Test–Retest Reliability of the Functional Electromechanical Dynamometer for Squat Exercise
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Study Design
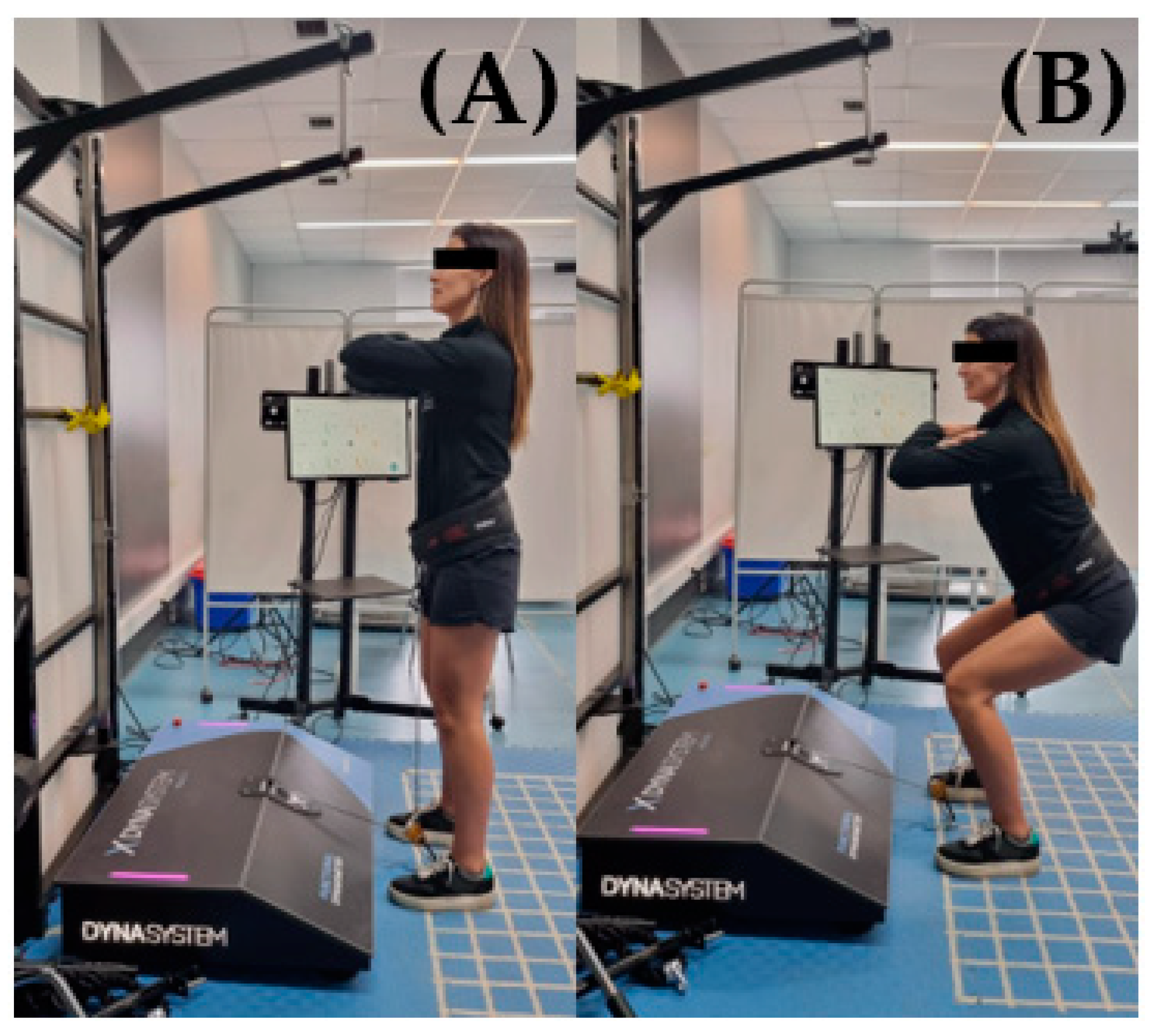
2.3. Materials
2.4. Familiarization Protocol and 1RM Determination
2.5. Evaluation Protocol
2.6. Statistical Analysis
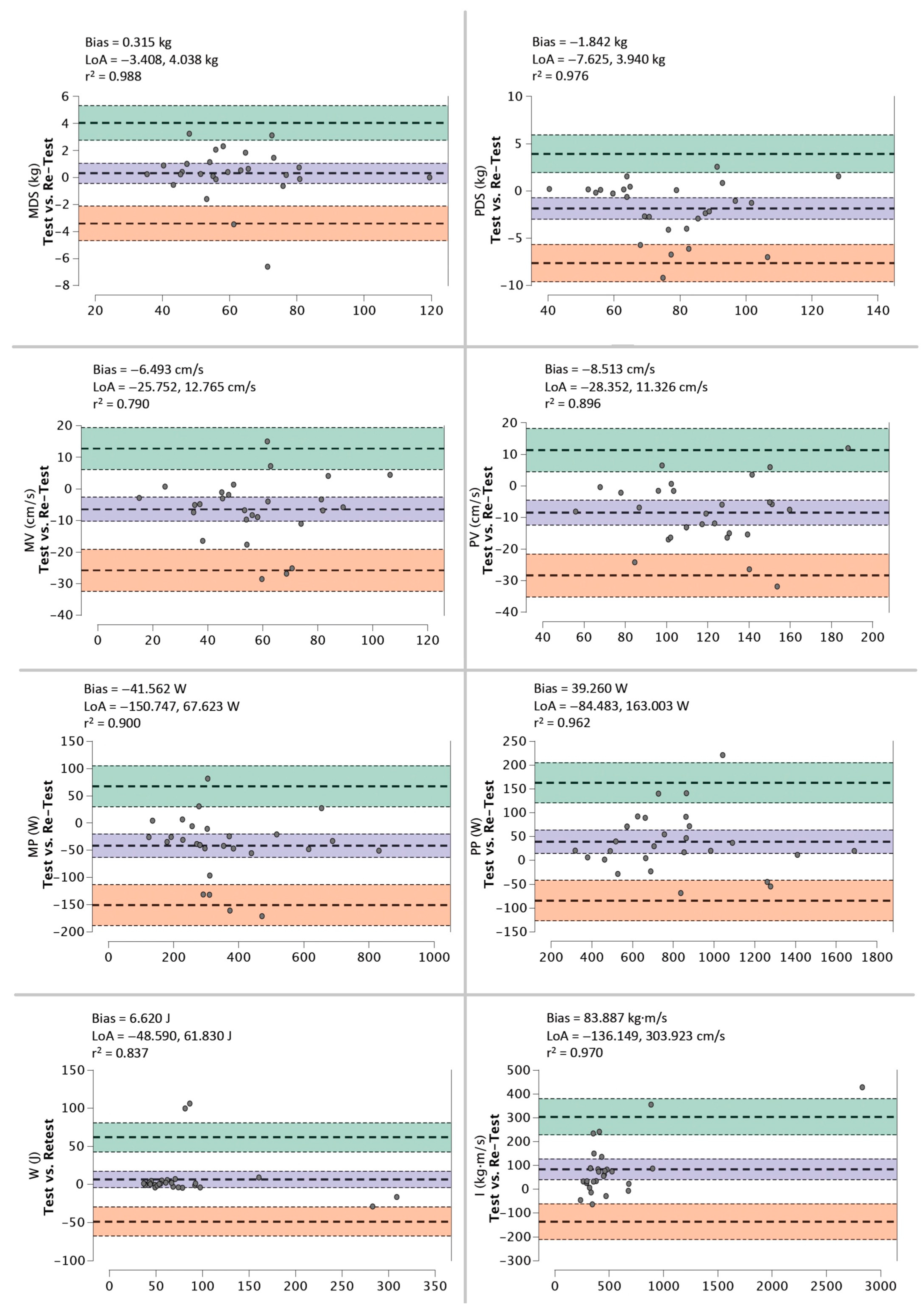
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lombardi, V.P. Beginning Weight Training: The Safe and Effective Way; WCB/McGraw-Hill: New York, NY, USA, 1989; ISBN 0697054969. [Google Scholar]
- Comfort, P.; Kasim, P. Optimizing squat technique. Strength Cond. J. 2007, 29, 10. [Google Scholar] [CrossRef]
- Schlegel, P.; Fialová, D. Deep squat–Should we be afraid? Stud. Sport. 2021, 15, 26–33. [Google Scholar] [CrossRef]
- Argaud, S.; Pairot de Fontenay, B.; Blache, Y.; Monteil, K. Does aging cause joint mechanics redistribution during squat jumping? Comput. Methods Biomech. Biomed. Engin. 2020, 23, S12–S14. [Google Scholar] [CrossRef]
- Soares, B.A.; Lacerda, A.C.R.; Nonato, L.F.; Corrêa, F.G.; da Silva Lage, V.K.; Camargos, A.C.R.; Mendonça, V.A.; Leite, H.R. Cardiopulmonary responses during dynamic squatting exercise with and without whole-body vibration in adolescents. Rev. Pesqui. Em. Fisioter. 2021, 11, 190–197. [Google Scholar] [CrossRef]
- Fanchamps, M.H.J.; Horemans, H.L.D.; Ribbers, G.M.; Stam, H.J.; Bussmann, J.B.J. The accuracy of the detection of body postures and movements using a physical activity monitor in people after a stroke. Sensors 2018, 18, 2167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Endo, Y.; Miura, M.; Sakamoto, M. The Relationship between the deep squat movement and the hip, knee and ankle range of motion and muscle strength. J. Phys. Ther. Sci. 2020, 32, 391–394. [Google Scholar] [CrossRef]
- Bosquet, L.; Porta-Benache, J.; Blais, J. Validity of a commercial linear encoder to estimate bench press 1 RM from the force-velocity relationship. J. Sport. Sci. Med. 2010, 9, 459–463. [Google Scholar]
