. [Google Scholar]
- Lubarsky, H.; Fava, N.D.M.N.; Souza Freitas, B.L.; Terin, U.C.; Oliveira, M.; Lamon, A.W.; Pichel, N.; Byrne, J.A.; Sabogal-Paz, L.P.; Fernandez-Ibañez, P. Biological Layer in Household Slow Sand Filters: Characterization and Evaluation of the Impact on Systems Efficiency. Water 2022, 14, 1078. [Google Scholar] [CrossRef]
- D’Alessio, M.; El-Swaify, G.; Yoneyama, B.; Ray, C. A low-cost water-treatment system for potable water supplies in developing countries and after a natural disaster: Ability to remove total coliforms and E-coli. Clean Technol. Environ. Policy 2016, 18, 925–934. [Google Scholar] [CrossRef]
- Wang, H.; Narihiro, T.; Straub, A.P.; Pugh, C.R.; Tamaki, H.; Moor, J.F.; Bradley, L.M.; Kamagata, Y.; Liu, W.-T.; Nguyen, T.H. MS2 Bacteriophage Reduction and Microbial Communities in Biosand Filters. Environ. Sci. Technol. 2014, 48, 6702–6709. [Google Scholar] [CrossRef]
- Hijnen, W.A.M.; Schijven, J.F.; Bonné, P.; Visser, A.; Medema, G.J. Elimination of viruses, bacteria and protozoan oocysts by slow sand filtration. Water Sci. Technol. 2004, 50, 147–154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bellamy, W.D. Filtration of Giardia Cysts and Other Substances: Volume 2, Slow Sand Filtration; Silverman, G.P., Hendricks, D.W., Eds.; U.S. Environmental Protection Agency, Water Engineering Research Laboratory: Cincinnati, OH, USA, 1985.
- Elliott, M.A.; Digiano, F.A.; Sobsey, M.D. Virus attenuation by microbial mechanisms during the idle time of a household slow sand filter. Water Res. 2011, 45, 4092–4102. [Google Scholar] [CrossRef] [PubMed]
- Ellis, K.V. Slow sand filtration. Crit. Rev. Environ. Control 1985, 15, 315–354. [Google Scholar] [CrossRef]
- Fogel, D.; Isaac-Renton, J.; Guasparini, R.; Moorehead, W.; Ongerth, J. Removing giardia and cryptosporidium by slow sand filtration. J. Am. Water Work. Assoc. 1993, 85, 77–84. [Google Scholar] [CrossRef]
- WeberShirk, M.L.; Dick, R.I. Biological mechanisms in slow sand filters. J. Am. Water Work. Assoc. 1997, 89, 72–83. [Google Scholar] [CrossRef]
- Bai, Y.; Liu, R.; Liang, J.; Qu, J. Integrated metagenomic and physiochemical analyses to evaluate the potential role of microbes in the sand filter of a drinking water treatment system. PLoS ONE 2013, 8, e61011. [Google Scholar] [CrossRef] [Green Version]
- Visscher, J.T.; Paramasiram, R.; Raman, A.; Heijnen, H.A. Slow Sand Filtration for Community Water Supply: Planning, Design, Construction, Operation and Maintenance; Technical paper series/IRC; IRC: The Hague, The Netherlands, 1987; Volume 24.
- Ngai, T.K.K.; Baker, D.L. Recent Advances in Household Biosand Filter Design; Progress in Slow Sand and Alternative Biofiltration Processes: Further Developments and Applications; Nakamoto, N., Graham, N., Collins, M.R., Gimbel, R., Eds.; International Water Association: Nagoya, Japan, 2014. [Google Scholar]
- Campos, L.C.; Su, M.F.J.; Graham, N.J.D.; Smith, S.R. Biomass development in slow sand filters. Water Res. 2002, 36, 4543–4551. [Google Scholar] [CrossRef]
- CAWST. BioSand Filter Construction Manual; CAWST (Centre for Affordable Water and Sanitation Technology): Calgary, AB, Canada, 2012. [Google Scholar]
- Galvis, G.; Latorre, J.; Galvis, A. Multi-stage filtration technology. Small Community Water Supplies IRC Tech. Pap. Ser. 2002, 40, 121–345. [Google Scholar]
- Kubare, M.; Haarhoff, J. Rational design of domestic biosand filters. J. Water Supply Res. Technol.-Aqua 2010, 59, 1–15. [Google Scholar] [CrossRef]
- Freitas, B.L.S.; Terin, U.C.; Fava, N.D.M.N.; Maciel, P.M.F.; Garcia, L.A.T.; Medeiros, R.C.; Oliveira, M.D.; Fernandez-Ibañez, P.; Byrne, J.A.; Sabogal-Paz, L.P. A critical overview of household slow sand filters for water treatment. Water Res. 2022, 208, 117870. [Google Scholar] [CrossRef]
- Kennedy, T.J.; Hernandez, E.A.; Morse, A.N.; Anderson, T.A. Hydraulic Loading Rate Effect on Removal Rates in a BioSand Filter: A Pilot Study of Three Conditions. Water Air Soil Pollut. 2012, 223, 4527–4537. [Google Scholar] [CrossRef]
- Bradley, I.; Straub, A.; Maraccini, P.; Markazi, S.; Nguyen, T.H. Iron oxide amended biosand filters for virus removal. Water Res. 2011, 45, 4501–4510. [Google Scholar] [CrossRef] [Green Version]
- Hussain, G.; Haydar, S.; Bari, A.J.; Aziz, J.A.; Anis, M.; Asif, Z. Evaluation of Plastic Household Biosand Filter (BSF) In Combination with Solar Disinfection (SODIS) For Water Treatment. J. Chem. Soc. Pak. 2015, 37, 352–362. [Google Scholar]
- Stauber, C.E.; Printy, E.R.; McCarty, F.A.; Liang, K.R.; Sobsey, M.D. Cluster randomized controlled trial of the plastic BioSand Water filter in Cambodia. Environ. Sci. Technol. 2012, 46, 722–728. [Google Scholar] [CrossRef]
