The Mechanism of miR-21-5p/TSP-1-Mediating Exercise on the Function of Endothelial Progenitor Cells in Aged Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects and Exercise Intervention
2.2. Cell Culture and Identification
2.3. Senescence-Associated Beta-Galactosidase Staining
2.4. Proliferation Assay
2.5. Migration Assay
2.6. Cell Transfection
2.7. Quantitative Real-Time Polymerase Chain Reaction Analysis
2.8. Western Blot
2.9. Statistical Analysis
3. Results
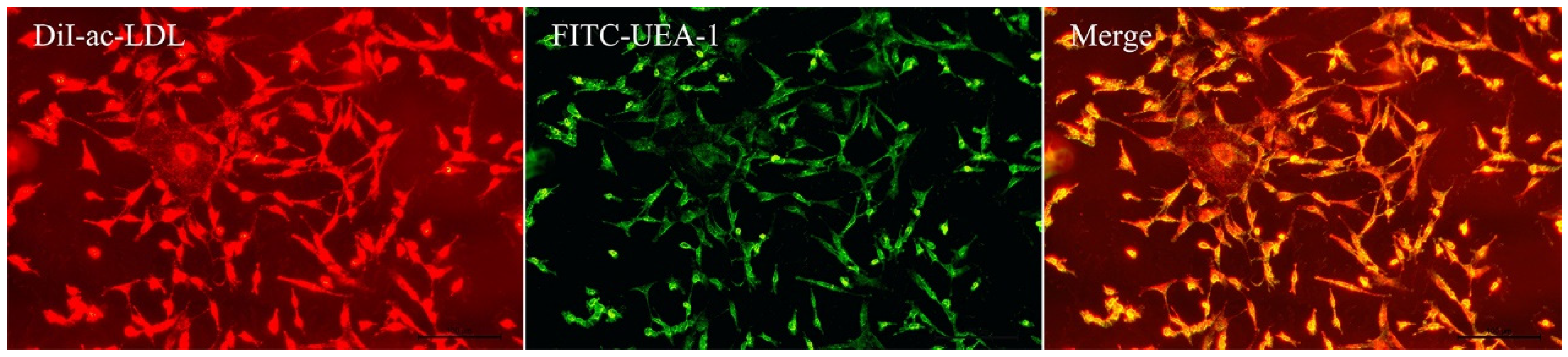
3.1. Identification of EPCs
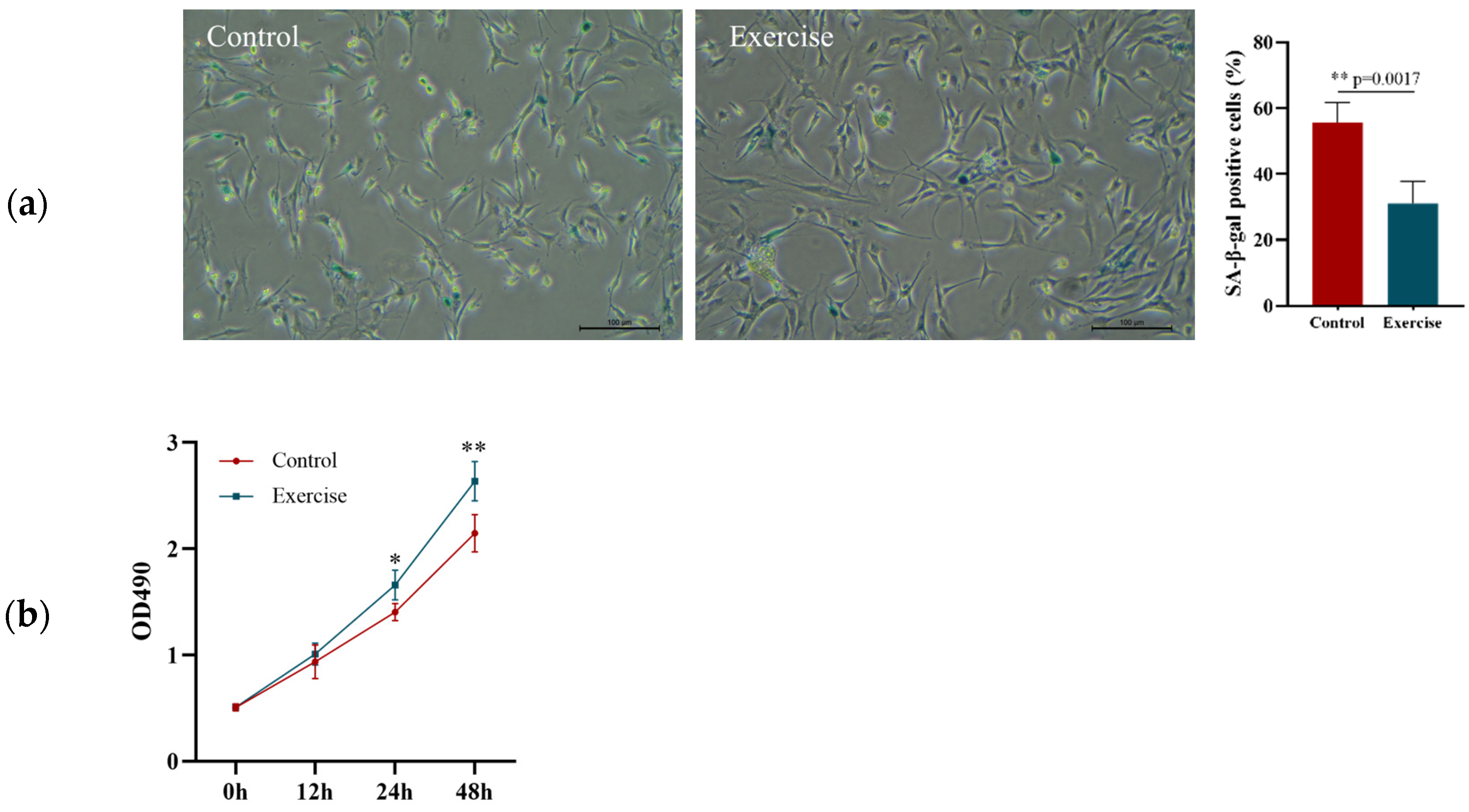
3.2. Effect of 8-Week Aerobic Exercise on the Senescence, Proliferation, and Migration of EPCs in Aged Rats
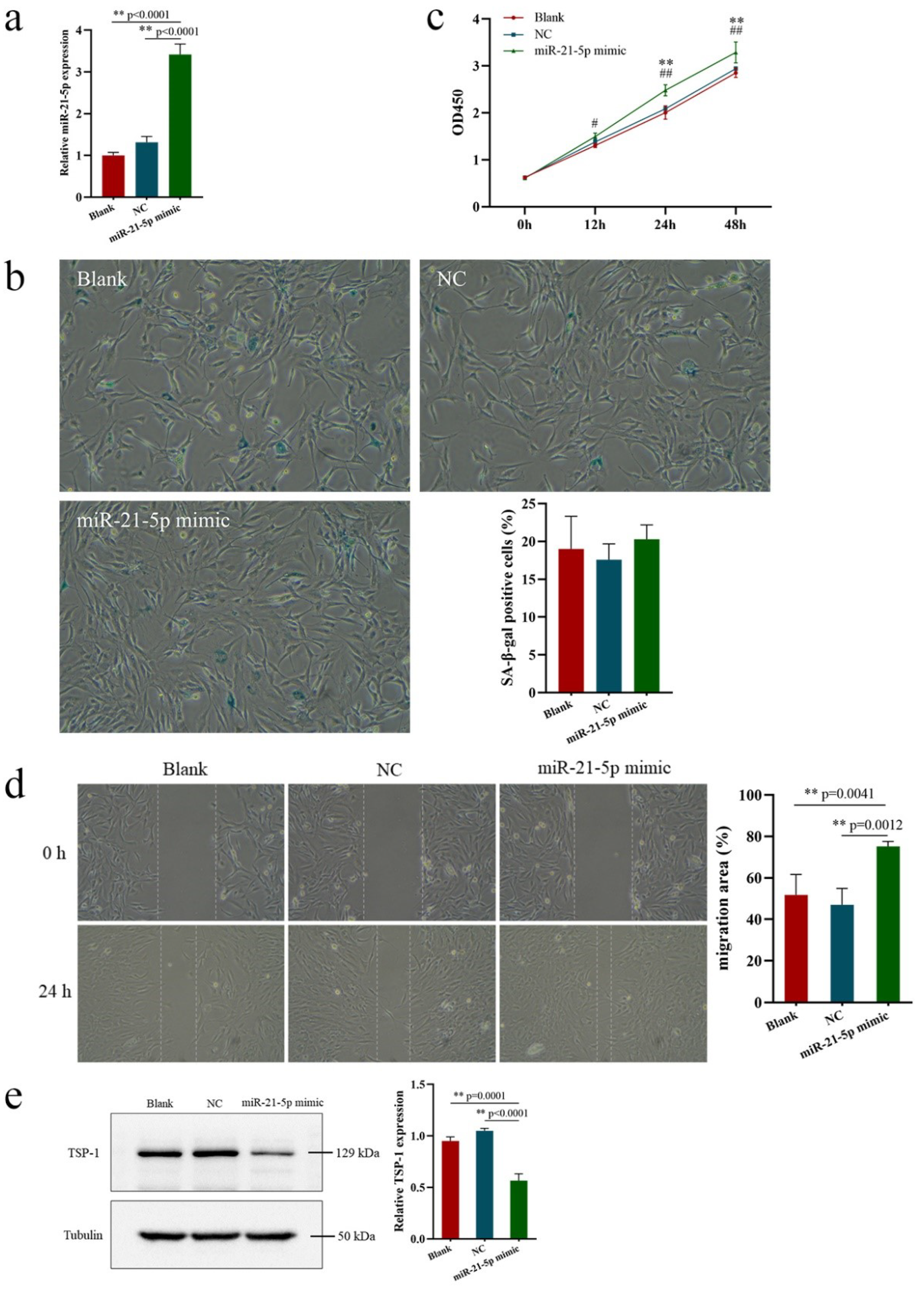
3.3. Effects of miR-21-5p Overexpression on EPCs Function and TSP-1 Expression
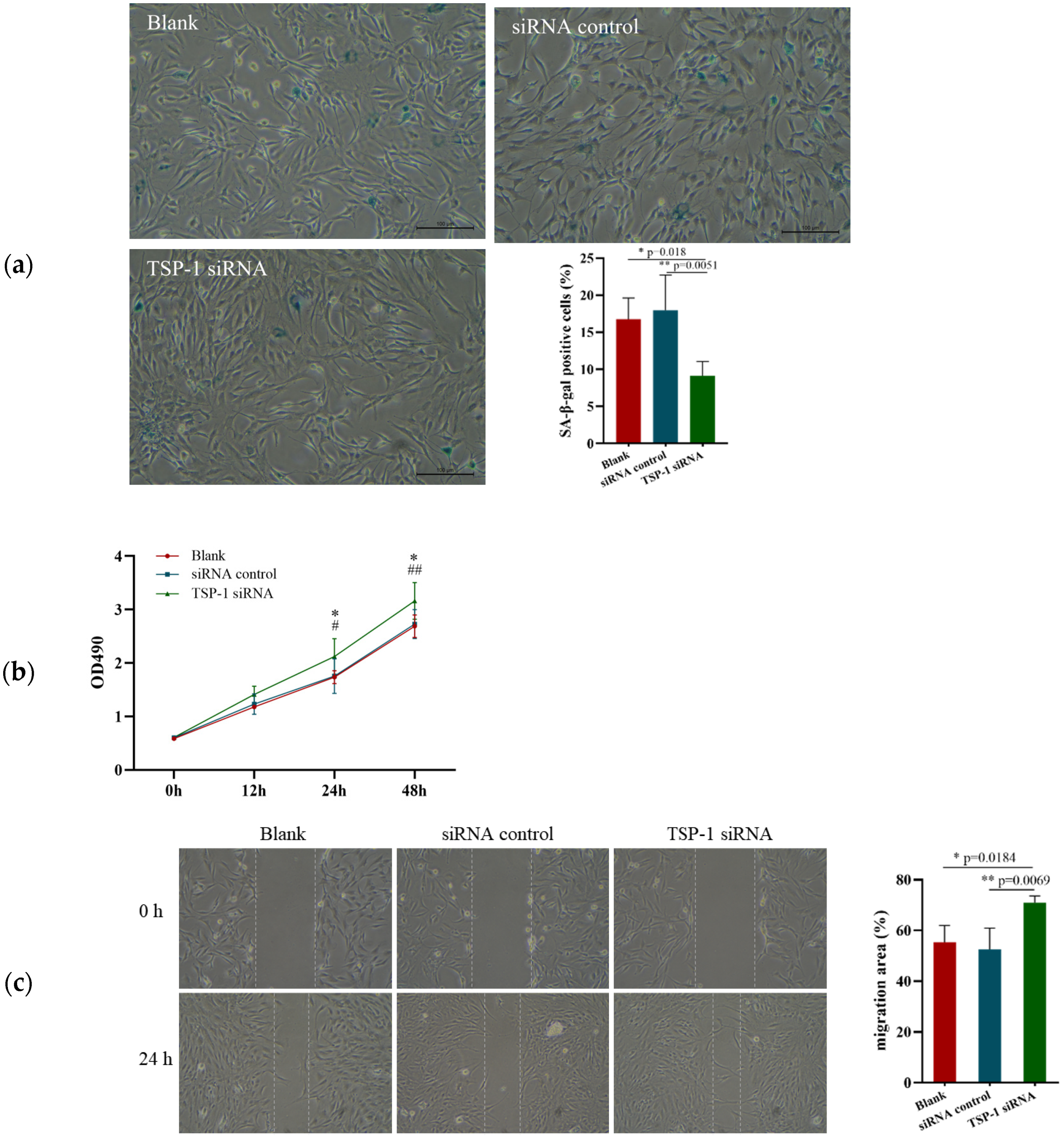
3.4. Effects of TSP-1 Expression on EPCs Function
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pi, X.; Xie, L.; Patterson, C. Emerging roles of vascular endothelium in metabolic homeostasis. Circ. Res. 2018, 123, 477–494. [Google Scholar] [CrossRef]
- Eelen, G.; de Zeeuw, P.; Simons, M.; Carmeliet, P. Endothelial cell metabolism in normal and diseased vasculature. Circ. Res. 2015, 116, 1231–1244. [Google Scholar] [CrossRef] [PubMed]
- Williamson, K.; Stringer, S.S.; Alexander, Y.M. Endothelial progenitor cells enter the aging arena. Front. Physiol. 2012, 3, 30. [Google Scholar] [CrossRef] [PubMed]
- Sandri, M.; Viehmann, M.; Adams, V.; Rabald, K.; Mangner, N.; Höllriegel, R.; Lurz, P.; Erbs, S.; Linke, A.; Kirsch, K.; et al. Chronic heart failure and aging—Effects of exercise training on endothelial function and mechanisms of endothelial regeneration: Results from the Leipzig Exercise Intervention in Chronic heart failure and Aging (LEICA) study. Eur. J. Prev. Cardiol. 2016, 23, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Möbius-Winkler, S.; Hilberg, T.; Menzel, K.; Golla, E.; Burman, A.; Schuler, G.; Adams, V. Time-dependent mobilization of circulating progenitor cells during strenuous exercise in healthy individuals. J. Appl. Physiol. 2009, 107, 1943–1950. [Google Scholar] [CrossRef]
