Evidence on Neurotoxicity after Intrauterine and Childhood Exposure to Organomercurials
Abstract
1. Introduction
2. Exposure to Organic Mercury
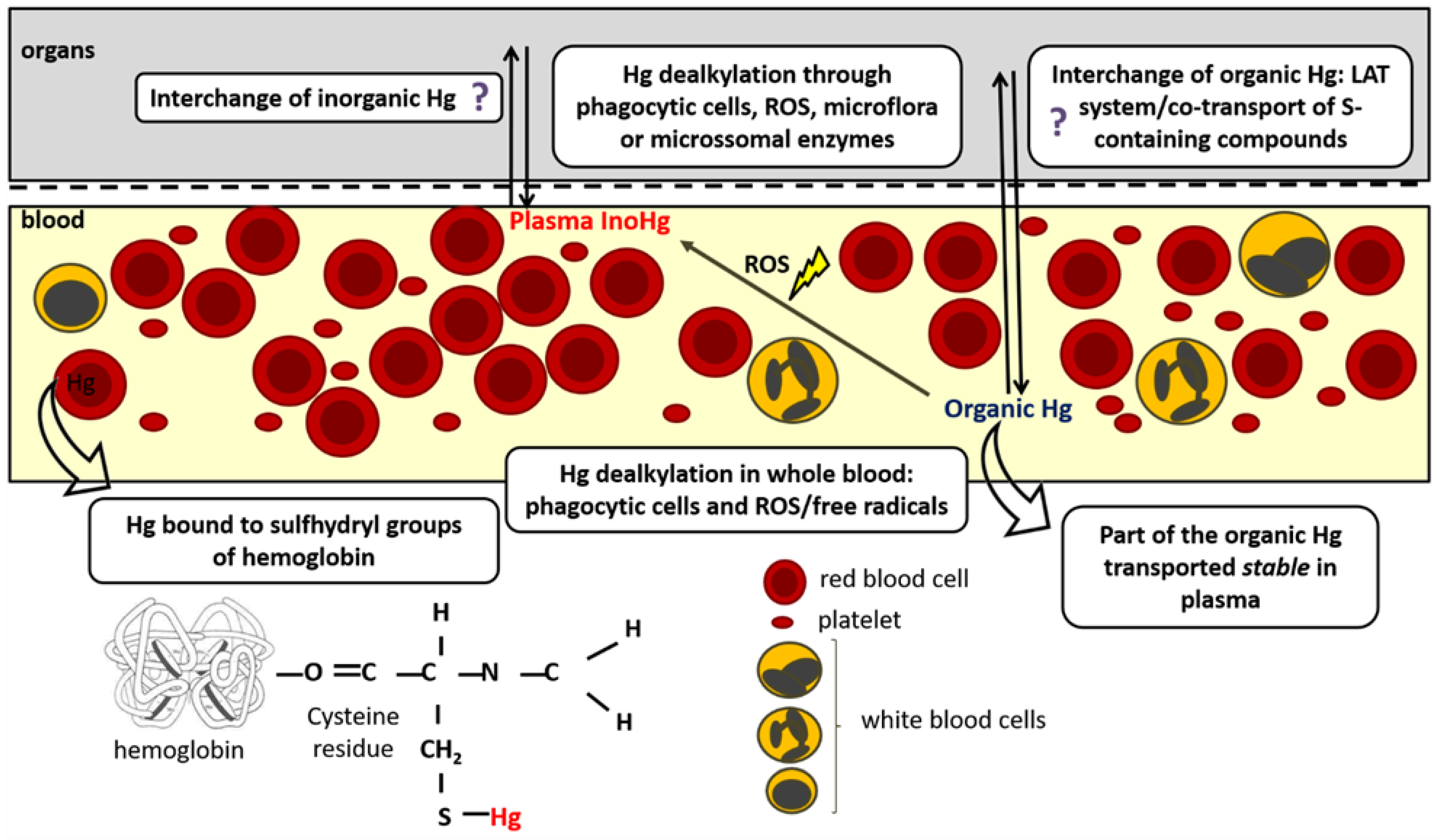
3. Organomercurials Kinetics
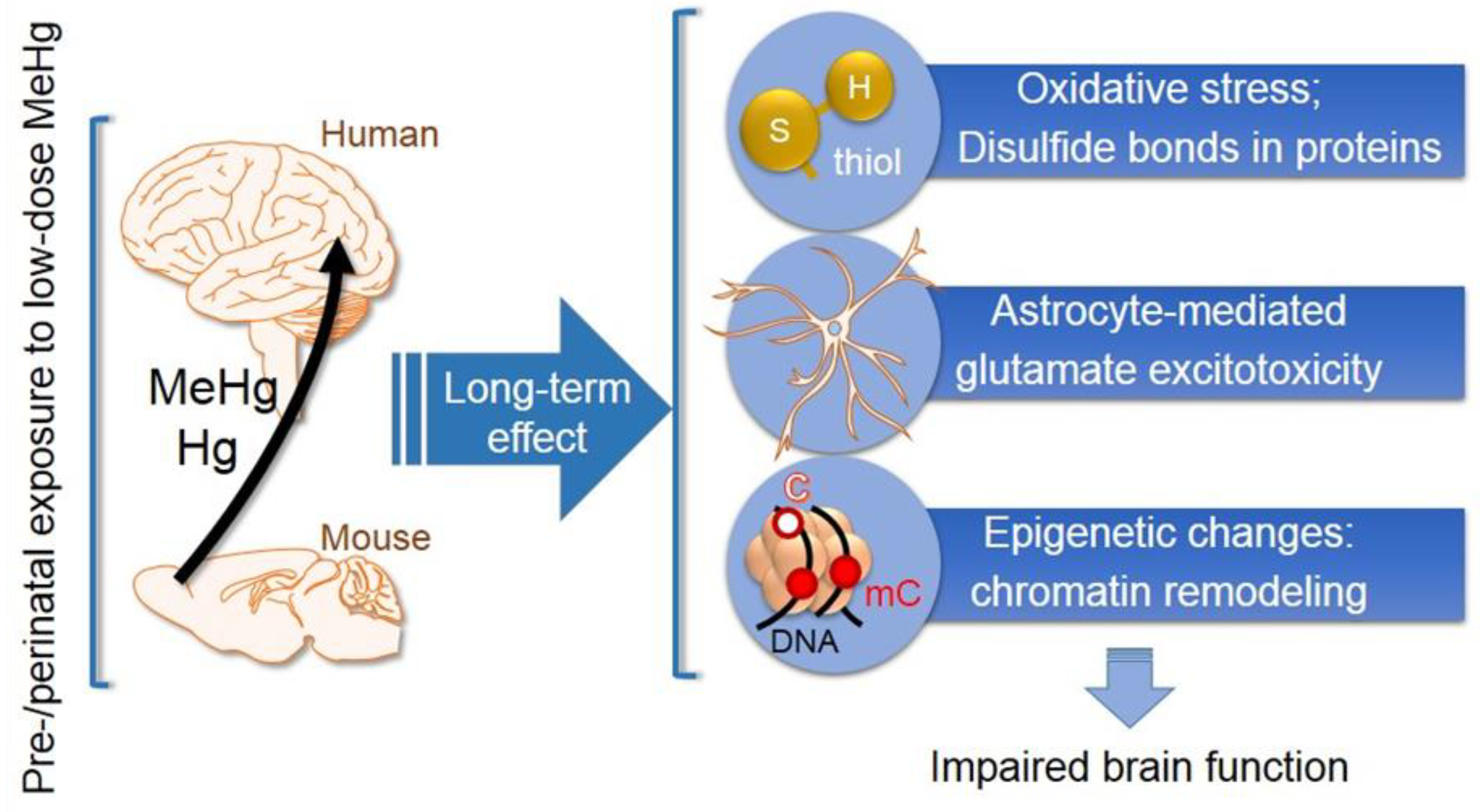
4. Neurotoxicity Effects following Intrauterine and Childhood Exposure to Methylmercury
5. Neurotoxicity Effects following Intrauterine and Childhood Exposure to Ethylmercury
| Biological System | Route | Exposure Design and Dose | Outcomes | Reference |
|---|---|---|---|---|
| FVBN_NJ mice | SC 1 | Pups were injected on postnatal day 1 (P1), P3, P5, and P9 with a 20-fold higher Hg dose than those used in current Chinese infant immunization schedule during the first 4 months of life (304, 238, 196, and 176 μg of Hg/kg, given respectively to each of the postnatal days) | The treated pups showed life neural development delay (eye-opening ratio), social interaction deficiency (autistic-like behavior), and inclination for depression. Neuropathological changes were also observed in the brain tissue of adult mice neonatally treated with thimerosal. High-throughput RNA sequencing of autistic-behaved mice brains revealed the alternation of a number of canonical pathways involving neuronal development and neuronal synaptic function as well as several gonadotropin hormone transcripts were strikingly up-regulated in thimerosal-treated males. | [164] |
| Spontaneously Hypertensive Rats (SHR) or Sprague–Dawley (SD) rats | SC 1 | Dams were exposed to 200 μg thimerosal per kg during pregnancy (gestational days 10 to 15) and lactation (post-natal days 5 to 10). | Delayed auditory (startle response) was verified in SD neonates; decreased motor learning was registered for males (both SHR and SD) and also for SD females. Also, a significant increase in cerebellar levels of the oxidative stress marker 3-nitrotyrosine was found in higher levels in SHR females as well as in SD males than in controls. The activity of cerebellar type 2 deiodinase (converts thyroxine to the active hormone, 3′,3,5-triiodothyronine (T3)) was significantly decreased in SHR males exposed to thimerosal with Odf4—a gene regulated by the levels of T3—found to be overexpressed in comparison to controls. | [18] |
| SJL/J mice | combination of SC 1 and IM 2 | Pups were injected on post-natal days 7, 9, 11, and 15 with: vehicle, 1 × thimerosal (cumulative dose = 39.8 μg Hg/kg representing the maximum Hg exposure, on a μg/kg basis, to which a child could have been exposed from vaccination if hepatitis B, diphtheria tetanus pertussis (DPT), and Hemophilus influenza B (HiB) were conserved with thimerosal) or 10 × thimerosal (10-fold higher cumulative dose: 390 μg/kg) | No significant behavioral alterations (i.e., social interaction, sensory gating, and anxiety) were produced by the treatment. | [24] |
| SJL/J, C57BL/6J, and BALB/cJ mice | IM 2 | Pups were treated at postnatal day (P)7, P9, P11, and P15 with (1) thimerosal-only (14.2, 10.8, 9.2, or 5.6 µg/kg of ethylmercury per postnatal immunization day) or (2) thimerosal vaccines (thimerosal-preserved diphtheria, tetanus, acellular pertussis (DTaP, Lederle), and Haemophilus influenza B (HiB, Lederle) vaccines—EtHg load the same as group (1); or (3) Control (PBS) | Thimerosal-treated animals—either through thimerosal administration or through its content as vaccines—had no significant differences in any parameters measured and results were then combined for the analysis. SJL/J mice are known to be autoimmune disease-sensitive and showed growth delay, decreased locomotion, amplified response to novelty, and densely packed, hyperchromic hippocampal neurons with altered glutamate receptors and transporters. Strains resistant to autoimmunity—C57BL/6J and BALB/cJ—were not affected by thimerosal. | [159] |
| Wistar rats of both sexes | IM 2 | Pups were injected on postnatal days 7, 9, 11 and 15 with one of the four different doses in study (12, 240, 1440, or 3000 µg Hg/kg) + vehicle | The locomotor activity was impaired in males at the lowest dose tested whereas in females this effect was only observed at the highest dose; animals of both sexes treated with the highest dose of thimerosal presented reduced rates of prosocial behavior and the frequency of asocial/antisocial interactions was increased and decreased, respectively, in males and females. For males, significant less striatal D2 receptors were found at the dose 12 µg Hg/kg while for females this was observed at 240 µg Hg/kg. No effects were documented considering spatial learning and memory. | [160] |
6. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Skerfving, S. Methylmercury exposure, mercury levels in blood and hair, and health status in Swedes consuming contaminated fish. Toxicology 1974, 2, 3–23. [Google Scholar] [CrossRef]
- Heron, J.; Golding, J. Thimerosal exposure in infants and developmental disorders: A prospective cohort study in the United Kingdom does not support a causal association. Pediatrics 2004, 114, 577–583. [Google Scholar] [CrossRef]
- Ynalvez, R.; Gutierrez, J.; Gonzalez-Cantu, H. Mini-review: Toxicity of mercury as a consequence of enzyme alteration. Biometals 2016, 29, 781–788. [Google Scholar] [CrossRef] [PubMed]
- Gochfeld, M. Cases of mercury exposure, bioavailability, and absorption. Ecotoxicol. Environ. Saf. 2003, 56, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Carocci, A.; Rovito, N.; Sinicropi, M.S.; Genchi, G. Mercury toxicity and neurodegenerative effects. Rev. Environ. Contam. Toxicol. 2014, 229, 1–18. [Google Scholar] [PubMed]
