Parental Perception of the Oral Health-Related Quality of Life of Children and Adolescents with Autism Spectrum Disorder (ASD)
Abstract
1. Introduction
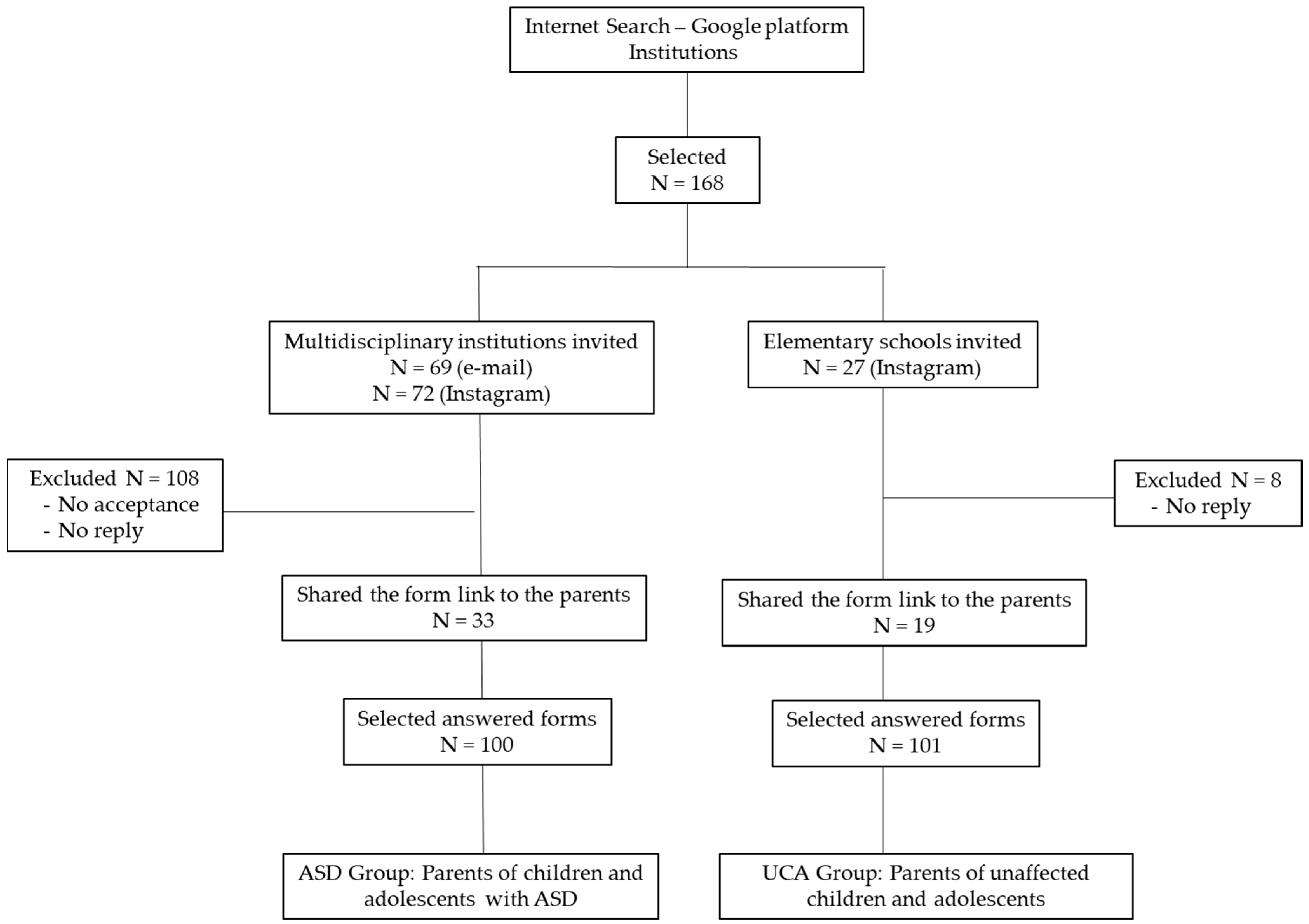
2. Materials and Methods
Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Psychiatric Association A (APA). Diagnostic and Statistical Manual of Mental Disorders: DSM-5, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar] [CrossRef]
- Nelson, L.P.; Getzin, A.; Graham, D.; Zhou, J.; Wagle, E.M.; McQuiston, J.; McLaughlin, S.; Govind, A.; Sadof, M.; Huntington, N.L. Unmet dental needs and barriers to care for children with significant special health care needs. Pediatr Dent. 2011, 1, 29–36. [Google Scholar]
- Nqcobo, C.; Ralephenya, T.; Kolisa, Y.M.; Esan, T.; Yengopal, V. Caregivers’ perceptions of the oral-health-related quality of life of children with special needs in Johannesburg, South Africa. Health SA 2019, 24, a1056. [Google Scholar] [CrossRef] [PubMed]
- Du, R.Y.; Yiu, C.K.Y.; King, N.M. Health- and oral health-related quality of life among preschool children with autism spectrum disorders. Eur. Arch. Paediatr. Dent. 2020, 3, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Ferrazzano, G.F.; Salerno, C.; Bravaccio, C.; Ingenito, A.; Sangianantoni, G.; Cantile, T. Autism spectrum disorders and oral health status: Review of the literature. Eur. J. Paediatr. Dent. 2020, 1, 9–12. [Google Scholar] [CrossRef]
- Bossù, M.; Trottini, M.; Corridore, D.; Di Giorgio, G.; Sfasciotti, G.L.; Palaia, G.; Ottolenghi, L.; Polimeni, A.; Di Carlo, S. Oral Health Status of Children with Autism in Central Italy. Appl. Sci. 2020, 7, 2247. [Google Scholar] [CrossRef]
- Zerman, N.; Zotti, F.; Chirumbolo, S.; Zangani, A.; Mauro, G.; Zoccante, L. Insights on dental care management and prevention in children with autism spectrum disorder (ASD). What is new? Front. Oral Health 2022, 3, 998831. [Google Scholar] [CrossRef]
- Barros, A.; Mascarenhas, P.; Botelho, J.; Machado, V.; Balixa, G.; Bandeira Lopes, L. Autism Spectrum Disorders and Malocclusions: Systematic Review and Meta-Analyses. J. Clin. Med. 2022, 11, 2727. [Google Scholar] [CrossRef]
- Granja, G.L.; Lacerda-Santos, J.T.; Firmino, R.T.; Jiao, R.; Martins, C.C.; Granville-Garcia, A.F.; Vargas-Ferreira, F. Occurrence of bruxism in individuals with autism spectrum disorder: A systematic review and meta-analysis. Spec. Care Dent. 2022, 5, 476–485. [Google Scholar] [CrossRef]
- Kammer, P.V.; Moro, J.S.; Soares, J.P.; Massignan, C.; Phadraig, C.M.G.; Bolan, M. Prevalence of tooth grinding in children and adolescents with neurodevelopmental disorders: A systematic review and meta-analysis. J. Oral Rehabil. 2022, 49, 671–685. [Google Scholar] [CrossRef]