- Cavalcante, S.L.; Lopes, S.; Bohn, L.; Cavero-Redondo, I.; Alvarez-Bueno, C.; Viamonte, S.; Ribeiro, F. Effects of exercise on endothelial progenitor cells in patients with cardiovascular disease: A systematic review and meta-analysis of randomized controlled trials. Rev. Port. Cardiol. 2019, 38, 817–827. [Google Scholar] [CrossRef]
- De Biase, C.; De Rosa, R.; Luciano, R.; DE Luca, S.; Capuano, E.; Trimarco, B.; Galasso, G. Effects of physical activity on endothelial progenitor cells (EPCs). Front. Physiol. 2013, 4, 414–430. [Google Scholar] [CrossRef]
- Pei, C.Z.; Liu, B.; Li, Y.T.; Fang, L.; Zhang, Y.; Li, Y.G.; Meng, S. MicroRNA-126 protects against vascular injury by promoting homing and maintaining stemness of late outgrowth endothelial progenitor cells. Stem Cell Res. Ther. 2020, 11, 28–40. [Google Scholar] [CrossRef]
- Wang, J.; Liu, H.; Chen, S.; Zhang, W.; Chen, Y.; Yang, Y. Moderate exercise has beneficial effects on mouse ischemic stroke by enhancing the functions of circulating endothelial progenitor cell-derived exosomes. Exp. Neurol. 2020, 330, 113325. [Google Scholar] [CrossRef]
- Ma, C.; Wang, J.; Liu, H.; Chen, Y.; Ma, X.; Chen, S.; Chen, Y.; Bihl, J.; Yang, Y. Moderate exercise enhances endothelial progenitor cell exosomes release and function. Med. Sci. Sports Exerc. 2018, 50, 2024–2032. [Google Scholar] [CrossRef]
- Mayourian, J.; Ceholski, D.K.; Gorski, P.A.; Mathiyalagan, P.; Murphy, J.; Salazar, S.I.; Stillitano, F.; Hare, J.M.; Sahoo, S.; Hajjar, R.J.; et al. Exosomal microRNA-21-5p mediates mesenchymal stem cell paracrine effects on human cardiac tissue contractility. Circ. Res. 2018, 122, 933–944. [Google Scholar] [CrossRef] [PubMed]