- Nitzsche, K.S.; Weigold, P.; Losekann-Behrens, T.; Kappler, A.; Behrens, S. Microbial community composition of a household sand filter used for arsenic, iron, and manganese removal from groundwater in Vietnam. Chemosphere 2015, 138, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Ngai, T.K.K.; Shrestha, R.R.; Dangol, B.; Maharjan, M.; Murcott, S.E. Design for sustainable development–Household drinking water filter for arsenic and pathogen treatment in Nepal. J. Environ. Sci. Health Part A-Toxic/Hazard. Subst. Environ. Eng. 2007, 42, 1879–1888. [Google Scholar] [CrossRef] [PubMed]
- Bellamy, W.D.; Hendricks, D.W.; Logsdon, G.S. Slow Sand Filtration: Influences of Selected Process Variables. J. Am. Water Work. Assoc. 1985, 77, 62–66. [Google Scholar] [CrossRef]
- Jenkins, M.W.; Tiwari, S.K.; Darby, J. Bacterial, viral and turbidity removal by intermittent slow sand filtration for household use in developing countries: Experimental investigation and modeling. Water Res. 2011, 45, 6227–6239. [Google Scholar] [CrossRef]
- Logan, A.J.; Stevik, T.K.; Siegrist, R.L.; Rønn, R.M. Transport and fate of Cryptosporidium parvum oocysts in intermittent sand filters. Water Res. 2001, 35, 4359–4369. [Google Scholar] [CrossRef] [PubMed]
- Schuler, P.F.; Ghosh, M.M.; Gopalan, P. Slow sand and diatomaceous-earth filtration of cysts and other particulates. Water Res. 1991, 25, 995–1005. [Google Scholar] [CrossRef]
- White, C.; Sangster, J.L.; Joy, S.R.; Dunekacke, A.; Pirog, M.; Jones, E.G.; Barelt-Hunt, S.L. Effect of Contaminated Filtration Sand on Performance of Household Biosand Filters. In Proceedings of the Third 2013 IEEE Global Humanitarian Technology Conference, San Jose/Silicon Valley, CA, USA, 20–23 October 2013; IEEE: New York, NY, USA, 2013; pp. 243–247. [Google Scholar]
- Grace, M.A.; Healy, M.G.; Clifford, E. Performance and surface clogging in intermittently loaded and slow sand filters containing novel media. J. Environ. Manage 2016, 180, 102–110. [Google Scholar] [CrossRef] [Green Version]
- Ahammed, M.M.; Davra, K. Performance evaluation of biosand filter modified with iron oxide-coated sand for household treatment of drinking water. Desalination 2011, 276, 287–293. [Google Scholar] [CrossRef]
- Tellen, V.; Nkeng, G.; Dentel, S. Improved Filtration Technology for Pathogen Reduction in Rural Water Supplies. Water 2010, 2, 285–306. [Google Scholar] [CrossRef]
- Mwabi, J.K.; Mamba, B.B.; Momba, M.N.B. Removal of Escherichia coli and Faecal Coliforms from Surface Water and Groundwater by Household Water Treatment Devices/Systems: A Sustainable Solution for Improving Water Quality in Rural Communities of the Southern African Development Community Region. Int. J. Environ. Res. Public Health 2012, 9, 139–170. [Google Scholar] [CrossRef]
- Okademi, N.; Odipo, O.; Khazenzi, J. Defluorination Effectiveness of Modified Biosand Filters. Afr. J. Educ. Sci. Technol. 2022, 7, 123–140. [Google Scholar]
- Ebrahimi, A.; Jafari, N.; Abdolahnejad, A. Application of Iranian natural zeolite and blast furnace slag as slow sand filters media for water softening. Int. J. Environ. Health Eng. 2014, 3, 26. [Google Scholar] [CrossRef]
- Hyde, E.M.; Lackey, L.W. The impact of loading frequency and copper as a biocide on biosand filter performance. J. Water Sanit. Hyg. Dev. 2013, 3, 355–364. [Google Scholar] [CrossRef]
- Urfer, D. Use of bauxite for enhanced removal of bacteria in slow sand filters. Water Sci. Technol. Water Supply 2017, 17, 1007–1015. [Google Scholar] [CrossRef]
- Rao, S.M.; Malini, R.; Lydia, A.; Lee, Y. Contaminants removal by bentonite amended slow sand filter. J. Water Chem. Technol. 2013, 35, 23–29. [Google Scholar] [CrossRef]
- Jasudkar, D.; Rakhunde, R.; Deshpande, L.; Labhasetwar, P.; Juneja, H.D. Arsenic Remediation from Drinking Water Using Fenton’s Reagent with Slow Sand Filter. Bull. Environ. Contam. Toxicol. 2012, 89, 1231–1234. [Google Scholar] [CrossRef] [PubMed]
- Bauer, R.; Dizer, H.; Graeber, I.; Rosenwinkel, K.-H.; López-Pilaa, J.M. Removal of bacterial fecal indicators, coliphages and enteric adenoviruses from waters with high fecal pollution by slow sand filtration. Water Res. 2011, 45, 439–452. [Google Scholar] [CrossRef]
- Kennedy, T.J.; Anderson, T.A.; Hernandez, E.A.; Morse, A.N. Determining the operational limits of the biosand filter. Water Sci. Technol.-Water Supply 2013, 13, 56–65. [Google Scholar] [CrossRef]
- Mahmood, Q.; Baig, S.A.; Nawab, B.; Shafqat, M.N.; Pervez, A.; Zeb, B.S. Development of low cost household drinking water treatment system for the earthquake affected communities in Northern Pakistan. Desalination 2011, 273, 316–320. [Google Scholar] [CrossRef]