- Jokovic, A.; Locker, D.; Stephens, M.; Kenny, D.; Tompson, B.; Guyatt, G. Validity and reliability of a questionnaire for measuring child oral-health-related quality of life. J. Dent. Res. 2002, 7, 459–463. [Google Scholar] [CrossRef]
- Sischo, L.; Broder, H.L. Oral health-related quality of life: What, why, how, and future implications. J. Dent. Res. 2011, 11, 1264–1270. [Google Scholar] [CrossRef] [PubMed]
- Jokovic, A.; Locker, D.; Tompson, B.; Guyatt, G. Questionnaire for measuring oral health-related quality of life in eight- to ten-year-old children. Pediatr. Dent. 2004, 6, 512–518. [Google Scholar]
- Barbosa, T.D.S.; Gavião, M.B. Evaluation of the Family Impact Scale for use in Brazil. J. Appl. Oral Sci. 2009, 5, 397–403. [Google Scholar] [CrossRef] [PubMed]
- de Souza Barbosa, T.; Steiner-Oliveira, C.; Gavião, M.B.D. Translation and Brazilian adaptation of the Parental-Caregiver perceptions questionnaire (P-CPQ). Saude Soc. 2010, 3, 698–708. [Google Scholar] [CrossRef]
- Barbosa, T.D.S.; Gavião, M.B.D. Validation of the Parental-Caregiver Perceptions Questionnaire: Agreement between parental and child reports. J. Public Health Dent. 2015, 75, 255–264. [Google Scholar] [CrossRef]
- Petersen, P.E. The World Oral Health Report 2003: Continuous improvement of oral health in the 21st century–the approach of the WHO Global Oral Health Programme. Community Dent. Oral Epidemiol. 2003, 1, 3–24. [Google Scholar] [CrossRef] [PubMed]
- Alyafei, N.A.; Jaleel, B.N.F.; Mathew, T. Exploring the barriers to oral health care perceived by parents/caregivers of childrenwith disabilities in Qatar. Dentistry 2020, 10, 1–6. [Google Scholar]
- Puthiyapurayil, J.; Anupam Kumar, T.V.; Syriac, G.; Kt, R.; Najmunnisa. Parental perception of oral health related quality of life and barriers to access dental care among children with intellectual needs in Kottayam, central Kerala-A cross sectional study. Spec. Care Dent. 2022, 2, 177–186. [Google Scholar] [CrossRef]
- Richa, Y.R.; Puranik, M.P. Oral health status and parental perception of child oral health related quality-of-life of children with autism in Bangalore, India. J. Indian Soc. Pedod. Prev. Dent. 2014, 2, 135–139. [Google Scholar] [CrossRef]
- Prakash, J.; Das, I.; Bindal, R.; Shivu, M.E.; Sidhu, S.; Kak, V.; Kumar, A. Parental perception of oral health-related quality of life in children with autism. An observational study. J. Fam. Med. Prim. Care 2021, 10, 3845–3850. [Google Scholar] [CrossRef]
- Pani, S.C.; Mubaraki, S.A.; Ahmed, Y.T.; Alturki, R.Y.; Almahfouz, S.F. Parental perceptions of the oral health-related quality of life of autistic children in Saudi Arabia. Spec. Care Dent. 2013, 1, 8–12. [Google Scholar] [CrossRef]