- Souza, R.W.A.; Fernandez, G.J.; Cunha, J.P.Q.; Piedade, W.P.; Soares, L.C.; Souza, P.A.T.; de Campos, D.H.S.; Okoshi, K.; Cicogna, A.C.; Dal-Pai-Silva, M.; et al. Regulation of cardiac microRNAs induced by aerobic exercise training during heart failure. Am. J. Physiol. Circ. Physiol. 2015, 309, 1629–1641. [Google Scholar] [CrossRef] [PubMed]
- van Almen, G.C.; Verhesen, W.; van Leeuwen, R.E.; van de Vrie, M.; Eurlings, C.; Schellings, M.W.; Schroen, B. MicroRNA-18 and microRNA-19 regulate CTGF and TSP-1 expression in age-related heart failure. Aging Cell 2011, 10, 769–779. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Yuan, Z.; Fei, Q.; Zhao, J. Investigation of thrombospondin-1 and transforming growth factor-β expression in the heart of aging mice. Exp. Ther. Med. 2012, 3, 433–436. [Google Scholar] [CrossRef] [PubMed]
- Ii, M.; Takenaka, H.; Asai, J.; Ibusuki, K.; Mizukami, Y.; Maruyama, K.; Yoon, Y.-S.; Wecker, A.; Luedemann, C.; Eaton, E.; et al. Endothelial progenitor thrombospondin-1 mediates diabetes-induced delay in reendothelialization following arterial injury. Circ. Res. 2006, 98, 697–704. [Google Scholar] [CrossRef]
- Wu, J.; He, Z.; Gao, X.; Wu, F.; Ding, R.; Ren, Y.; Jiang, Q.; Fan, M.; Liang, C.; Wu, Z. Oxidized high-density lipoprotein impairs endothelial progenitor cells’ function by activation of CD36-MAPK-TSP-1 pathways. Antioxid. Redox Signal. 2015, 22, 308–324. [Google Scholar] [CrossRef]
- Hu, H.; Wang, B.; Jiang, C.; Li, R.; Zhao, J. Endothelial progenitor cell-derived exosomes facilitate vascular endothelial cell repair through shuttling miR-21-5p to modulate Thrombospondin-1 expression. Clin. Sci. 2019, 133, 1629–1644. [Google Scholar] [CrossRef]