- Stauber, C.E.; Elliott, M.A.; Koksal, F.; Ortiz, G.M.; DiGiano, F.A.; Sobsey, M.D. Characterisation of the biosand filter for E. coli reductions from household drinking water under controlled laboratory and field use conditions. Water Sci. Technol. 2006, 54, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Muhammad, N.; Ellis, K.; Parr, J.; Smith, M.D. Optimization of Slow sand Filtration. In Reaching the Unreached–Challenges for the 21st Century: Proceedings of the 22nd WEDC Conference, New Delhi, India, 1996; WEDC, Loughborough University: Leicestershire, UK, 1996; pp. 283–285. [Google Scholar]
- Nair, A.T.; Ahammed, M.M.; Davra, K. Influence of operating parameters on the performance of a household slow sand filter. Water Sci. Technol.-Water Supply 2014, 14, 643–649. [Google Scholar] [CrossRef]
- Mangoua-Allali, A.L.C.; Coulibaly, L. Effect of flow rate on the performance of Biosand Filter. Int. J. Biol. Chem. Sci. 2021, 15, 2224–2232. [Google Scholar] [CrossRef]
- Tundia, K.R.; Ahammed, M.M.; George, D. The effect of operating parameters on the performance of a biosand filter: A statistical experiment design approach. Water Sci. Technol.-Water Supply 2016, 16, 775–782. [Google Scholar] [CrossRef]
- Napotnik, J.A.; Baker, D.; Jellison, K.L. Influence of sand depth and pause period on microbial removal in traditional and modified biosand filters. Water Res. 2020, 189, 116577. [Google Scholar] [CrossRef] [PubMed]
- Bellamy, W.D.; Silverman, G.P.; Hendricks, D.W.; Logsdon, G.S. Removing Giardia Cysts with Slow Sand Filtration. J. Am. Water Work. Assoc. 1985, 77, 52–60. [Google Scholar] [CrossRef]
- Young-Rojanschi, C.; Madramootoo, C. Intermittent versus continuous operation of biosand filters. Water Res. 2014, 49, 1–10. [Google Scholar] [CrossRef]
- McKenzie, E.R.; Jenkins, M.W.; Tiwari, S.S.K.; Darby, J.; Saenyi, W.; Gichaba, C.M. In-home performance and variability of biosand filters treating turbid surface and rain water in rural Kenya. J. Water Sanit. Hyg. Dev. 2013, 3, 189–198. [Google Scholar] [CrossRef]
- Aslan, S.; Cakici, H. Biological denitrification of drinking water in a slow sand filter. J. Hazard. Mater. 2007, 148, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Moreira Neto, R.F.; Calijuri, M.L.; Carvalho, I.D.C.; Santiago, A.D.F. Rainwater treatment in airports using slow sand filtration followed by chlorination: Efficiency and costs. Resour. Conserv. Recycl. 2012, 65, 124–129. [Google Scholar] [CrossRef]
- Hijnen, W.A.M.; Dullemont, Y.J.; Schijven, J.F.; Hanzens-Brower, A.J.; Rosielle, M.; Medema, G. Removal and fate of Cryptosporidium paruum, Clostridium perfringens and small-sized centric diatoms (Stephanodiscus hantzschii) in slow sand filters. Water Res. 2007, 41, 2151–2162. [Google Scholar] [CrossRef] [PubMed]
- Pereira, S.P.; Martins, F.D.C.; Gomes, L.N.L.; Sales, M.D.V.; De Padua, V.L. Removal of cyanobacteria by slow sand filtration for drinking water. J. Water Sanit. Hyg. Dev. 2012, 2, 133–145. [Google Scholar] [CrossRef]
- Kawata, K. Slow sand filtration for cercarial control in North Cameroon village water supply. Water Sci. Technol. 1982, 14, 491–498. [Google Scholar] [CrossRef]
- Yildiz, B.S. Performance assessment of modified biosand filter with an extra disinfection layer. J. Water Supply Res. Technol.-Aqua 2016, 65, 266–276. [Google Scholar] [CrossRef] [Green Version]
- Tiwari, S.S.K.; Schmidt, W.P.; Darby, J.; Kariuki, Z.G.; Jenkins, M.W. Intermittent slow sand filtration for preventing diarrhea among children in Kenyan households using unimproved water sources: Randomized controlled trial. Trop. Med. Int. Health 2009, 14, 1374–1382. [Google Scholar] [CrossRef] [Green Version]
- Stauber, C.E.; Kominek, B.; Liang, K.R.; Osman, M.K.; Sobsey, M.D. Evaluation of the Impact of the Plastic BioSand Filter on Health and Drinking Water Quality in Rural Tamale, Ghana. Int. J. Environ. Res. Public Health 2012, 9, 3806–3823. [Google Scholar] [CrossRef]
- Ngai, T.K.K.; Coff, B.; Baker, D.; Lentz, R. Global Review of the Adoption, Use, and Performance of the Biosand Filter; Progress in Slow Sand and Alternative Biofiltration Processes: Further Developments and, Applications; Nakamoto, N., Graham, N., Collins, M.R., Gimbel, R., Eds.; International Water Association: Nagoya, Japan, 2014. [Google Scholar]
- Yahya, M.; Cluff, C.; Gerba, C. Virus removal by slow sand filtration and nanofiltration. Wal. Sci. Tech. 1993, 27, 445–448. [Google Scholar] [CrossRef]
- Wang, H.T.; Li, M.M.; Brockman, K.; Nguyen, T.H. Reduction of MS2 bacteriophage and rotavirus in biosand filters. Environ. Sci.-Water Res. Technol. 2016, 2, 483–491. [Google Scholar] [CrossRef]
- Anderson, W.B.; DeLoyde, J.L.; Van Dyke, M.I.; Huck, P.M. Influence of design and operating conditions on the removal of MS2 bacteriophage by pilot-scale multistage slow sand filtration. J. Water Supply Res. Technol.-Aqua 2009, 58, 450–462. [Google Scholar] [CrossRef]
- WHO. Water Quality and Health Review of Turbidity: Information for Regulators and Water Suppliers; World Health Organization: Geneva, Switzerland, 2017.