- Eslami, N.; Movahed, T.; Asadi, M. Parents’ Perceptions of the Oral Health-related Quality of Life of their Autistic Children in Iran. J. Clin. Pediatr. Dent. 2018, 6, 422–426. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; Shi, H.; Wang, H.; Wang, M.; Chen, F. Oral Health Status of Chinese Children with Autism Spectrum Disorders. Front. Psychiatry 2020, 11, 398. [Google Scholar] [CrossRef] [PubMed]
- Baghdadi, Z.D.; Muhajarine, N. Effects of Dental Rehabilitation under General Anesthesia on Children’s Oral-Health-Related Quality of Life: Saudi Arabian Parents’ Perspectives. Dent. J. 2014, 1, 1–13. [Google Scholar] [CrossRef] [PubMed]
- El-Meligy, O.; Maashi, M.; Al-Mushayt, A.; Al-Nowaiser, A.; Al-Mubark, S. The Effect of Full-Mouth Rehabilitation on Oral Health-Related Quality of Life for Children with Special Health Care Needs. J. Clin. Pediatr. Dent. 2016, 1, 53–61. [Google Scholar] [CrossRef]
- de Almeida, J.S.; Fernandes, R.F.; Andrade, Á.C.B.; Almeida, B.D.C.; Amorim, A.N.D.S.; Lustosa, J.H.D.C.M.; Mendes, R.F.; Prado, R.R., Júnior. Impact of dental treatment on the oral health-related quality of life of children and adolescents with Autism Spectrum Disorder. Spec. Care Dent. 2021, 6, 658–669. [Google Scholar] [CrossRef]
- Goursand, D.; Ferreira, M.C.; Pordeus, I.A.; Mingoti, S.A.; Veiga, R.T.; Paiva, S.M. Development of a short form of the Brazilian Parental-Caregiver Perceptions Questionnaire using exploratory and confirmatory factor analysis. Qual. Life Res. 2013, 22, 393–402. [Google Scholar] [CrossRef]
- Kramer, M.S.; Feinstein, A.R. Clinical biostatistics LIV- The biostatistics of concordance. Clin. Pharmacol. Ther. 1989, 3, 309. [Google Scholar] [CrossRef]
- Lewis, C.; Vigo, L.; Novak, L.; Klein, E.J. Listening to Parents: A Qualitative Look at the Dental and Oral Care Experiences of Children with Autism Spectrum Disorder. Pediatric Dent. 2015, 7, E98–E104. [Google Scholar]
- Abanto, J.; Carvalho, T.S.; Mendes, F.M.; Wanderley, M.T.; Bönecker, M.; Raggio, D.P. Impact of oral diseases and disorders on oral health-related quality of life of preschool children. Community Dent. Oral Epidemiol. 2011, 2, 105–114. [Google Scholar] [CrossRef]
- Shaw, K.A.; Maenner, M.J.; Bakian, A.V.; Bilder, D.A.; Durkin, M.S.; Furnier, S.M.; Hughes, M.M.; Patrick, M.; Pierce, K.; Salinas, A.; et al. Early Identification of Autism Spectrum Disorder Among Children Aged 4 Years-Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2018. MMWR Surveill Summ. 2021, 10, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Maenner, M.J.; Shaw, K.A.; Bakian, A.V.; Bilder, D.A.; Durkin, M.S.; Esler, A.; Furnier, S.M.; Hallas, L.; Hall-Lande, J.; Hudson, A.; et