- Liu, W.; Wang, Z.; Xia, Y.; Kuang, H.; Liu, S.; Li, L.; Tang, C.; Yin, D. The balance of apoptosis and autophagy via regulation of the AMPK signal pathway in aging rat striatum during regular aerobic exercise. Exp. Gerontol. 2019, 124, 110647. [Google Scholar] [CrossRef]
- Hur, J.; Yoon, C.-H.; Kim, H.-S.; Choi, J.-H.; Kang, H.-J.; Hwang, K.-K.; Oh, B.-H.; Lee, M.-M.; Park, Y.-B. Characterization of two types of endothelial progenitor cells and their different contributions to neovasculogenesis. Arter. Thromb. Vasc. Biol. 2004, 24, 288–293. [Google Scholar] [CrossRef]
- Xia, W.H.; Li, J.; Su, C.; Yang, Z.; Chen, L.; Wu, F.; Tao, J. Physical exercise attenuates age-associated reduction in endothelium-reparative capacity of endothelial progenitor cells by increasing CXCR4/JAK-2 signaling in healthy men. Aging Cell 2012, 11, 111–119. [Google Scholar] [CrossRef]
- Li, F.; Bai, M.; Xu, J.; Zhu, L.; Liu, C.; Duan, R. Long-Term Exercise Alters the Profiles of Circulating Micro-RNAs in the Plasma of Young Women. Front. Physiol. 2020, 11, 372. [Google Scholar] [CrossRef]
- Valenti, M.T.; Deiana, M.; Cheri, S.; Dotta, M.; Zamboni, F.; Gabbiani, D.; Schena, F.; Carbonare, L.D.; Mottes, M. Physical exercise modulates miR-21-5p, miR-129-5p, miR-378-5p, and miR-188-5p expression in progenitor cells promoting osteogenesis. Cells 2019, 8, 742. [Google Scholar] [CrossRef]
- Ge, X.; Huang, S.; Gao, H.; Han, Z.; Chen, F.; Zhang, S.; Wang, Z.; Kang, C.; Jiang, R.; Yue, S.; et al. miR-21-5p alleviates leakage of injured brain microvascular endothelial barrier in vitro through suppressing inflammation and apoptosis. Brain Res. 2016, 1650, 31–40. [Google Scholar] [CrossRef]
- Ma, S.; Zhang, A.; Li, X.; Zhang, S.; Liu, S.; Zhao, H.; Wu, S.; Chen, L.; Ma, C.; Zhao, H. MiR-21-5p regulates extracellular matrix degradation and angiogenesis in TMJOA by targeting Spry1. Arthritis Res. Ther. 2020, 22, 99. [Google Scholar] [CrossRef]