- Clements, C.J.; Ball, L.K.; Ball, R.; Pratt, D. Thiomersal in vaccines. Lancet 2000, 355, 1279–1280. [Google Scholar] [CrossRef] [PubMed]
- Dorea, J.G. Exposure to environmental neurotoxic substances and neurodevelopment in children from Latin America and the Caribbean. Environ. Res. 2021, 192, 110199. [Google Scholar] [CrossRef]
- Lohren, H.; Blagojevic, L.; Fitkau, R.; Ebert, F.; Schildknecht, S.; Leist, M.; Schwerdtle, T. Toxicity of organic and inorganic mercury species in differentiated human neurons and human astrocytes. J. Trace Elem. Med. Biol. Organ Soc. Miner. Trace Elem. GMS 2015, 32, 200–208. [Google Scholar] [CrossRef]
- Eriksson, P.; Fischer, C.; Stenerlow, B.; Fredriksson, A.; Sundell-Bergman, S. Interaction of gamma-radiation and methyl mercury during a critical phase of neonatal brain development in mice exacerbates developmental neurobehavioural effects. Neurotoxicology 2010, 31, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Fujimura, M.; Zhao, W.; Wang, W. Neurobehavioral effects, c-Fos/Jun expression and tissue distribution in rat offspring prenatally co-exposed to MeHg and PFOA: PFOA impairs Hg retention. Chemosphere 2013, 91, 758–764. [Google Scholar] [CrossRef] [PubMed]
- Pletz, J.; Sanchez-Bayo, F.; Tennekes, H.A. Dose-response analysis indicating time-dependent neurotoxicity caused by organic and inorganic mercury-Implications for toxic effects in the developing brain. Toxicology 2016, 347–349, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Kuban, P.; Pelcova, P.; Margetinova, J.; Kuban, V. Mercury speciation by CE: An update. Electrophoresis 2009, 30, 92–99. [Google Scholar] [CrossRef]
- Clarkson, T.W.; Magos, L. The toxicology of mercury and its chemical compounds. Crit. Rev. Toxicol. 2006, 36, 609–662. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, M.F.H.; Morais, C.; Barbosa, F.; Gobe, G.C. Thimerosal in childhood vaccines contributes to accumulating mercury toxicity in the kidney. Toxicol. Environ. Chem. 2013, 95, 1424–1447. [Google Scholar] [CrossRef]
- Ball, L.K.; Ball, R.; Pratt, R.D. An assessment of thimerosal use in childhood vaccines. Pediatrics 2001, 107, 1147–1154. [Google Scholar] [CrossRef] [PubMed]
- Dorea, J.G. Safety of thimerosal in vaccines: For whom and how many doses? Therapie 2011, 66, 95–96. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.P. Mercury, vaccines, and autism: One controversy, three histories. Am. J. Public Health 2008, 98, 244–253. [Google Scholar] [CrossRef]
- Suneja, T.; Belsito, D.V. Thimerosal in the detection of clinically relevant allergic contact reactions. J. Am. Acad. Dermatol. 2001, 45, 23–27. [Google Scholar] [CrossRef]
- Kern, J.K.; Haley, B.E.; Geier, D.A.; Sykes, L.K.; King, P.G.; Geier, M.R. Thimerosal exposure and the role of sulfation chemistry and thiol availability in autism. Int. J. Environ. Res. Public Health 2013, 10, 3771–3800. [Google Scholar] [CrossRef]
- Sulkowski, Z.L.; Chen, T.; Midha, S.; Zavacki, A.M.; Sajdel-Sulkowska, E.M. Maternal thimerosal exposure results in aberrant cerebellar oxidative stress, thyroid hormone metabolism, and motor behavior in rat pups; sex- and strain-dependent effects. Cerebellum 2012, 11, 575–586. [Google Scholar] [CrossRef]
- Wilcox, C.R.; Rowe, R.; Mobley, D.C.; Willcox, M.; Jones, C.E. Routine vaccinations during pregnancy: An update. Br. J. Gen. Pract. 2020, 70, 142–143. [Google Scholar] [CrossRef] [PubMed]
- Dórea, J.G. Low-dose Thimerosal (ethyl-mercury) is still used in infants’ vaccines: Should we be concerned with this form of exposure? J. Trace Elem. Med. Biol. 2018, 49, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Lü, H.; Song, G.; Zhang, J.; Li J and Ma, Y. Analysis and evaluation to mercury content in the blood and hair of neonates in wujiang. J. Pediatr. Pharm. 2012, 18, 5–7. [Google Scholar]
- Brown, I.; Austin, D. Maternal Transfer of Mercury to the Developing Embryo/Fetus: Is there a Safe Level? Toxicol. Environ. Chem. 2012, 94, 1610–1627. [Google Scholar] [CrossRef]
- Lebel, J.; Mergler, D.; Branches, F.; Lucotte, M.; Amorim, M.; Larribe, F.; Dolbec, J. Neurotoxic effects of low-level methylmercury contamination in the Amazonian Basin. Environ. Res. 1998, 79, 20–32. [Google Scholar] [CrossRef] [PubMed]
- Berman, R.F.; Pessah, I.N.; Mouton, P.R.; Mav, D.; Harry, J. Low-level neonatal thimerosal exposure: Further evaluation of altered neurotoxic potential in SJL mice. Toxicol. Sci. 2008, 101, 294–309. [Google Scholar] [CrossRef]
- Delong, G. A positive association found between autism prevalence and childhood vaccination uptake across The U.S. Population. J. Toxicol. Environ. Health A 2011, 74, 903–916. [Google Scholar] [CrossRef] [PubMed]
- Bose, R.; Onishchenko, N.; Edoff, K.; Janson Lang, A.M.; Ceccatelli, S. Inherited effects of low-dose exposure to methylmercury in neural stem cells. Toxicol. Sci. 2012, 130, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Ida-Eto, M.; Oyabu, A.; Ohkawara, T.; Tashiro, Y.; Narita, N.; Narita, M. Prenatal exposure to organomercury, thimerosal, persistently impairs the serotonergic and dopaminergic systems in the rat brain: Implications for association with developmental disorders. Brain Dev. 2013, 35, 261–264. [Google Scholar] [CrossRef]
- Bensefa-Colas, L.; Andujar, P.; Descatha, A. Intoxication par le mercure. Rev. Med. Interne 2011, 32, 416–424. [Google Scholar] [CrossRef]