al. Prevalence and Characteristics of Autism Spectrum Disorder Among Children Aged 8 Years—Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2018. MMWR Surveill Summ. 2021, 11, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Loomes, R.; Hull, L.; Mandy, W.P.L. What Is the Male-to-Female Ratio in Autism Spectrum Disorder? A Systematic Review and Meta-Analysis. J. Am. Acad Child Adolesc. Psychiatry 2017, 6, 466–474. [Google Scholar] [CrossRef] [PubMed]
- Hyman, S.L.; Levy, S.E.; Myers, S.M. Identification, Evaluation, and Management of Children With Autism Spectrum Disorder. Pediatrics 2020, 1, e20193447. [Google Scholar] [CrossRef] [PubMed]
- van’t Hof, M.; Tisseur, C.; van Berckelear-Onnes, I.; van Nieuwenhuyzen, A.; Daniels, A.M.; Deen, M.; Hoek, H.W.; Ester, W.A. Age at autism spectrum disorder diagnosis: A systematic review and meta-analysis from 2012 to 2019. Autism 2021, 4, 862–873. [Google Scholar] [CrossRef]
- World Health Organization. Autism. Available online: https://www.who.int/news-room/fact-sheets/detail/autism-spectrum-disorders (accessed on 16 November 2022).
- Popow, C.; Ohmann, S.; Plener, P. Practitioner’s review: Medication for children and adolescents with autism spectrum disorder (ASD) and comorbid conditions. Neuropsychiatrie 2021, 3, 113–134. [Google Scholar] [CrossRef] [PubMed]
- Molteni, M.; Nobile, M.; Cattaneo, D.; Radice, S.; Clementi, E. Potential benefits and limits of psychopharmacological therapies in pervasive developmental disorders. CCP 2014, 4, 365–376. [Google Scholar] [CrossRef]
- Stein, L.I.; Polido, J.C.; Mailloux, Z.; Coleman, G.G.; Cermak, S.A. Oral care and sensory sensitivities in children with autism spectrum disorders. Spec. Care Dent. 2011, 3, 102–110. [Google Scholar] [CrossRef]
- Gao, L.; Liu, X.N. Status Quo and Advanced Progress in Oral Health Care and Treatment of Children with Autism Spectrum Disorder: A Literatiure Review. Chin. J. Dent. Res. Off. J. Sci. Sect. Chin. Stomatol. Assoc. 2022, 25, 251–259. [Google Scholar] [CrossRef]
- Seiverling, L.; Williams, K.E.; Hendy, H.M.; Adams, W.; Yusupova, S.; Kaczor, A. Sensory Eating Problems Scale (SEPS) for children: Psychometrics and associations with mealtime problems behaviors. Appetite 2019, 133, 223–230. [Google Scholar] [CrossRef]
- Moorthy, L.; Dixit, U.B.; Kole, R.C.; Gajre, M.P. Dietary Sugar Exposure and Oral Health Status in Children with Autism Spectrum Disorder: A Case-control Study. J. Autism Dev. Disord. 2022, 6, 2523–2534. [Google Scholar] [CrossRef] [PubMed]
- Du, R.Y.; Yiu, C.K.; King, N.M.; Wong, V.C.; McGrath, C.P. Oral health among preschool children with autism spectrum disorders: A case-control study. Autism 2015, 6, 746–751. [Google Scholar] [CrossRef] [PubMed]