- Li, Y.; Chen, X.; Jin, R.; Chen, L.; Dang, M.; Cao, H.; Dong, Y.; Cai, B.; Bai, G.; Gooding, J.J.; et al. Injectable hydrogel with MSNs/microRNA-21-5p delivery enables both immunomodification and enhanced angiogenesis for myocardial infarction therapy in pigs. Sci. Adv. 2021, 7, 2375–2548. [Google Scholar] [CrossRef]
- Wu, F.; Huang, W.; Tan, Q.; Guo, Y.; Cao, Y.; Shang, J.; Ping, F.; Wang, W.; Li, Y. ZFP36L2 regulates myocardial ischemia/reperfusion injury and attenuates mitochondrial fusion and fission by LncRNA PVT1. Cell Death Dis. 2021, 12, 614. [Google Scholar] [CrossRef]
- Mi, X.-L.; Gao, Y.-P.; Hao, D.-J.; Zhang, Z.-J.; Xu, Z.; Li, T.; Li, X.-W. Prognostic value of circulating microRNA-21-5p and microRNA-126 in patients with acute myocardial infarction and infarct-related artery total occlusion. Front. Cardiovasc. Med. 2022, 9, 947721. [Google Scholar] [CrossRef]
- Han, Y.; Cai, X.; Pan, M.; Gong, J.; Cai, W.; Lu, D.; Xu, C. MicroRNA-21-5p acts via the PTEN/Akt/FOXO3a signaling pathway to prevent cardiomyocyte injury caused by high glucose/high fat conditions. Exp. Ther. Med. 2022, 23, 230. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, J.; Long, K.; Qiu, W.; Wang, Y.; Hu, Z.; Liu, C.; Luo, Y.; Jiang, A.; Jin, L.; et al. Overexpression of exosomal cardioprotective miRNAs mitigates hypoxia-Induced H9c2 cells apoptosis. Int. J. Mol. Sci. 2017, 18, 711. [Google Scholar] [CrossRef]
- Qiao, L.; Hu, S.; Liu, S.; Zhang, H.; Ma, H.; Huang, K.; Li, Z.; Su, T.; Vandergriff, A.; Tang, J.; et al. microRNA-21-5p dysregulation in exosomes derived from heart failure patients impairs regenerative potential. J. Clin. Investg. 2019, 129, 2237–2250. [Google Scholar] [CrossRef]
- Luther, K.M.; Haar, L.; McGuinness, M.; Wang, Y.; Lynch, I.V.T.L.; Phan, A.; Song, Y.; Shen, Z.; Gardner, G.; Kuffel, G.; et al. Exosomal miR-21a-5p mediates cardioprotection by mesenchymal stem cells. J. Mol. Cell Cardiol. 2018, 119, 125–137. [Google Scholar] [CrossRef] [PubMed]