- Harada, M. Minamata disease: Methylmercury poisoning in Japan caused by environmental pollution. Crit. Rev. Toxicol. 1995, 25, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Sanfeliu, C.; Sebastià, J.; Cristòfol, R.; Rodríguez-Farré, E. Neurotoxicity of organomercurial compounds. Neurotox. Res. 2003, 5, 283–305. [Google Scholar] [CrossRef] [PubMed]
- Geier, D.A.; King, P.G.; Hooker, B.S.; Dórea, J.G.; Kern, J.K.; Sykes, L.K.; Geier, M.R. Thimerosal: Clinical, epidemiologic and biochemical studies. Clin. Chim. Acta 2015, 444, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Zalups, R.K.; Bridges, C.C. Relationships between the renal handling of DMPS and DMSA and the renal handling of mercury. Chem. Res. Toxicol. 2012, 25, 1825–1838. [Google Scholar] [CrossRef]
- Dorea, J.G.; Farina, M.; Rocha, J.B. Toxicity of ethylmercury (and Thimerosal): A comparison with methylmercury. J. Appl. Toxicol. 2013, 33, 700–711. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, M.F.; Oliveira Souza, J.M.; Grotto, D.; Batista, B.L.; de Oliveira Souza, V.C.; Barbosa, F., Jr. A systematic study of the disposition and metabolism of mercury species in mice after exposure to low levels of thimerosal (ethylmercury). Environ. Res. 2014, 134, 218–227. [Google Scholar] [CrossRef]
- Zuidema, J.; Kadir, F.; Titulaer, H.A.C.; Oussoren, C. Release and absorption rates of intramuscularly and subcutaneously injected pharmaceuticals (II). Int. J. Pharm. 1994, 105, 189–207. [Google Scholar] [CrossRef]
- Rodrigues, J.L.; Serpeloni, J.M.; Batista, B.L.; Souza, S.S.; Barbosa, F., Jr. Identification and distribution of mercury species in rat tissues following administration of thimerosal or methylmercury. Arch. Toxicol. 2010, 84, 891–896. [Google Scholar] [CrossRef]
- Zimmermann, L.T.; Santos, D.B.; Naime, A.A.; Leal, R.B.; Dorea, J.G.; Barbosa, F., Jr.; Aschner, M.; Rocha, J.B.T.; Farina, M. Comparative study on methyl- and ethylmercury-induced toxicity in C6 glioma cells and the potential role of LAT-1 in mediating mercurial-thiol complexes uptake. Neurotoxicology 2013, 38, 1–8. [Google Scholar] [CrossRef]
- Clarkson, T.W. The three modern faces of mercury. Environ. Health Perspect. 2002, 110 (Suppl. S1), 11–23. [Google Scholar] [CrossRef]
- Silbergeld, E.; Stern, A.; Domingo, J.L.; Horvat, M.; Nyland, J.; Barbosa, J.F. Children’s Exposure to Mercury Compounds; World Health Organization Press: Geneva, Switzerland, 2011; p. 104. [Google Scholar]
- Ancora, S.; Rossi, R.; Simplicio, P.D.; Lusini, L.; Leonzio, C. In vitro study of methylmercury in blood of bottlenose dolphins (Tursiops truncatus). Arch. Environ. Contam. Toxicol. 2002, 42, 348–353. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Shishido, S.; Ishihara, N. Interaction of inorganic to organic mercury in their metabolism in human body. Int. Arch. Occup. Environ. Health 1976, 38, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Magos, L.; Webb, M. Differences in distribution and excretion of selenium and cadmium or mercury after their simultaneous administration subcutaneously in equimolar doses. Arch. Toxicol. 1976, 36, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Berglund, M.; Lind, B.; Bjornberg, K.A.; Palm, B.; Einarsson, O.; Vahter, M. Inter-individual variations of human mercury exposure biomarkers: A cross-sectional assessment. Environ. Health 2005, 4, 20. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, M.F.; Grotto, D.; Barbosa, F., Jr. Inorganic and methylmercury levels in plasma are differentially associated with age, gender, and oxidative stress markers in a population exposed to mercury through fish consumption. J. Toxicol. Environ. Health A 2014, 77, 69–79. [Google Scholar] [CrossRef]
- De Marco, K.C.; Braga, G.U.; Barbosa, F., Jr. Determination of the effects of eNOS gene polymorphisms (T-786C and Glu298Asp) on nitric oxide levels in a methylmercury-exposed population. J. Toxicol. Environ. Health A 2011, 74, 1323–1333. [Google Scholar] [CrossRef] [PubMed]
- De Marco, K.C.; Passos, C.J.; Sertorio, J.; Tanus-Santos, J.E.; Barbosa, F., Jr. Environmental exposure to methylmercury is associated with a decrease in nitric oxide production. Basic Clin. Pharm. Toxicol. 2010, 106, 411–415. [Google Scholar] [CrossRef]
- Grotto, D.; Valentini, J.; Fillion, M.; Passos, C.J.; Garcia, S.C.; Mergler, D.; Barbosa, F., Jr. Mercury exposure and oxidative stress in communities of the Brazilian Amazon. Sci. Total Environ. 2010, 408, 806–811. [Google Scholar] [CrossRef]
- Blair, A.; Clark, B.; Clarke, A.J.; Wood, P. Tissue concentrations of mercury after chronic dosing of squirrel monkeys with thiomersal. Toxicology 1975, 3, 171–176. [Google Scholar] [CrossRef]
- Burbacher, T.M.; Shen, D.D.; Liberato, N.; Grant, K.S.; Cernichiari, E.; Clarkson, T. Comparison of blood and brain mercury levels in infant monkeys exposed to methylmercury or vaccines containing thimerosal. Environ. Health Perspect. 2005, 113, 1015–1021. [Google Scholar] [CrossRef]
- Magos, L. The Absorption, Distribution and Excretion of Methyl Mercury. In The Toxicity of Methyl Mercury; Eccles, C.U., Annau, Z., Eds.; The Johns Hopkins University Press: Baltimore, MD, USA, 1986; pp. 24–44. [Google Scholar]
- Zareba, G.; Cernichiari, E.; Hojo, R.; Nitt, S.M.; Weiss, B.; Mumtaz, M.M.; Jones, D.E.; Clarkson, T.W. Thimerosal distribution and metabolism in neonatal mice: Comparison with methyl mercury. J. Appl. Toxicol. 2007, 27, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Suda, I.; Suda, M.; Hirayama, K. Degradation of methyl and ethyl mercury by singlet oxygen generated from sea water exposed to sunlight or ultraviolet light. Arch. Toxicol. 1993, 67, 365–368. [Google Scholar] [CrossRef]