- Li, J.; Zhou, Q.; Campos, L.C. The application of GAC sandwich slow sand filtration to remove pharmaceutical and personal care products. Sci. Total Environ. 2018, 635, 1182–1190. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Mehrez, R.; Jekel, M.; Ernst, M. Effect of slow sand filtration of treated wastewater as pre-treatment to UF. Desalination 2009, 249, 591–595. [Google Scholar] [CrossRef]
- Joshi, N.S.; Kelkar, P.S.; Dhage, S.S.; Paramasivam, R.; Gadkari, S.K. Water quality changes during slow sand filtration. Indian J. Environ. Health 1982, 24, 261–276. [Google Scholar]
- Demir, N.M. Experimental Study of Factors that Affect Iron and Manganese Removal in Slow Sand Filters and Identification of Responsible Microbial Species. Pol. J. Environ. Stud. 2016, 25, 1453–1465. [Google Scholar] [CrossRef] [PubMed]
- Subari, F.; Kamaruzzaman, M.A.; Abdullah, S.R.S.; Hasan, H.A.; Othman, A.R. Simultaneous removal of ammonium and manganese in slow sand biofilter (SSB) by naturally grown bacteria from lake water and its diverse microbial community. J. Environ. Chem. Eng. 2018, 6, 6351–6358. [Google Scholar] [CrossRef]
| Recommended by | Bed Depth (m) | Effective Medium Size (mm) | Darcy Filtration Rate (m/h) | Sand Uniformity Coefficient | Support Bed Depth (m) | Supernatant Water Depth (m) | Type of Filter |
|---|---|---|---|---|---|---|---|
| [14] | 1.2 | 0.15–0.35 | 0.1–0.4 | 3.0 | 1–1.5 | SSF | |
| [26] | 0.9 | 0.15–0.3 | 0.1–0.2 | 0.3–0.5 | 1 | SSF | |
| Ten States Standards USA (1987) [8] | 0.8 | 0.3–0.45 | 0.08–0.24 | 0.4–0.6 | 0.9 | SSF | |
| [27,28] | 0.6–1 | 0.1–0.3 | 0.1–0.3 | <3 | SSF | ||
| [29] | >0.5 | 0.15–0.2 | 0.4 | 1.5–2.5 | Concrete BSF | ||
| [27] | >0.4 | 0.15–0.3 | 0.16–1.1 | 1.5–5 | BSF |
| Parameter | Setting | Filter Design | Operation | Removal Efficiency | Reference | |||
|---|---|---|---|---|---|---|---|---|
| Material | Medium (Depth) | Dimensions/Area | Mode | Residence Time/Flow Rate | ||||
| Cryptosporidium parvum | Pilot | 0.28 mm sand (1.5 m) | 2.5 m2 | Continuous | 0.3 m/h | 4.7 log10 | [68] | |
| Pilot | 0.3 mm sand | 2.56 m2, depth = 1.5 m | Continuous | 0.3 m/h | >5 log10 | [19] | ||
| Lab | PVC pipe | 0.27 mm sand (0.9 m) | Diameter = 0.286 m | Continuous | 0.15–0.4 m/h | >99.99% | [42] | |
| Field | 0.2–0.3 mm sand (1.05 m) | 43 × 6 m | Continuous | 0.19–0.4 m/h | Average 48% | [23] | ||
| Lab | Acrylic lysimeters | 0.16 mm sand | Diameter = 0.15 m | Intermittent | 0.04–0.1 m/h | >3 log10 | [41] | |
| Giardia cysts | Pilot | PVC pipe | 0.29–0.62 mm sand (0.48–0.97 m) | Diameter = 0.3 m | Continuous | 0.04–0.4 m/h | >98% | [20,39,63] |
| Phytoplankton | Lab | 10 mm thick acrylic | 0.18–2.83 sand (0.9 m) | 0.2 × 0.2 m; | Continuous | 1.8–7.1 m/day | Up to 97% | [69] |
| Schistome Cercariae | Lab | PVC pipe | 0.1–0.4 mm sand (0.6–1.2 m) | Diameter = 0.152 m | Continuous | 0.04–0.4 m/h | 88.2–100% | [22,70] |
| Parameter | Setting | Filter Design | Operation | Removal Efficiency | Reference | |||
|---|---|---|---|---|---|---|---|---|
| Material | Medium (Depth) | Dimensions/Area | Mode | Residence Time/Flow Rate | ||||
| E. coli | Lab | Columns | 0.17 mm sand (0.55 m) | Dia = 10 cm | Continuous | R. time = 1 day | 3.71 log10 | [64] |
| Lab | Columns | 0.17 mm sand (0.55 m) | Dia = 10 cm | Batch | R. time = 1 day | 1.67 log10 | ||
| Lab | Perspex cylinders | Sand (0.6 m) | 0.09 m2, depth = 3 m | Continuous | 0.2 m/h | >99.3% | [15] | |
| Pilot | 0.3 mm sand | 2.56 m2, depth = 1.5 m | Continuous | 0.3 m/h | 2–4.2 log10 | [19] | ||
| Field | 0.13–0.6 mm sand | Length = 1–1.5 m | Continuous | 0.25–0.4 m/h | 1.5–3.7 log10 | |||
| Field | Plastic filter | <1 mm sand (0.45 m) | 60 L, depth = 0.76 m | Batch | Public use | 0–97% (mean 93%) | [57] | |
| Lab | Plastic filter | <1 mm sand (0.45 m) | 60 L, depth = 0.76 m | Batch | 40 L/day | 63–99% (mean 94%) | ||