- AlOtaibi, A.; Ben Shaber, S.; AlBatli, A.; AlGhamdi, T.; Murshid, E. A systematic review of population-based gingival health studies among children and adolescents with autism spectrum disorder. Saudi Dent. J. 2021, 7, 370–374. [Google Scholar] [CrossRef] [PubMed]
- Antunes, L.A.A.; Castilho, T.; Marinho, M.; Fraga, R.S.; Antunes, L.S. Childhood bruxism: Related factors and impact on oral health-related quality of life. Spec. Care Dent. 2016, 36, 7–12. [Google Scholar] [CrossRef]
- Knorst, J.K.; Sfreddo, C.S.; de F Meira, G.; Zanatta, F.B.; Vettore, M.V.; Ardenghi, T.M. Socioeconomic status and oral health-related quality of life: A systematic review and meta-analysis. Community Dent. Oral Epidemiol. 2021, 2, 95–102. [Google Scholar] [CrossRef]
- Jokovic, A.; Locker, D.; Stephens, M.; Kenny, D.; Tompson, B.; Guyatt, G. Measuring parental perceptions of child oral health-related quality of life. J Public Health Dent. 2003, 63, 67–72. [Google Scholar] [CrossRef]
- Locker, D.; Jokovic, A.; Stephens, M.; Kenny, D.; Tompson, B.; Guyatt, G. Family impact of child oral and oro-facial conditions. Community Dent. Oral Epidemiol. 2002, 30, 438–448. [Google Scholar] [CrossRef]
| ASD Group (n = 100) | UCA Group (n = 101) | p Value | ||
|---|---|---|---|---|
| Age | Mean (SD) | 8.38 (2.38) | 9.11 (1.96) | Mann–Whitney p = 0.019 |
| Median (25th; 75th) | 8 (6.0; 10.0) | 9 (8.0; 11.0) | ||
| Range | 6–14 | 6–14 | ||
| Sociodemographic [n (%)] | ||||
| Sex | Male | 65 (65) | 55 (54.5) | χ2 p = 0.128 |
| Female | 35 (35) | 46 (45.5) | ||
| ASD diagnosis (age) | <2 years | 36 (36) | - | χ2 goodness of fit p < 0.001 |
| 2–6 Years | 54 (54) | - | ||
| >6 years | 10 (10) | - | ||
| ASD levels | Level 1 | 37 (37) | - | χ2 goodness of fit p < 0.001 |
| Level 2 | 51 (51) | - | ||
| Level 3 | 12 (12) | - | ||
| Medication use | Yes | 53 (53) | 6 (5.90) | χ2 p < 0.01 |
| No | 47 (47) | 95 (94.1) | ||
| Parent’s education | Elementary School | 24 (24) | 15 (14.9) | χ2 p < 0.01 |
| High school | 16 (16) | 55 (53.5) | ||
| College | 44 (44) | 26 (25.8) | ||
| Postgraduate studies | 16 (16) | 5 (5) | ||
| Employment status | Working | 79 (79) | 82 (81.2) | χ2 p = 0.698 |
| Unemployed | 21 (21) | 19 (18.8) | ||
| Family income | ≤1 minimum wage | 17 (17) | 8 (7.9) | χ2 p < 0.001 |
| 2–3 minimum wage | 34 (34) | 67 (66.3) | ||
| ≥4 minimum wage | 49 (49) | 26 (25.7) | ||
| Respondent | Mother | 90 (90) | 94 (93.1%) | Fisher’s exact test p = 0.062 p < 0.001 * |
| Father | 10 (10) | 4 (4.0%) | ||
| Other | 0 | 3 (3.0%) | ||
| Feeding [n (%)] | ||||
| Mealtime | Yes | 81 (81) | 76 (75.2) | χ2 p = 0.324 |
| No | 19 (19) | 25 (24.8) | ||
| Bottle feeding | Don’t use | 66 (66) | 94 (93) | Fisher’s exact test p < 0.001 |