- Straka, E.; Ellinger, I.; Balthasar, C.; Scheinast, M.; Schatz, J.; Szattler, T.; Bleichert, S.; Saleh, L.; Knöfler, M.; Zeisler, H.; et al. Mercury toxicokinetics of the healthy human term placenta involve amino acid transporters and ABC transporters. Toxicology 2015, 340, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Kerper, L.E.; Ballatori, N.; Clarkson, T.W. Methylmercury transport across the blood-brain barrier by an amino acid carrier. Am. J. Physiol. 1992, 262 Pt 2, R761–R765. [Google Scholar] [CrossRef] [PubMed]
- Aschner, M.; Aschner, J.L. Mercury neurotoxicity: Mechanisms of blood-brain barrier transport. Neurosci. Biobehav. Rev. 1990, 14, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Branco, V.; Caito, S.; Farina, M.; Teixeira da Rocha, J.; Aschner, M.; Carvalho, C. Biomarkers of mercury toxicity: Past, present, and future trends. J. Toxicol. Environ. Health B Crit. Rev. 2017, 20, 119–154. [Google Scholar] [CrossRef]
- Bridges, C.C.; Zalups, R.K. Molecular and ionic mimicry and the transport of toxic metals. Toxicol. Appl. Pharm. 2005, 204, 274–308. [Google Scholar] [CrossRef]
- Bridges, C.C.; Zalups, R.K. Transport of inorganic mercury and methylmercury in target tissues and organs. J. Toxicol. Environ. Health B Crit. Rev. 2010, 13, 385–410. [Google Scholar] [CrossRef]
- Ek, C.J.; Dziegielewska, K.M.; Habgood, M.D.; Saunders, N.R. Barriers in the developing brain and Neurotoxicology. Neurotoxicology 2012, 33, 586–604. [Google Scholar] [CrossRef]
- Heggland, I.; Kaur, P.; Syversen, T. Uptake and efflux of methylmercury in vitro: Comparison of transport mechanisms in C6, B35 and RBE4 cells. Toxicol. Vitr. 2009, 23, 1020–1027. [Google Scholar] [CrossRef]
- Vimy, M.J.; Takahashi, Y.; Lorscheider, F.L. Maternalfetal distribution of mercury (203Hg) released from dental amalgam fillings. Am. J. Physiol. 1990, 258 Pt 2, R939–R945. [Google Scholar] [PubMed]
- Takahashi, Y.; Tsuruta, S.; Hasegawa, J.; Kameyama, Y.; Yoshida, M. Release of mercury from dental amalgam fillings in pregnant rats and distribution of mercury in maternal and fetal tissues. Toxicology 2001, 163, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Bridges, C.C.; Joshee, L.; Zalups, R.K. Placental and fetal disposition of mercuric ions in rats exposed to methylmercury: Role of Mrp2. Reprod. Toxicol. 2012, 34, 628–634. [Google Scholar] [CrossRef] [PubMed]
- Kajiwara, Y.; Yasutake, A.; Adachi, T.; Hirayama, K. Methylmercury transport across the placenta via neutral amino acid carrier. Arch. Toxicol. 1996, 70, 310–314. [Google Scholar] [CrossRef]
- Magos, L.; Brown, A.W.; Sparrow, S.; Bailey, E.; Snowden, R.T.; Skipp, W.R. The comparative toxicology of ethyl- and methylmercury. Arch. Toxicol. 1985, 57, 260–267. [Google Scholar] [CrossRef]
- Magos, L. Neurotoxic character of thimerosal and the allometric extrapolation of adult clearance half-time to infants. J. Appl. Toxicol. 2003, 23, 263–269. [Google Scholar] [CrossRef]
- Rowland, I.R.; Davies, M.J.; Evans, J.G. The effect of the gastrointestinal flora on tissue content of mercury and organomercurial neurotoxicity in rats given methylmercuric chloride. Dev. Toxicol. Environ. Sci. 1980, 8, 79–82. [Google Scholar]
- Rowland, I.R.; Robinson, R.D.; Doherty, R.A. Effects of diet on mercury metabolism and excretion in mice given methylmercury: Role of gut flora. Arch. Environ. Health 1984, 39, 401–408. [Google Scholar] [CrossRef]
- Suda, I.; Takahashi, H. Degradation of methyl and ethyl mercury into inorganic mercury by other reactive oxygen species besides hydroxyl radical. Arch. Toxicol. 1992, 66, 34–39. [Google Scholar] [CrossRef]
- Suda, I.; Totoki, S.; Takahashi, H. Degradation of methyl and ethyl mercury into inorganic mercury by oxygen free radical-producing systems: Involvement of hydroxyl radical. Arch. Toxicol. 1991, 65, 129–134. [Google Scholar] [CrossRef]
- Suda, I.; Totoki, S.; Uchida, T.; Takahashi, H. Degradation of methyl and ethyl mercury into inorganic mercury by various phagocytic cells. Arch. Toxicol. 1992, 66, 40–44. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, A.M.; Chan, H.M. Characterization of demethylation of methylmercury in cultured astrocytes. Chemosphere 2008, 74, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Pehkonen, S.O.; Lin, C.J. Degradation of monomethylmercury chloride by hydroxyl radicals in simulated natural waters. Water Res. 2003, 37, 2496–2504. [Google Scholar] [CrossRef]
- De Magalhães Silva, M.; de Araújo Dantas, M.D.; da Silva Filho, R.C.; Dos Santos Sales, M.V.; de Almeida Xavier, J.; Leite, A.C.R.; Grillo, L.A.M.; de Barros, W.A.; de Fátima, Â.; Figueiredo, I.M. Toxicity of thimerosal in biological systems: Conformational changes in human hemoglobin, decrease of oxygen binding capacity, increase of protein glycation and amyloid’s formation. Int. J. Biol. Macromol. 2020, 154, 661–671. [Google Scholar] [CrossRef] [PubMed]
- Sales, M.V.S.; da Silva Filho, R.C.; Silva, M.M.; Rocha, J.L.; Freire, R.O.; Tanabe, E.L.D.L.; Silva, E.C.O.; Fonseca, E.J.S.; Figueiredo, I.M.; Rocha, U.; et al. Consequences of thimerosal on human erythrocyte hemoglobin: Assessing functional and structural protein changes induced by an organic mercury compound. J. Trace Elem. Med. Biol. 2022, 71, 126928. [Google Scholar] [CrossRef] [PubMed]