- Julovi, S.M.; Sanganeria, B.; Minhas, N.; Ghimire, K.; Nankivell, B.; Rogers, N.M. Blocking thrombospondin-1 signaling via CD47 mitigates renal interstitial fibrosis. Lab. Investg. 2020, 100, 1184–1196. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Chen, K.; Gao, L.; Zheng, Y.; Yang, Y.-G. Thrombospondin-1 signaling through CD47 inhibits cell cycle progression and induces senescence in endothelial cells. Cell Death Dis. 2016, 7, e2368. [Google Scholar] [CrossRef] [PubMed]
- Mikuła-Pietrasik, J.; Sosińska, P.; Janus, J.; Rubiś, B.; Brewińska-Olchowik, M.; Piwocka, K.; Książek, K. Bystander senescence in human peritoneal mesothelium and fibroblasts is related to thrombospondin-1-dependent activation of transforming growth factor-β1. Int. J. Biochem. Cell Biol. 2013, 45, 2087–2096. [Google Scholar] [CrossRef] [PubMed]
- Rogers, N.M.; Zhang, Z.J.; Wang, J.-J.; Thomson, A.W.; Isenberg, J.S. CD47 regulates renal tubular epithelial cell self-renewal and proliferation following renal ischemia reperfusion. Kidney Int. 2016, 90, 334–347. [Google Scholar] [CrossRef] [PubMed]
- Meijles, D.N.; Sahoo, S.; Al Ghouleh, I.; Amaral, J.H.; Bienes-Martinez, R.; Knupp, H.E.; Attaran, S.; Sembrat, J.C.; Nouraie, S.M.; Rojas, M.M.; et al. The matricellular protein TSP1 promotes human and mouse endothelial cell senescence through CD47 and Nox1. Sci. Signal. 2017, 10, 1784–1799. [Google Scholar] [CrossRef]
- Taraboletti, G.; Roberts, D.; Liotta, L.A.; Giavazzi, R. Platelet thrombospondin modulates endothelial cell adhesion, motility, and growth: A potential angiogenesis regulatory factor. J. Cell Biol. 1990, 111, 765–772. [Google Scholar] [CrossRef]
- Good, D.J.; Polverini, P.J.; Rastinejad, F.; Le Beau, M.M.; Lemons, R.S.; Frazier, W.A.; Bouck, N.P. A tumor suppressor-dependent inhibitor of angiogenesis is immunologically and functionally indistinguishable from a fragment of thrombospondin. Proc. Natl. Acad. Sci. USA 1990, 87, 6624–6628. [Google Scholar] [CrossRef]