| Once a day | 7 (7) | 2 (2) | ||
| Twice a day | 22 (22) | 5 (5) | ||
| Three times a day | 5 (5) | 0 | ||
| Sugar consumption | Don’t consume | 0 | 0 | χ2 p = 0.493 |
| Once a day | 46 (46) | 44 (43.6) | ||
| 2–3 times a day | 36 (36) | 32 (31.7) | ||
| More than 3 times a day | 18 (18) | 25 (24.8) | ||
| Oral habits and dental care [n (%)] | ||||
| Tooth brushing frequency | Don’t brush | 8 (8) | 0 | Fisher’s exact test p < 0.001 |
| Once a day | 42 (42) | 24 (23.8) | ||
| Twice a day | 23 (23) | 44 (43.6) | ||
| Three times a day | 27 (27) | 33 (32.7) | ||
| Responsible for tooth brushing | Parents | 75 (75) | 7 (6.9) | χ2 p < 0.001 |
| Child | 3 (3) | 33 (32.7) | ||
| Both | 22 (22) | 61 (60.4) | ||
| Dentist appointment | Yes | 62 (62) | 84 (83.2) | χ2 p < 0.001 |
| No | 38 (38) | 17 (16.8) | ||
| Premature tooth loss | Yes | 40 (40) | 8 (7.9) | χ2 p < 0.001 |
| No | 60 (60) | 93 (92.1) | ||
| Gingival bleeding | Yes | 12 (12) | 12 (11.9) | χ2 p = 0.979 |
| No | 88 (88) | 89 (88.1) | ||
| Dental Trauma | Yes | 32 (32) | 33 (32.7) | χ2 p = 0.919 |
| No | 68 (68) | 68 (67.3) | ||
| Deleterious oral habits | No | 70 (70) | 86 (85.1) | Fisher’s exact test p = 0.004 |
| Digital suction | 2 (2) | 3 (3) | ||
| Pacifier | 1 (1) | 1 (1) | ||
| Feeding bottle | 20 (20) | 6 (5.9) | ||
| Onychophagia | 7 (7) | 5 (5) | ||
| Mouth breathing | Yes | 56 (56) | 40 (39.6) | χ2 p = 0.020 |
| No | 44 (44) | 61 (60.4) | ||
| Teeth grinding | Never | 52 (52) | 73 (72.3) | χ2 p = 0.005 |
| Sometimes | 39 (39) | 26 (25.7) | ||
| Many times | 9 (9) | 2 (2) | ||
| ASD Group (N = 100) | UCA Group (N = 101) | p Values 1 | Effect Size 2 | ||
|---|---|---|---|---|---|
| 16-P-CPQ total scale | Mean (SD) | 8.51 (5.84) | 6.12 (4.58) | p = 0.002 | 0.254 |
| Median (25th; 75th) | 6.5 (4; 11) | 5 (4; 8) | |||
| Range | 0–23 | 1–23 | |||
| Subscales | |||||
| OS | Mean (SD) | 3.58 (2.69) | 3.03 (2.61) | p = 0.096 | 0.134 |
| Median (25th; 75th) | 4.00 (2.00; 5.00) | 2.00 (1.00–5.00) | |||
| Range | 0–11 | 0–11 | |||
| FL | Mean (SD) | 3.27 (2.44) | 2.34 (1.94) | p = 0.007 | 0.215 |
| Median (25th; 75th) | 3.00 (2.00; 5.00) | 2.00 (1.00; 3.00) | |||
| Range | 0–9 | 0–9 | |||
| EWB | Mean (SD) | 0.95 (1.32) | 0.49 (0.88) | p = 0.021 | 0.157 |
| Median (25th; 75th) | 0 (0; 2) | 0 (0; 1) | |||
| Range | 0–5 | 0–3 | |||
| SWB | Mean (SD) | 0.71 (1.13) | 0.27 (0.65) | p = 0.002 | 0.195 |
| Median (25th; 75th) | 0 (0; 1) | 0 (0; 0) | |||
| Range | 0–5 | 0–3 | |||
| Global Perception | Mean (SD) | 2.78 (1.85) | 1.74 (1.29) | p < 0.001 | 0.316 |
| Median (25th; 75th) | 3.00 (1.00; 4.00) | 2.00 (1.00; 2.00) | |||
| Range | 0–6 | 0–6 | |||
| 4-FIS | Mean (SD) | 4.08 (3.21) | 2.95 (2.52) | p = 0.025 | 0.182 |