- Charleston, J.S.; Body, R.L.; Mottet, N.K.; Vahter, M.E.; Burbacher, T.M. Autometallographic determination of inorganic mercury distribution in the cortex of the calcarine sulcus of the monkey Macaca fascicularis following long-term subclinical exposure to methylmercury and mercuric chloride. Toxicol. Appl. Pharm. 1995, 132, 325–333. [Google Scholar] [CrossRef]
- Pedersen, M.B.; Hansen, J.C.; Mulvad, G.; Pedersen, H.S.; Gregersen, M.; Danscher, G. Mercury accumulations in brains from populations exposed to high and low dietary levels of methyl mercury. Concentration, chemical form and distribution of mercury in brain samples from autopsies. Int. J. Circumpolar Health 1999, 58, 96–107. [Google Scholar]
- Vahter, M.E.; Mottet, N.K.; Friberg, L.T.; Lind, S.B.; Charleston, J.S.; Burbacher, T.M. Demethylation of methyl mercury in different brain sites of Macaca fascicularis monkeys during long-term subclinical methyl mercury exposure. Toxicol. Appl. Pharm. 1995, 134, 273–284. [Google Scholar] [CrossRef]
- USEPA. Mercury Study Report to Congress; Office of Air Quality Planning and Standards, Office of Research and Development: Washington, DC, USA, 1997.
- Evans, R.D.; Hickie, B.; Rouvinen-Watt, K.; Wang, W. Partitioning and kinetics of methylmercury among organs in captive mink (Neovison vison): A stable isotope tracer study. Environ. Toxicol. Pharm. 2016, 42, 163–169. [Google Scholar] [CrossRef]
- Arvidson, B.; Arvidsson, J.; Johansson, K. Mercury deposits in neurons of the trigeminal ganglia after insertion of dental amalgam in rats. Biometals 1994, 7, 261–263. [Google Scholar] [CrossRef]
- Pamphlett, R.; Kum Jew, S. Heavy metals in locus ceruleus and motor neurons in motor neuron disease. Acta Neuropathol. Commun. 2013, 1, 81. [Google Scholar] [CrossRef]
- Pamphlett, R.; Waley, P. Uptake of inorganic mercury by the human brain. Acta Neuropathol. 1996, 92, 525–527. [Google Scholar] [CrossRef] [PubMed]
- Pamphlett, R.; Waley, P. Motor neuron uptake of low dose inorganic mercury. J. Neurol. Sci. 1996, 135, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.J.; Fisher, H.L.; Sumler, M.R.; Mushak, P.; Hall, L.L. Sexual differences in the excretion of organic and inorganic mercury by methyl mercury-treated rats. Environ. Res. 1987, 43, 203–216. [Google Scholar] [CrossRef] [PubMed]
- Komsta-Szumska, E.; Czuba, M.; Reuhl, K.R.; Miller, D.R. Demethylation and excretion of methyl mercury by the guinea pig. Environ. Res. 1983, 32, 247–257. [Google Scholar] [CrossRef]
- Ishihara, N. Excretion of methyl mercury in human feces. Arch. Environ. Health 2000, 55, 44–47. [Google Scholar] [CrossRef]
- Pichichero, M.E.; Cernichiari, E.; Lopreiato, J.; Treanor, J. Mercury concentrations and metabolism in infants receiving vaccines containing thiomersal: A descriptive study. Lancet 2002, 360, 1737–1741. [Google Scholar] [CrossRef]
- Pichichero, M.E.; Gentile, A.; Giglio, N.; Alonso, M.M.; Fernandez Mentaberri, M.V.; Zareba, G.; Clarkson, T.; Gotelli, C.; Gotelli, M.; Yan, L.; et al. Mercury levels in premature and low birth weight newborn infants after receipt of thimerosal-containing vaccines. J. Pediatr. 2009, 155, 495–499. [Google Scholar] [CrossRef]
- Barregard, L.; Rekic, D.; Horvat, M.; Elmberg, L.; Lundh, T.; Zachrisson, O. Toxicokinetics of mercury after long-term repeated exposure to thimerosal-containing vaccine. Toxicol. Sci. 2011, 120, 499–506. [Google Scholar] [CrossRef]
- Yaginuma-Sakurai, K.; Murata, K.; Iwai-Shimada, M.; Nakai, K.; Kurokawa, N.; Tatsuta, N.; Satoh, H. Hair-to-blood ratio and biological half-life of mercury: Experimental study of methylmercury exposure through fish consumption in humans. J. Toxicol. Sci. 2012, 37, 123–130. [Google Scholar] [CrossRef]
- Magos, L.; Butler, W.H. The kinetics of methylmercury administered repeatedly to rats. Arch. Toxicol. 1976, 35, 25–39. [Google Scholar] [CrossRef] [PubMed]
- Petersson, K.; Dock, L.; Soderling, K.; Vahter, M. Distribution of mercury in rabbits subchronically exposed to low levels of radiolabeled methyl mercury. Pharm. Toxicol. 1991, 68, 464–468. [Google Scholar] [CrossRef] [PubMed]
- Castoldi, A.F.; Onishchenko, N.; Johansson, C.; Coccini, T.; Roda, E.; Vahter, M.; Ceccatelli, S.; Manzo, L. Neurodevelopmental toxicity of methylmercury: Laboratory animal data and their contribution to human risk assessment. Regul. Toxicol. Pharm. 2008, 51, 215–229. [Google Scholar] [CrossRef] [PubMed]
- Mottet, N.K.; Shaw, C.M.; Burbacher, T.M. Health risks from increases in methylmercury exposure. Environ. Health Perspect. 1985, 63, 133–140. [Google Scholar] [CrossRef]
- Greener, Y.; Kochen, J.A. Methyl mercury toxicity in the chick embryo. Teratology 1983, 28, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Bertossi, M.; Girolamo, F.; Errede, M.; Virgintino, D.; Elia, G.; Ambrosi, L.; Roncali, L. Effects of methylmercury on the microvasculature of the developing brain. Neurotoxicology 2004, 25, 849–857. [Google Scholar] [CrossRef]
- Hastings, F.L.; Lucier, G.W.; Klein, R. Methylmercury-cholinesterase interactions in rats. Environ. Health Perspect. 1975, 12, 127–130. [Google Scholar] [CrossRef]
- Aschner, M.; Clarkson, T.W. Mercury 203 distribution in pregnant and nonpregnant rats following systemic infusions with thiol-containing amino acids. Teratology 1987, 36, 321–328. [Google Scholar] [CrossRef]
- Stringari, J.; Nunes, A.K.; Franco, J.L.; Bohrer, D.; Garcia, S.C.; Dafre, A.L.; Milatovic, D.; Souza, D.O.; Rocha, J.B.; Aschner, M.; et al. Prenatal methylmercury exposure hampers glutathione antioxidant system ontogenesis and causes long-lasting oxidative stress in the mouse brain. Toxicol. Appl. Pharm. 2008, 227, 147–154. [Google Scholar] [CrossRef]