- Bagavandoss, P.; Wilks, J. Specific inhibition of endothelial cell proliferation by thrombospondin. Biochem. Biophys. Res. Commun. 1990, 170, 867–872. [Google Scholar] [CrossRef]
- Tie, L.; Chen, L.-Y.; Chen, D.-D.; Xie, H.-H.; Channon, K.M.; Chen, A.F. GTP cyclohydrolase I prevents diabetic-impaired endothelial progenitor cells and wound healing by suppressing oxidative stress/thrombospondin-1. Am. J. Physiol. Endocrinol. Metab. 2014, 306, E1120–E1131. [Google Scholar] [CrossRef]
- Qin, Q.; Qian, J.; Ge, L.; Shen, L.; Jia, J.; Jin, J.; Ge, J. Effect and mechanism of thrombospondin-1 on the angiogenesis potential in human endothelial progenitor cells: An in vitro study. PLoS One 2014, 9, e88213. [Google Scholar] [CrossRef]
- Kaur, S.; Soto-Pantoja, D.R.; Stein, E.V.; Liu, C.; Elkahloun, A.G.; Pendrak, M.L.; Nicolae, A.; Singh, S.P.; Nie, Z.; Levens, D.; et al. Thrombospondin-1 signaling through CD47 inhibits self-renewal by regulating c-Myc and other stem cell transcription factors. Sci. Rep. 2013, 3, 1673. [Google Scholar] [CrossRef]
- Ligi, I.; Simoncini, S.; Tellier, E.; Vassallo, P.F.; Sabatier, F.; Guillet, B.; Lamy, E.; Sarlon, G.; Quemener, C.; Bikfalvi, A.; et al. A switch toward angiostatic gene expression impairs the angiogenic properties of endothelial progenitor cells in low birth weight preterm infants. Blood 2011, 118, 1699–1709. [Google Scholar] [CrossRef]


| Week | Mon | Tue | Wed | Thur | Fri |
|---|---|---|---|---|---|
| 1 | 15 × 20 | 15 × 20 | 15 × 20 | 15 × 20 | 15 × 20 |
| 2 | 16 × 30 | 16 × 30 | 16 × 30 | 16 × 30 | 16 × 30 |
| 3 | 17 × 40 | 17 × 40 | 17 × 40 | 17 × 40 | 17 × 40 |
| 4 | 18 × 50 | 18 × 50 | 18 × 50 | 18 × 50 | 18 × 50 |
| 5 | 20 × 50 | 20 × 50 | 20 × 50 | 20 × 50 | 20 × 50 |
| 6 | 20 × 50 | 20 × 50 | 20 × 50 | 20 × 50 | 20 × 50 |