| Lab | Plastic containers | 0.23 mm sand (0.45 m) | p. vol. = 19.1 L, dia = 40 cm, depth = 73 cm | Batch | 20–40 L/day | <1 log10–2 log10 | [45] | |
| Lab | Plastic containers | 0.23 mm sand (0.45 m) 10 cm iron-oxide-coated sand | p. vol. = 19.1 L, dia = 40 cm, depth = 73 cm | Batch | 20–40 L/day | >2 log10 to >3 log10 | ||
| Pilot | Concrete BSF | 0.19 to 0.22 mm sand (0.45 m) | dia 15 cm, depth 90 cm | Batch | 20 L/day | 96.3–97.9% | [56] | |
| Lab | Plastic bucket | Sand (0.175 m) | 25 L bucket, depth 41 cm | Batch | 20 L/day | 1.6–3.7 log10 | [47] | |
| Lab | Plastic bucket | Sand (0.175 m) 10 cm of 3 mm zeolites | 25 L bucket, depth 41 cm | Batch | 20 L/day | 1.3–3.7 log10 | ||
| Lab | Concrete BSF | 0.84–3.3 mm sand (0.44 m); 155 g chipped copper | Concrete BSFs (CAWST); 70 L | Batch | 20 L/day | 52–99.99% | [50] | |
| Lab | Concrete BSF | 0.84–3.3 mm sand (0.44 m) | Concrete BSFs (CAWST); 70 L | Batch | 20 L/day | 20–99.99% | ||
| Lab | Concrete BSF | 0.84–3.3 mm sand (0.44 m); 155 g chipped Cu | Concrete BSFs (CAWST); 70 L | Batch | 20 L/3 day | 45–99.99% | ||
| Lab | Concrete BSF | 0.84–3.3 mm sand (0.44 m) | Concrete BSFs (CAWST); 70 L | Batch | 20 L/3 day | 22–99.99% | ||
| Lab | Concrete | 0.18–0.3 mm sand | Concrete BSFs (CAWST) [29] | Batch | 10 L/36 h–30 L/12 h | Optimum 99.4% | [61] | |
| Pilot | Polyethylene barrels | 0.15–0.30 mm sand (0.57 m) | Dia 0.57 m, depth 0.88 m | Continuous | 0.05 m/h | 0–99.64% | [17] | |
| Field | Concrete | Sand (0.55 m) | Depth 95 cm, dia 35 cm, vol. 53.5 L | 18 L/day | >95% by day 7 | [43] | ||
| Lab | Concrete | Contaminated sand (0.55 m) | Concrete BSFs (CAWST) | 10 L/day | 38 to over 99% | |||
| Lab | Concrete | Sand (0.55 m) | Concrete BSFs (CAWST) | 10 L/day | 71–99% | |||
| Lab | PVC columns | 0.3–2.33 mm sand (0.45 m) | Depth 85 cm, dia 14.9 cm | Intermittent | 8 L/12 h | 97.8% | [71] | |
| Lab | PVC columns | 0.3–2.33 mm sand (0.45 m); 21 g brass | Depth 85 cm, dia 14.9 cm | Intermittent | 8 L/12 h | 98.2% | ||
| Lab | PVC columns | 0.3–2.33 mm sand (0.45 m); 39 g zerovalent iron | Depth 85 cm, dia 14.9 cm | Intermittent | 8 L/12 h | 97.33% | ||
| Total Coliform | Lab | Perspex cylinders | Sand (0.6 m) | 0.09 m2, d = 3 m | Continuous | 4.8 m/day | >96.3% | [15] |
| Pilot | PVC pipe | 0.29–0.62 mm sand (0.48–0.97 m) | diameter = 0.3 m | Continuous | 0.12 m/h | 60.1–99.994% | [20,39] | |
| Field | KanchanTM Arsenic Filter (KAF) | Gravel, sand, and iron nails | Plastic or concrete bucket | Intermittent | Public use 10–15 L/h | 60–99% | [38] | |
| Field | Concrete BSF | 0.14 mm sand (0.5 m) | BushProof BSF: 0.95 m tall, 36 cm diameter | Intermittent | Public use | 1.25 log10 | [72] | |
| Field | Concrete BSF | 0.14 mm sand (0.5 m) | BushProof BSF: 0.95 m tall, 36 cm diameter | Intermittent | 1.30 log10 | |||
| Lab | Concrete BSF | 0.3 mm sand (0.5 m); 0.5 kg zerovalent iron | Concrete BSFs (CAWST) | Batch | 99% | [46] | ||
| Lab | Concrete BSF | 0.3 mm sand (0.5 m) | Concrete BSFs (CAWST) | Batch | 0–95% | |||
| Pilot | Concrete BSF | 0.19 to 0.22 mm sand (0.45 m) | Dia 15 cm, depth 90 cm | Batch | 20 L/day | 97.2–97.4% | [56] | |
| Lab | 10 mm thick acrylic | 0.18–2.83 sand (0.9 m) | 0.2 × 0.2 m | Continuous | 1.8–7.1 m/day | 50–99.98% | [69] | |
| Lab | Concrete BSF | 0.84–3.3 mm sand (0.44 m); 155 g chipped copper | Concrete BSFs (CAWST); 70 L | Batch | 20 L/day | 33.3–99.8% | [50] | |
| Lab | Concrete BSF | 0.84–3.3 mm sand (0.44 m) | Concrete BSFs (CAWST); 70 L | Batch | 20 L/day | 30–99.6% | ||
| Lab | Concrete BSF | 0.84–3.3 mm sand (0.44 m); 155 g chipped Cu | Concrete BSFs (CAWST); 70 L | Batch | 20 L/3 day | 13.3–99.8% | ||
| Lab | Concrete BSF | 0.84–3.3 mm sand (0.44 m) | Concrete BSFs (CAWST); 70 L | Batch | 20 L/3 day | 25.35–99.99% | ||