- Caito, S.; Zeng, H.; Aschner, J.L.; Aschner, M. Methylmercury alters the activities of Hsp90 client proteins, prostaglandin E synthase/p23 (PGES/23) and nNOS. PLoS ONE 2014, 9, e98161. [Google Scholar] [CrossRef]
- Aschner, M. Neuron-astrocyte interactions: Implications for cellular energetics and antioxidant levels. Neurotoxicology 2000, 21, 1101–1107. [Google Scholar] [PubMed]
- Charleston, J.S.; Body, R.L.; Bolender, R.P.; Mottet, N.K.; Vahter, M.E.; Burbacher, T.M. Changes in the number of astrocytes and microglia in the thalamus of the monkey Macaca fascicularis following long-term subclinical methylmercury exposure. Neurotoxicology 1996, 17, 127–138. [Google Scholar] [PubMed]
- Kaur, P.; Aschner, M.; Syversen, T. Glutathione modulation influences methyl mercury induced neurotoxicity in primary cell cultures of neurons and astrocytes. Neurotoxicology 2006, 27, 492–500. [Google Scholar] [CrossRef] [PubMed]
- Mori, N.; Yasutake, A.; Hirayama, K. Comparative study of activities in reactive oxygen species production/defense system in mitochondria of rat brain and liver, and their susceptibility to methylmercury toxicity. Arch. Toxicol. 2007, 81, 769–776. [Google Scholar] [CrossRef]
- Gawryluk, J.W.; Wang, J.F.; Andreazza, A.C.; Shao, L.; Young, L.T. Decreased levels of glutathione, the major brain antioxidant, in post-mortem prefrontal cortex from patients with psychiatric disorders. Int. J. Neuropsychopharmacol. 2011, 14, 123–130. [Google Scholar] [CrossRef]
- Moretti, M.; Colla, A.; de Oliveira Balen, G.; dos Santos, D.B.; Budni, J.; de Freitas, A.E.; Farina, M.; Severo Rodrigues, A.L. Ascorbic acid treatment, similarly to fluoxetine, reverses depressive-like behavior and brain oxidative damage induced by chronic unpredictable stress. J. Psychiatr. Res. 2012, 46, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Salim, S.; Asghar, M.; Taneja, M.; Hovatta, I.; Chugh, G.; Vollert, C.; Vu, A. Potential contribution of oxidative stress and inflammation to anxiety and hypertension. Brain Res. 2011, 1404, 63–71. [Google Scholar] [CrossRef]
- Karpova, N.N.; Lindholm, J.S.; Kulesskaya, N.; Onishchenko, N.; Vahter, M.; Popova, D.; Ceccatelli, S.; Castren, E. TrkB overexpression in mice buffers against memory deficits and depression-like behavior but not all anxiety- and stress-related symptoms induced by developmental exposure to methylmercury. Front. Behav. Neurosci. 2014, 8, 315. [Google Scholar] [CrossRef]
- Khan, J.Y.; Black, S.M. Developmental changes in murine brain antioxidant enzymes. Pediatr. Res. 2003, 54, 77–82. [Google Scholar] [CrossRef]
- Umemori, J.; Winkel, F.; Castren, E.; Karpova, N.N. Distinct effects of perinatal exposure to fluoxetine or methylmercury on parvalbumin and perineuronal nets, the markers of critical periods in brain development. Int. J. Dev. Neurosci. 2015, 44, 55–64. [Google Scholar] [CrossRef]
- Kosaka, T.; Katsumaru, H.; Hama, K.; Wu, J.Y.; Heizmann, C.W. GABAergic neurons containing the Ca2+-binding protein parvalbumin in the rat hippocampus and dentate gyrus. Brain Res. 1987, 419, 119–130. [Google Scholar] [CrossRef]
- Hensch, T.K. Critical period plasticity in local cortical circuits. Nat. Rev. Neurosci. 2005, 6, 877–888. [Google Scholar] [CrossRef] [PubMed]
- Cabungcal, J.H.; Steullet, P.; Kraftsik, R.; Cuenod, M.; Do, K.Q. Early-life insults impair parvalbumin interneurons via oxidative stress: Reversal by N-acetylcysteine. Biol. Psychiatry 2013, 73, 574–582. [Google Scholar] [CrossRef] [PubMed]
- Cabungcal, J.H.; Steullet, P.; Morishita, H.; Kraftsik, R.; Cuenod, M.; Hensch, T.K.; Do, K.Q. Perineuronal nets protect fast-spiking interneurons against oxidative stress. Proc. Natl. Acad. Sci. USA 2013, 110, 9130–9135. [Google Scholar] [CrossRef] [PubMed]
- Carulli, D.; Pizzorusso, T.; Kwok, J.C.; Putignano, E.; Poli, A.; Forostyak, S.; Andrews, M.; Deepa, S.; Glant, T.T.; Fawcett, J. Animals lacking link protein have attenuated perineuronal nets and persistent plasticity. Brain 2010, 133 Pt 8, 2331–2347. [Google Scholar] [CrossRef] [PubMed]
- Balmer, T.S.; Carels, V.M.; Frisch, J.L.; Nick, T.A. Modulation of perineuronal nets and parvalbumin with developmental song learning. J. Neurosci. 2009, 29, 12878–12885. [Google Scholar] [CrossRef]
- Suttkus, A.; Morawski, M.; Arendt, T. Protective Properties of Neural Extracellular Matrix. Mol. Neurobiol. 2016, 53, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Ceccatelli, S.; Bose, R.; Edoff, K.; Onishchenko, N.; Spulber, S. Long-lasting neurotoxic effects of exposure to methylmercury during development. J. Intern. Med. 2013, 273, 490–497. [Google Scholar] [CrossRef]
- Costa, L.G.; Aschner, M.; Vitalone, A.; Syversen, T.; Soldin, O.P. Developmental neuropathology of environmental agents. Annu. Rev. Pharmacol. Toxicol. 2004, 44, 87–110. [Google Scholar] [CrossRef]
- Grandjean, P.; Landrigan, P.J. Developmental neurotoxicity of industrial chemicals. Lancet 2006, 368, 2167–2178. [Google Scholar] [CrossRef]
- Debes, F.; Budtz-Jorgensen, E.; Weihe, P.; White, R.F.; Grandjean, P. Impact of prenatal methylmercury exposure on neurobehavioral function at age 14 years. Neurotoxicol. Teratol. 2006, 28, 363–375. [Google Scholar] [CrossRef]
- Yorifuji, T.; Tsuda, T.; Inoue, S.; Takao, S.; Harada, M. Long-term exposure to methylmercury and psychiatric symptoms in residents of Minamata, Japan. Environ. Int. 2011, 37, 907–913. [Google Scholar] [CrossRef] [PubMed]