| Median (25th; 75th) | 3 (2.00; 6.25) | 3 (1.00; 4.00) | |||
| Range | 0–11 | 0–11 |
| ASD Group N (%) | UCA Group N (%) | ||
|---|---|---|---|
| Global rating of oral health | |||
| Excellent | 12 (12.0) | 14 (13.86) | Fisher’s exact test p < 0.001 |
| Very good | 26 (26.0) | 51 (50.50) | |
| Good | 24 (24.0) | 30 (29.70) | |
| Fair | 32 (32.0) | 3 (2.97) | |
| Poor | 6 (6.0) | 3 (2.97) | |
| Global rating of overall well-being (affected) | |||
| Not at all | 47 (47.0) | 75 (74.36) | χ2 p < 0.001 * |
| Very little | 29 (29.0) | 15 (14.85) | |
| Some | 17 (17.0) | 4 (1.98) | |
| A lot | 7 (7.0) | 7 (6.93) | |
| Very much | 0 | 0 | |
| ASD Group | UCA Group | |||
|---|---|---|---|---|
| McDonald’s ω | ICC (95% CI) (n = 10) | McDonald’s ω | ICC (95% CI) (n = 10) | |
| 16-P-CPQ total | 0.778 | 0.982 (0.932–0.995) | 0.773 | 0.996 (0.985–0.999) |
| Subscales | ||||
| OS | 0.684 | 0.995 (0.980–0.999) | 0.692 | 1 |
| FL | 0.638 | 0.969 (0.887–0.992) | 0.502 | 1 |
| EWB | 0.471 | 0.444 | ||
| SWB | 0.380 | 0.469 | ||
| Global perception | 0.708 | 1 | 0.227 | 0.974 (0.906–0.993) |
| 4-FIS | 0.743 | 0.982 (0.932–0.995) | 0.715 | 0.935 (0.776–0.983) |
| Global Oral Health (n = 201) | Global Well-Being Teeth (n = 201) | |||
|---|---|---|---|---|
| R * | p Value | R | p Value | |
| 16-P-CPQ total | 0.421 | <0.001 | 0.662 | <0.001 |
| Subscales | ||||
| OS | 0.301 | <0.001 | 0.366 | <0.001 |
| FL | 0.393 | <0.001 | 0.692 | <0.001 |
| EWB | 0.295 | <0.001 | 0.431 | <0.001 |
| SWB | 0.214 | 0.002 | 0.356 | <0.001 |
| 4-FIS | 0.339 | <0.001 | 0.578 | <0.001 |
| Predictor | Estimate | Odds Ratio | p Value | 95% CI | R2N | |
|---|---|---|---|---|---|---|
| Dependent variable: 16-P-CPQ total dichotomized (Median 6) | ||||||
| Groups | ASD—UCA | 0.775 | 2.17 | 0.008 | 1.22–3.86 | 0.047 |
| Age | 0.148 | 1.159 | 0.026 | 1.02–1.32 | 0.034 | |
| Employment status | Unemployed—Working | 0.521 | 1.683 | 0.143 | 0.84–3.38 | 0.014 |
| Responsible for tooth brushing | Parent—child/adolescent | 1.001 | 2.721 | 0.024 | 1.14–6.50 | 0.037 |
| Both—child/adolescent | 0.633 | 1.882 | 0.156 | 0.79–4.51 | ||
| Mealtime | No–Yes | 0.653 | 1.921 | 0.058 | 0.98–3.77 | 0.024 |
| Dentist appointment | No–Yes | 0.526 | 1.692 | 0.100 | 0.90–3.17 | 0.018 |
| Dental Trauma | Yes–No | 0.575 | 1.778 | 0.060 | 0.98–3.24 | 0.024 |
| Teeth grinding | Yes–No | 0.505 | 1.657 | 0.088 | 0.93–2.96 | 0.019 |
| Gingival bleeding | No–Yes | −0.663 | 0.515 | 0.130 | 0.22–1.22 | 0.053 |
| Tooth brushing frequency | 0–1x/day—2–3x/day | 0.849 | 2.337 | 0.005 | 1.30–4.21 | 0.053 |
| Dependent variable: 4-FIS dichotomized (Median 3) | ||||||
| Parent´s Education | Elementary School–High School | −0.665 | 0.514 | 0.109 | 0.23–1.16 | 0.020 |