| Field | Concrete | Sand (0.55 m) | Depth 95 cm, dia 35 cm, vol. 53.5 L | 18 L/day | >95% by day 7 | [43] | ||
| Lab | Concrete | Contaminated sand (0.55 m) | Concrete BSFs (CAWST) | 10 L/day | 64 to over 99% | |||
| Lab | Concrete | Sand (0.55 m) | Concrete BSFs (CAWST) | 10 L/day | 83–99% | |||
| Lab | Plastic bucket | As per CASWT specifications [29]; 0.14 mm sand (0.15 m) | 30 L, dia 30 cm, depth 32 cm | Batch | 20 L/12 h | Up to 1.5–2.2 log10 | [35] | |
| Pilot | Polyethylene barrels | 0.15–0.30 mm sand (0.57 m) | Dia 0.57 m, depth 0.88 m | Continuous | 0.05 m/h | 40.73–99.99% | [17] | |
| Lab | Concrete | 0.18–0.3 mm sand | Concrete BSFs (CAWST) [29] | Batch | 10 L/36 h–30 L/12 h | Optimum 99.7% | [61] | |
| Lab | PVC columns | 0.3–2.33 mm sand (0.45 m) | Depth 85 cm, dia 14.9 cm | Intermittent | 8 L/12 h | 91.29% | [71] | |
| Lab | PVC columns | 0.3–2.33 mm sand (0.45 m); 21 g brass | Depth 85 cm, dia 14.9 cm | Intermittent | 8 L/12 h | 90.11% | ||
| Lab | PVC columns | 0.3–2.33 mm sand (0.45 m); 39 g zerovalent iron | Depth 85 cm, dia 14.9 cm | Intermittent | 8 L/12 h | 96.93% | ||
| Fecal Coliform | Pilot | PVC pipe | 0.29–0.62 mm sand (0.48–0.97 m) | Diameter = 0.3 m | Continuous | 0.04–0.4 m/h | 98.45–99.84% | [20,63] |
| Lab | PVC | 0.117–0.52 mm sand | Dia = 0.305 m | Batch | R. time 5–16 h (0.1–0.3 m/h) | 1.4 log10 | [40] | |
| Lab | Concrete BSF | 0.3 mm sand (0.5 m); 0.5 kg zerovalent iron | Concrete BSFs (CAWST) | Batch | 99% | [46] | ||
| Lab | Concrete BSF | 0.3 mm sand (0.5 m) | Concrete BSFs (CAWST) | Batch | 0–95% | |||
| Lab | Plastic bucket | Sand (0.175 m) | 25 L bucket, depth 41 cm | Batch | 20 L/day | 2.2–3.7 log10 | [47] | |
| Lab | Plastic bucket | Sand (0.175 m); 10 cm of 3 mm zeolites | 25 L bucket, depth 41 cm | Batch | 20 L/day | 2.7–4 log10 | ||
| Lab | Plastic BSF | 0.15–0.4 mm sand (0.43 m) | 60 L BSF (HydrAid) | Batch | 20 L/day | 0.88–1.72 log10 | [55] | |
| Field | Concrete BSFs (n = 155) | 0.15 mm sand (0.5 m) | Depth 0.95 m, dia 36 cm | Batch | Public use 20 L/8 h | 1.41 log10 | [65] | |
| Lab | Plastic bucket | As per CASWT specifications [29]; 0.14 mm sand (0.15 m) | 30 L, dia 30 cm, depth 32 cm | Batch | 20 L/12 h | Up to 1.8–1.95 log10 | [35] | |
| Fecal Streptococci | Lab | Concrete BSF | 0.3 mm sand (0.5 m); 0.5 kg zerovalent iron | Concrete BSFs (CAWST) | Batch | 99% | [46] | |
| Lab | Concrete BSF | 0.3 mm sand (0.5 m) | Concrete BSFs (CAWST) | Batch | 0–95% | |||
| Campylobacter | Field | 0.13–0.6 mm sand | L = 1 to 1.5 m | Continuous | 0.25–0.4 m/h | 3.2–4.1 log10 | [19] | |
| Parameter | Setting | Filter Design | Operation | Removal Efficiency | Reference | |||
|---|---|---|---|---|---|---|---|---|
| Material | Medium (Depth) | Dimensions/Area | Mode | Residence Time/Flow Rate | ||||
| Enteric Viruses (e.g., Polio Virus) | Lab | PVC pipe | 0.27 mm sand (0.9 m) | Diameter = 0.286 m | Continuous | 0.15–0.4 m/h | >99.83% | [42] |
| Field | 0.2–0.3 mm sand (1.05 m) | 43 × 6 m | Continuous | 0.19–0.4 m/h | Average 93% | [23] | ||
| Pilot | Pond | (1.8 m) sample at 120 cm | 3.25 × 6.85 m | Continuous | 0.29 m/d | >1.8 log10 | [54] | |
| Bacteriophage (MS-2) | Lab | Perspex cylinders | Sand (0.6 m) | 0.09 m2, depth = 3 m | Continuous | 0.2 m/h | 99.75–99.996% | [15] |
| Pilot | 0.3 mm sand | 2.56 m2, depth = 1.5 m | Continuous | 0.3 m/h | 2.1 ± 0.6 log10 | [19] | ||
| Field | Plastic | Sand (4 ft) | 5 × 10 ft, depth = 6 ft | Continuous | 99% | [75] | ||
| Pilot | Pond | (1.8 m) sample at 90 cm | 3.25 × 6.85 m | Continuous | 0.5–2 m/d | 2.5–4.01 log10 (mean) | [54] | |
| Lab | Plastic BSF | 0.15–0.4 mm sand (0.45 m) | 60 L BSF (HydrAid) | Batch | 20 L/day | 1–2 log10 | [34] | |
| Lab | Plastic BSF | 0.15–0.4 mm sand (0.45 m); 5.54 kg mild iron filings | 60 L BSF (HydrAid) | Batch | 20 L/day | 3–4 2 log10 | ||