- Sahay, A.; Hen, R. Adult hippocampal neurogenesis in depression. Nat. Neurosci. 2007, 10, 1110–1115. [Google Scholar] [CrossRef] [PubMed]
- Onishchenko, N.; Karpova, N.; Sabri, F.; Castren, E.; Ceccatelli, S. Long-lasting depression-like behavior and epigenetic changes of, B.D.N.F gene expression induced by perinatal exposure to methylmercury. J. Neurochem. 2008, 106, 1378–1387. [Google Scholar] [CrossRef] [PubMed]
- Onishchenko, N.; Karpova, N.N.; Castrén, E. Epigenetics of Environmental Contaminants. In Methylmercury and Neurotoxicity; Ceccatelli, S., Aschner, M., Eds.; Springer: New York, NY, USA, 2012; pp. 199–218. [Google Scholar]
- Karpova, N.N.; Sales, A.J.; Joca, S.R. Epigenetic Basis of Neuronal and Synaptic Plasticity. Curr. Top. Med. Chem. 2017, 17, 771–793. [Google Scholar] [CrossRef] [PubMed]
- Szyf, M. Nongenetic inheritance and transgenerational epigenetics. Trends Mol. Med. 2015, 21, 134–144. [Google Scholar] [CrossRef]
- Debes, F.; Weihe, P.; Grandjean, P. Cognitive deficits at age 22 years associated with prenatal exposure to methylmercury. Cortex 2016, 74, 358–369. [Google Scholar] [CrossRef]
- Dórea, J.G.; Marques, R.C. Mercury levels and human health in the Amazon Basin. Ann. Hum. Biol. 2016, 43, 349–359. [Google Scholar] [CrossRef]
- Marques, R.C.; Garrofe Dórea, J.; Rodrigues Bastos, W.; de Freitas Rebelo, M.; de Freitas Fonseca, M.; Malm, O. Maternal mercury exposure and neuro-motor development in breastfed infants from Porto Velho (Amazon), Brazil. Int. J. Hyg. Environ. Health 2007, 210, 51–60. [Google Scholar] [CrossRef]
- Orlando, M.S.; Dziorny, A.C.; Harrington, D.; Love, T.; Shamlaye, C.F.; Watson, G.E.; van Wijngaarden, E.; Davidson, P.W.; Myers, G.J. Associations between prenatal and recent postnatal methylmercury exposure and auditory function at age 19 years in the Seychelles Child Development Study. Neurotoxicol. Teratol. 2014, 46, 68–76. [Google Scholar] [CrossRef]
- Ethier, A.A.; Muckle, G.; Bastien, C.; Dewailly, E.; Ayotte, P.; Arfken, C.; Jacobson, S.W.; Jacobson, J.L.; Saint-Amour, D. Effects of environmental contaminant exposure on visual brain development: A prospective electrophysiological study in school-aged children. Neurotoxicology 2012, 33, 1075–1085. [Google Scholar] [CrossRef]
- Murata, K.; Sakamoto, M.; Nakai, K.; Weihe, P.; Dakeishi, M.; Iwata, T.; Liu, X.J.; Ohno, T.; Kurosawa, T.; Kamiya, K.; et al. Effects of methylmercury on neurodevelopment in Japanese children in relation to the Madeiran study. Int. Arch. Occup. Environ. Health 2004, 77, 571–579. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.; Lin, C.C.; Hwang, Y.H.; Hsieh, W.S.; Liao, H.F.; Chen, P.C. Mercury, APOE, and children’s neurodevelopment. Neurotoxicology 2013, 37, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Julvez, J.; Smith, G.D.; Golding, J.; Ring, S.; Pourcain, B.S.; Gonzalez, J.R.; Grandjean, P. Prenatal methylmercury exposure and genetic predisposition to cognitive deficit at age 8 years. Epidemiology 2013, 24, 643–650. [Google Scholar] [CrossRef]
- Morris, G.; Puri, B.K.; Frye, R.E.; Maes, M. The Putative Role of Environmental Mercury in the Pathogenesis and Pathophysiology of Autism Spectrum Disorders and Subtypes. Mol. Neurobiol. 2018, 55, 4834–4856. [Google Scholar] [CrossRef] [PubMed]
- Biamonte, F.; Latini, L.; Giorgi, F.S.; Zingariello, M.; Marino, R.; De Luca, R.; D’Ilio, S.; Majorani, C.; Petrucci, F.; Violante, N.; et al. Associations among exposure to methylmercury, reduced Reelin expression, and gender in the cerebellum of developing mice. Neurotoxicology 2014, 45, 67–80. [Google Scholar] [CrossRef] [PubMed]
- Cinca, I.; Dumitrescu, I.; Onaca, P.; Serbanescu, A.; Nestorescu, B. Accidental ethyl mercury poisoning with nervous system, skeletal muscle, and myocardium injury. J. Neurol. Neurosurg. Psychiatry 1980, 43, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Dórea, J.G. Abating Mercury Exposure in Young Children Should Include Thimerosal-Free Vaccines. Neurochem. Res. 2017, 42, 2673–2685. [Google Scholar] [CrossRef]
- Aschner, M.; Ceccatelli, S. Are neuropathological conditions relevant to ethylmercury exposure? Neurotox. Res. 2010, 18, 59–68. [Google Scholar] [CrossRef]
- Hviid, A.; Stellfeld, M.; Wohlfahrt, J.; Melbye, M. Association between thimerosal-containing vaccine and autism. JAMA 2003, 290, 1763–1766. [Google Scholar] [CrossRef]
- Madsen, K.M.; Lauritsen, M.B.; Pedersen, C.B.; Thorsen, P.; Plesner, A.M.; Andersen, P.H.; Mortensen, P.B. Thimerosal and the occurrence of autism: Negative ecological evidence from Danish population-based data. Pediatrics 2003, 112 Pt 1, 604–606. [Google Scholar] [CrossRef] [PubMed]
- Parker, S.K.; Schwartz, B.; Todd, J.; Pickering, L.K. Thimerosal-containing vaccines and autistic spectrum disorder: A critical review of published original data. Pediatrics 2004, 114, 793–804. [Google Scholar] [CrossRef] [PubMed]
- Tozzi, A.E.; Bisiacchi, P.; Tarantino, V.; De Mei, B.; D’Elia, L.; Chiarotti, F.; Salmaso, S. Neuropsychological performance 10 years after immunization in infancy with thimerosal-containing vaccines. Pediatrics 2009, 123, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Thompson, W.W.; Price, C.; Goodson, B.; Shay, D.K.; Benson, P.; Hinrichsen, V.L.; Lewis, E.; Eriksen, E.; Ray, P.; Marcy, S.M.; et al. Early thimerosal exposure and neuropsychological outcomes at 7 to 10 years. N. Engl. J. Med. 2007, 357, 1281–1292. [Google Scholar] [CrossRef] [PubMed]