| College–High School | −0.029 | 0.971 | 0.931 | 0.50–1.88 | ||
| Postgraduate–High School | −0.0671 | 0.935 | 0.893 | 0.35–2.48 | ||
| Family income | ≤1 minimum wage—2–3 minimum wages | 0.705 | 2.023 | 0.122 | 0.83–4.94 | 0.016 |
| ≥4 minimum wage—2–3 minimum wages | 0.112 | 1.119 | 0.715 | 0.61–2.04 | ||
| Tooth brushing frequency | 0–1x/day—2–3x/day | 0.442 | 1.556 | 0.133 | 0.87–2.77 | 0.015 |
| Predictor (Independents) | Estimate | Odds Ratio | p-Value | 95% CI | Collinearity | |
|---|---|---|---|---|---|---|
| Dependent variable: 16-P-CPQ dichotomized; Overall Model Test: χ2 = 35.9, p < 0.001. R2N = 0.221 | ||||||
| Intercept | −3.233 | 0.040 | <0.001 | 0.008–0.193 | VIF | Tolerance |
| Age | 0.213 | 1.237 | 0.007 | 1.06–1.44 | 1.10 | 0.908 |
| Tooth brushing frequency | ||||||
| 0–1x/day—2–3x/day | 0.793 | 2.210 | 0.025 | 1.10–4.42 | 1.10 | 0.908 |
| Teeth grinding | ||||||
| Yes—No | 0.790 | 2.204 | 0.028 | 1.09–4.46 | 1.12 | 0.894 |
| Gingival bleeding | ||||||
| Yes—No | 1.206 | 3.341 | 0.030 | 1.12–9.96 | 1.18 | 0.851 |
| Dependent variable: 4-FIS dichotomized; Overall Model Test: χ2 = 11.4, p = 0.076, R2N = 0.074 | ||||||
| Parent’s education | ||||||
| Elementary School—High School | −1.158 | 0.314 | 0.014 | 0.13–0.79 | 1.14 | 0.876 |
| Family income | ||||||
| ≤1 minimum wage— 2–3 minimum wage | 1.115 | 3.049 | 0.029 | 1.12–8.28 | 1.2 | 0.830 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Silva, A.C.F.; Barbosa, T.d.S.; Gavião, M.B.D. Parental Perception of the Oral Health-Related Quality of Life of Children and Adolescents with Autism Spectrum Disorder (ASD). Int. J. Environ. Res. Public Health 2023, 20, 1151. https://doi.org/10.3390/ijerph20021151
da Silva ACF, Barbosa TdS, Gavião MBD. Parental Perception of the Oral Health-Related Quality of Life of Children and Adolescents with Autism Spectrum Disorder (ASD). International Journal of Environmental Research and Public Health. 2023; 20(2):1151. https://doi.org/10.3390/ijerph20021151
Chicago/Turabian Styleda Silva, Anna Cecília Farias, Taís de Souza Barbosa, and Maria Beatriz Duarte Gavião. 2023. "Parental Perception of the Oral Health-Related Quality of Life of Children and Adolescents with Autism Spectrum Disorder (ASD)" International Journal of Environmental Research and Public Health 20, no. 2: 1151. https://doi.org/10.3390/ijerph20021151
APA Styleda Silva, A. C. F., Barbosa, T. d. S., & Gavião, M. B. D. (2023). Parental Perception of the Oral Health-Related Quality of Life of Children and Adolescents with Autism Spectrum Disorder (ASD). International Journal of Environmental Research and Public Health, 20(2), 1151. https://doi.org/10.3390/ijerph20021151