- Caserotti, P.; Aagaard, P.; Buttrup Larsen, J.; Puggaard, L. Explosive heavy-resistance training in old and very old adults: Changes in rapid muscle force, strength and power. Scand. J. Med. Sci. Sport. 2008, 18, 773–782. [Google Scholar] [CrossRef]
- García-Ramos, A.; Janicijevic, D.; González-Hernández, J.M.; Keogh, J.W.L.; Weakley, J. Reliability of the velocity achieved during the last repetition of sets to failure and its association with the velocity of the 1-repetition maximum. PeerJ 2020, 8, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Son, S.M.; Kang, K.W.; Lee, N.K.; Nam, S.H.; Kwon, J.W.; Kim, K. Influence of isokinetic strength training of unilateral ankle on ipsilateral one-legged standing balance of adults. J. Phys. Ther. Sci. 2013, 25, 1313–1315. [Google Scholar] [CrossRef] [Green Version]
- Dvir, Z.; Müller, S. Multiple-joint isokinetic dynamometry: A critical review. J. Strength Cond. Res. 2020, 34, 587–601. [Google Scholar] [CrossRef] [PubMed]
- Kemmler, W.; Teschler, M.; Weißenfels, A.; Bebenek, M.; Fröhlich, M.; Kohl, M.; von Stengel, S. Effects of whole-body electromyostimulation versus high-intensity resistance exercise on body composition and strength: A randomized controlled study. Evid. Based Complement. Altern. Med. 2016, 2016, 9236809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baur, H.; Müller, S.; Hirschmüller, A.; Huber, G.; Mayer, F. Reactivity, stability, and strength performance capacity in motor sports. Br. J. Sport. Med. 2006, 40, 906–911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cale’-Benzoor, M.; Dickstein, R.; Arnon, M.; Ayalon, M. Strength enhancement with limited range closed kinetic chain isokinetic exercise of the upper extremity. Isokinet. Exerc. Sci. 2014, 22, 37–46. [Google Scholar] [CrossRef]