- Barile, J.P.; Kuperminc, G.P.; Weintraub, E.S.; Mink, J.W.; Thompson, W.W. Thimerosal exposure in early life and neuropsychological outcomes 7-10 years later. J. Pediatr. Psychol. 2012, 37, 106–118. [Google Scholar] [CrossRef] [PubMed]
- Gadad, B.S.; Li, W.; Yazdani, U.; Grady, S.; Johnson, T.; Hammond, J.; Gunn, H.; Curtis, B.; English, C.; Yutuc, V.; et al. Administration of thimerosal-containing vaccines to infant rhesus macaques does not result in autism-like behavior or neuropathology. Proc. Natl. Acad. Sci. USA 2015, 112, 12498–12503. [Google Scholar] [CrossRef] [PubMed]
- Curtis, B.; Liberato, N.; Rulien, M.; Morrisroe, K.; Kenney, C.; Yutuc, V.; Ferrier, C.; Marti, C.N.; Mandell, D.; Burbacher, T.M.; et al. Examination of the safety of pediatric vaccine schedules in a non-human primate model: Assessments of neurodevelopment, learning, and social behavior. Environ. Health Perspect. 2015, 123, 579–589. [Google Scholar] [CrossRef]
- Deer, B. Autism Research: What Makes an Expert? BMJ 2007, 334, 666–667. [Google Scholar] [CrossRef]
- Price, O.R.; Thorbek, P. In Response: Challenges and opportunities for landscape ecotoxicology and spatially explicit risk assessment—An industry perspective. Environ. Toxicol. Chem. 2014, 33, 1194–1196. [Google Scholar] [CrossRef]
- Taylor, L.E.; Swerdfeger, A.L.; Eslick, G.D. Vaccines are not associated with autism: An evidence-based meta-analysis of case-control and cohort studies. Vaccine 2014, 32, 3623–3629. [Google Scholar] [CrossRef]
- Uno, Y.; Uchiyama, T.; Kurosawa, M.; Aleksic, B.; Ozaki, N. Early exposure to the combined measles-mumps-rubella vaccine and thimerosal-containing vaccines and risk of autism spectrum disorder. Vaccine 2015, 33, 2511–2516. [Google Scholar] [CrossRef]
- Mitkus, R.J.; King, D.B.; Walderhaug, M.O.; Forshee, R.A. A comparative pharmacokinetic estimate of mercury in, U.S. Infants following yearly exposures to inactivated influenza vaccines containing thimerosal. Risk Anal. 2014, 34, 735–750. [Google Scholar] [CrossRef]
- Yoshimasu, K.; Kiyohara, C.; Takemura, S.; Nakai, K. A meta-analysis of the evidence on the impact of prenatal and early infancy exposures to mercury on autism and attention deficit/hyperactivity disorder in the childhood. Neurotoxicology 2014, 44, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Modabbernia, A.; Velthorst, E.; Reichenberg, A. Environmental risk factors for autism: An evidence-based review of systematic reviews and meta-analyses. Mol. Autism 2017, 8, 13. [Google Scholar] [CrossRef] [PubMed]
- Hornig, M.; Chian, D.; Lipkin, W.I. Neurotoxic effects of postnatal thimerosal are mouse strain dependent. Mol. Psychiatry 2004, 9, 833–845. [Google Scholar] [CrossRef] [PubMed]
- Olczak, M.; Duszczyk, M.; Mierzejewski, P.; Meyza, K.; Majewska, M.D. Persistent behavioral impairments and alterations of brain dopamine system after early postnatal administration of thimerosal in rats. Behav. Brain Res. 2011, 223, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, P. The Laboratory Rat: Relating Its Age with Human’s. Int. J. Prev. Med. 2013, 4, 624–630. [Google Scholar] [PubMed]
- Olczak, M.; Duszczyk, M.; Mierzejewski, P.; Majewska, M.D. Neonatal administration of a vaccine preservative, thimerosal, produces lasting impairment of nociception and apparent activation of opioid system in rats. Brain Res. 2009, 1301, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Olczak, M.; Duszczyk, M.; Mierzejewski, P.; Wierzba-Bobrowicz, T.; Majewska, M.D. Lasting neuropathological changes in rat brain after intermittent neonatal administration of thimerosal. Folia Neuropathol. 2010, 48, 258–269. [Google Scholar]
- Li, X.; Qu, F.; Xie, W.; Wang, F.; Liu, H.; Song, S.; Chen, T.; Zhang, Y.; Zhu, S.; Wang, Y.; et al. Transcriptomic analyses of neurotoxic effects in mouse brain after intermittent neonatal administration of thimerosal. Toxicol. Sci. 2014, 139, 452–465. [Google Scholar] [CrossRef]
- Yoshida, M.; Lee, J.-Y.; Shimizu-Furusawa, H.; Satoh, M.; Watanabe, C. Neurobehavioral toxicity related to the exposure of weaning mice to low-level mercury vapor and methylmercury and influence of aging. Fundam. Toxicol. Sci. 2016, 3, 185–193. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azevedo, L.F.; Karpova, N.; Rocha, B.A.; Barbosa Junior, F.; Gobe, G.C.; Hornos Carneiro, M.F. Evidence on Neurotoxicity after Intrauterine and Childhood Exposure to Organomercurials. Int. J. Environ. Res. Public Health 2023, 20, 1070. https://doi.org/10.3390/ijerph20021070
Azevedo LF, Karpova N, Rocha BA, Barbosa Junior F, Gobe GC, Hornos Carneiro MF. Evidence on Neurotoxicity after Intrauterine and Childhood Exposure to Organomercurials. International Journal of Environmental Research and Public Health. 2023; 20(2):1070. https://doi.org/10.3390/ijerph20021070
Chicago/Turabian StyleAzevedo, Lara Ferreira, Nina Karpova, Bruno Alves Rocha, Fernando Barbosa Junior, Glenda Carolyn Gobe, and Maria Fernanda Hornos Carneiro. 2023. "Evidence on Neurotoxicity after Intrauterine and Childhood Exposure to Organomercurials" International Journal of Environmental Research and Public Health 20, no. 2: 1070. https://doi.org/10.3390/ijerph20021070
APA StyleAzevedo, L. F., Karpova, N., Rocha, B. A., Barbosa Junior, F., Gobe, G. C., & Hornos Carneiro, M. F. (2023). Evidence on Neurotoxicity after Intrauterine and Childhood Exposure to Organomercurials. International Journal of Environmental Research and Public Health, 20(2), 1070. https://doi.org/10.3390/ijerph20021070