| Lab | Concrete BSF | Sand | Concrete BSFs (CAWST) | Batch | 20 L/day | Average = 2 log10 | ||
| Lab | Concrete BSF | Sand; 5.54 kg zerovalent iron | Concrete BSFs (CAWST) | Batch | 20 L/day | 7 log10 | ||
| Lab | Concrete BSF | Sand; 0.26 kg steel wool | Concrete BSFs (CAWST) | Batch | 20 L/day | >5 log10 | ||
| Lab | Columns | 224.1 g sand; 10% iron | Dia = 2.5 cm, vol = 146.7–161.5 cm3 | Batch | R. time = 1 day | >5 log10 (mean) | ||
| Lab | Columns | 224.1 g sand | Dia = 2.5 cm, vol = 146.7–161.5 cm3 | Continuous | 1.36 mL/min | 1 log10 (mean) | ||
| Lab | Columns | 0.27 mm sand (0.52 m) | Dia = 4.4 cm | Batch | 450 mL/day | Up to 0.061 log10 per hour | [21] | |
| Lab | Columns | 0.17 mm sand (0.55 m) | Dia = 10 cm | Continuous | R. time = 1 day | 2.25 log10 | [64] | |
| Lab | Columns | 0.17 mm sand (0.55 m) | Dia = 10 cm | Batch | R. time = 1 day | 0.85 log10 | ||
| Lab | Concrete BSF | Sand (0.55 m) | Concrete BSFs (CAWST) | Batch | R. time 24 ± 8.5 h | 4–7 log10 | [18] | |
| Lab | PVC pipe | 0.35 mm sand (0.55 m) | Dia = 4 inch | Intermittent | R. time 20–29 h | Average 5 log10 | [76] | |
| Lab | PVC column | 0.35 mm sand (0.4–0.9 m) | MS Filter Inc | Continuous | 0.1–0.4 m/h | 0.2–2.2 log10 | [77] | |
| Lab | PVC | 0.117–0.52 mm sand | Dia = 0.305 m | Batch | R. time 5–16 h (0.1–0.3 m/h) | 0.54 log10 | [40] | |
| Rotavirus surrogate | Lab | PVC pipe | 0.35 mm sand (0.55 m) | Dia = 4 inch | Intermittent | R. time 20–29 h | 1.2–5.36 log10 | [76] |
| Field | Plastic | Sand (4 ft) | 5 × 10 ft, depth = 6 ft | Continuous | 99.9% | [75] | ||
| Lab | Columns | 224.1 g sand; 10% iron | Dia = 2.5 cm, vol = 146.7–161.5 cm3 | Batch | R. time = 1 day | 5.2 log10 (mean) | [34] | |
| Lab | Columns | 224.1 g sand | Dia = 2.5 cm, vol = 146.7–161.5 cm3 | Continuous | 1.36 mL/min | 1.1 log10 (mean) | ||
| Lab | Columns | 0.27 mm sand (0.52 m) | Dia = 4.4 cm | Batch | 450 mL/day | Up to 0.053 log10 per hour | [21] | |
| Setting | Filter Design | Operation | Removal Efficiency | Reference | |||
|---|---|---|---|---|---|---|---|
| Material | Medium (Depth) | Dimensions/Area | Mode | Residence Time/Flow Rate | |||
| Field | 0.2–0.3 mm sand (1.05 m) | 43 × 6 m | Continuous | 0.19–0.4 m/h | Average 55%, effluent < 1.1 NTU | [23] | |
| Lab | Columns | 0.17 mm sand (0.55 m) | Dia = 10 cm | Continuous | R. time = 1 day | 2.25 log10 | [64] |
| Lab | Columns | 0.17 mm sand (0.55 m) | Dia = 10 cm | Batch | R. time = 1 day | 0.85 log10 | |
| Lab | PVC | 0.117–0.52 mm sand | Dia = 0.305 m | Batch | R. time 5–16 h (0.1–0.3 m/h) | 89% | [40] |
| Field | Plastic filter | <1 mm sand (0.45 m) | 60 L, depth = 0.76 m | Batch | Public use | Mean effluent = 1.3 NTU | [57] |
| Lab | Concrete BSF | 0.3 mm sand (0.5 m); 0.5 kg zerovalent iron | Concrete BSFs (CAWST) | Batch | 91.5–95% | [46] | |
| Lab | Concrete BSF | 0.3 mm sand (0.5 m) | Concrete BSFs (CAWST) | Batch | 50–70% | ||
| Lab | Plastic containers | 0.23 mm sand (0.45 m) | p. vol. = 19.1 L, dia = 40 cm, depth = 73 cm | Batch | 20–40 L/day | 93%, effluent = 1 NTU | [45] |
| Lab | Plastic containers | 0.23 mm sand (0.45 m); 10 cm iron-oxide-coated sand | p. vol. = 19.1 L, dia = 40 cm, depth = 73 cm | Batch | 20–40 L/day | 92%, effluent = 1 NTU | |
| Pilot | Concrete BSF | 0.19 to 0.22 mm sand (0.45 m) | dia 15 cm, depth 90 cm | Batch | 20 L/day | 96.2–96.6%, effluent = 0.6–1.5 NTU | [56] |
| Lab | Plastic bucket | Sand (0.175 m) | 25 L bucket, depth 41 cm | Batch | 20 L/day | 80% | [47] |
| Lab | Plastic bucket | Sand (0.175 m); 10 cm of 3 mm zeolites | 25 L bucket, depth 41 cm | Batch | 20 L/day | 93% | |
| Lab | 10 mm thick acrylic | 0.18–2.83 sand (0.9 m) | 0.2 × 0.2 m | Continuous | 1.8–7.1 m/day | Effluent > 2 NTU (in 95% of samples) | [69] |