| 7 | 22 × 50 | 22 × 50 | 22 × 50 | 22 × 50 | 22 × 50 |
| 8 | 22 × 50 | 22 × 50 | 22 × 50 | 22 × 50 | 22 × 50 |
| Gene | Sequences (5′-3′) |
|---|---|
| rno-miR-21-5p mimic | Forward: UAGCUUAUCAGACUGAUGUUGA |
| Reverse: AACAUCAGUCUGAUAAGCUAUU | |
| rno-mimic NC | Forward: UUCUCCGAACGUGUCACGUTT |
| Reverse: ACGUGACACGUUCGGAGAATT | |
| TSP-1-rat-589 | Forward: GCAUCUUCACAAGGGAUUUT |
| Reverse: AAAUCCCUUGUGAAGAUGCTT | |
| TSP-1-rat-1951 | Forward: CCAACAAACAGGUGUGCAATT |
| Reverse: UUGCACACCUGUUUGUUGGTT | |
| TSP-1-rat-3317 | Forward: GCAUGACCCUCGUCACAUUTT |
| Reverse: AAUGUGACGAGGGUCAUGCTT | |
| siRNA control | Forward: UUCUCCGAACGUGUCACGUTT |
| Reverse: ACGUGACACGUUCGGAGAATT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Xie, K.; Sun, X.; Zhang, C.; He, H. The Mechanism of miR-21-5p/TSP-1-Mediating Exercise on the Function of Endothelial Progenitor Cells in Aged Rats. Int. J. Environ. Res. Public Health 2023, 20, 1255. https://doi.org/10.3390/ijerph20021255
Chen X, Xie K, Sun X, Zhang C, He H. The Mechanism of miR-21-5p/TSP-1-Mediating Exercise on the Function of Endothelial Progenitor Cells in Aged Rats. International Journal of Environmental Research and Public Health. 2023; 20(2):1255. https://doi.org/10.3390/ijerph20021255
Chicago/Turabian StyleChen, Xiaoke, Kejia Xie, Xinzheng Sun, Chengzhu Zhang, and Hui He. 2023. "The Mechanism of miR-21-5p/TSP-1-Mediating Exercise on the Function of Endothelial Progenitor Cells in Aged Rats" International Journal of Environmental Research and Public Health 20, no. 2: 1255. https://doi.org/10.3390/ijerph20021255
APA StyleChen, X., Xie, K., Sun, X., Zhang, C., & He, H. (2023). The Mechanism of miR-21-5p/TSP-1-Mediating Exercise on the Function of Endothelial Progenitor Cells in Aged Rats. International Journal of Environmental Research and Public Health, 20(2), 1255. https://doi.org/10.3390/ijerph20021255