| Field | Concrete BSFs (n = 155) | 0.15 mm sand (0.5 m) | Depth 0.95 m, dia 36 cm | Batch | Public use 20 L/8 h | Average 32.5% | [65] |
| Lab | Plastic BSF | 0.15–0.4 mm sand (0.43 m) | 60 L BSF (HydrAid) | Batch | 20 L/day | 87–91%, effluent = 0.9–1.1 NTU (average) | [55] |
| Lab | Plastic bucket | As per CASWT specifications [29]; 0.14 mm sand (0.15 m) | 30 L, dia 30 cm, depth 32 cm | Batch | 20 L/12 h | 94.5%, effluent average 0.9 NTU | [35] |
| Pilot | Polyethylene barrels | 0.15–0.30 mm sand (0.57 m) | Dia 0.57 m, depth 0.88 m | Continuous | 0.05 m/h | 0–82.63% | [17] |
| Lab | Concrete | 0.18–0.3 mm sand | Concrete BSFs (CAWST)[29] | Batch | 10 L/36 h–30 L/12 h | Effluent < 1.5 NTU | [61] |
| Lab | PVC columns | 0.3–2.33 mm sand (0.45 m) | Depth 85 cm, dia 14.9 cm | Intermittent | 8 L/12 h | 88.71% | [71] |
| Lab | PVC columns | 0.3–2.33 mm sand (0.45 m); 21 g brass | Depth 85 cm, dia 14.9 cm | Intermittent | 8 L/12 h | 88.5% | |
| Lab | PVC columns | 0.3–2.33 mm sand (0.45 m); 39 g zerovalent iron | Depth 85 cm, dia 14.9 cm | Intermittent | 8 L/12 h | 91.5% | |
| Parameter | Setting | Filter Design | Operation | Removal Efficiency | Reference | |||
|---|---|---|---|---|---|---|---|---|
| Material | Medium (Depth) | Dimensions/Area | Mode | Residence Time/Flow Rate | ||||
| Natural Organic Matter (NOM) | Lab | Concrete BSF | 0.3 mm sand (0.5 m) | Concrete BSFs (CAWST) | Batch | Average 47.64% | [46] | |
| Lab | Concrete BSF | 0.15 mm sand (0.5 m) | Concrete BSFs (CAWST) | Batch | Average 54.0% | [46] | ||
| COD | Sand (fine/coarse) | 50–74% | [22,81] | |||||
| Lab | Filter column | Sand (0.3 m) and GAC (0.2 m) | Height 0.65 m, | Continuous | 5–10 cm/h | 65.8% | [79] | |
| TOC | Layered filter | Bayer residue, zeolite, and fly ash | Continuous and intermittent | 71% | [44] | |||
| Lab | Filter column | Sand (0.3 m) and GAC (0.2 m) | Height 0.65 m | Continuous | 5–10 cm/h | 90.3% | [79] | |
| PPCPs | Lab | Filter column | Sand (0.4 m) and GAC (0.1 m) | Height 0.65 m | Continuous | 10 cm/h | 90.3% | [79] |
| Parameter | Setting | Filter Design | Operation | Removal Efficiency | Reference | |||
|---|---|---|---|---|---|---|---|---|
| Material | Medium (Depth) | Dimensions/Area | Mode | Residence Time/Flow Rate | ||||
| Nitrate | Lab | Stainless steel | 0.5 mm sand (0.8 m) | Depth = 1 m, diameter = 0.1 m | Continuous | 0.015–0.06 m/h | 94–100% | [66] |
| NH4 | Layered filter | Bayer residue, zeolite, and fly ash | Continuous and intermittent | 88% | [44] | |||
| Arsenic | Field | KanchanTM Arsenic Filter (KAF) | Gravel, sand, and iron nails | Plastic or concrete bucket | Intermittent | Public use 10–15 L/h | 88–95% | [38] |
| Iron | Field | KanchanTM Arsenic Filter (KAF) | Gravel, sand, and iron nails | Plastic or concrete bucket | Intermittent | Public use 10–15 L/h | >93% | [38] |
| Aluminum | Layered filter | Bayer residue, zeolite, and fly ash | Continuous and intermittent | 95% | [44] | |||
| Lab | PVC column | 1.2 mm and 6.7 mm sand | 0.25 m dia | Continuous | 6 L/min, and 9.45 h HRT | 98% | [83] | |
| Managnese | Lab | PVC column | 1.2 mm and 6.7 mm sand | 0.25 m dia | Continuous | 6 L/min, and 9.45 h HRT | 88% | [83] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maiyo, J.K.; Dasika, S.; Jafvert, C.T. Slow Sand Filters for the 21st Century: A Review. Int. J. Environ. Res. Public Health 2023, 20, 1019. https://doi.org/10.3390/ijerph20021019
Maiyo JK, Dasika S, Jafvert CT. Slow Sand Filters for the 21st Century: A Review. International Journal of Environmental Research and Public Health. 2023; 20(2):1019. https://doi.org/10.3390/ijerph20021019
Chicago/Turabian StyleMaiyo, John K., Sruthi Dasika, and Chad T. Jafvert. 2023. "Slow Sand Filters for the 21st Century: A Review" International Journal of Environmental Research and Public Health 20, no. 2: 1019. https://doi.org/10.3390/ijerph20021019