- Feiring, D.C.; Ellenbecker, T.S.; Derscheid, G.L. Test-retest reliability of the biodex isokinetic dynamometer. J. Orthop. Sport. Phys. Ther. 1990, 11, 298–300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- LaStayo, P.C.; Marcus, R.L.; Dibble, L.E.; Smith, S.B.; Beck, S.L. Eccentric exercise versus usual-care with older cancer survivors: The impact on muscle and mobility-an exploratory pilot study. BMC Geriatr. 2011, 11, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Bily, W.; Franz, C.; Trimmel, L.; Loefler, S.; Cvecka, J.; Zampieri, S.; Kasche, W.; Sarabon, N.; Zenz, P.; Kern, H. Effects of leg-press training with moderate vibration on muscle strength, pain, and function after total knee arthroplasty: A randomized controlled trial. Arch. Phys. Med. Rehabil. 2016, 97, 857–865. [Google Scholar] [CrossRef]
- Chamorro, C.; de La Fuente, C.; Jerez, D.; Campos, C.; Chirosa, L.J. Reliability of shoulder rotators isometric strength test using a novel pulley electromechanical dynamometer. influence of the assessment position. Asian J. Sport. Med. 2018, 9, 1–9. [Google Scholar] [CrossRef]
- Jerez-Mayorga, D.; Huerta-Ojeda, Á.; Chirosa-Ríos, L.J.; Guede-Rojas, F.; Guzmán-Guzmán, I.P.; Intelangelo, L.; Miranda-Fuentes, C.; Delgado-Floody, P. Test–retest reliability of functional electromechanical dynamometer on five sit-to-stand measures in healthy young adults. Int. J. Environ. Res. Public Health 2021, 18, 6829. [Google Scholar] [CrossRef]
- Müller, S.; Baur, H.; König, T.; Hirschmüller, A.; Mayer, F. Reproducibility of isokinetic single-and multi-joint strength measurements in healthy and injured athletes. Isokinet. Exerc. Sci. 2007, 15, 295–302. [Google Scholar] [CrossRef]
- Baena-Raya, A.; Díez-Fernández, D.M.; García-Ramos, A.; Soriano-Maldonado, A.; Rodríguez-Pérez, M.A. concurrent validity and reliability of a functional electromechanical dynamometer to assess isometric mid-thigh pull performance. Proc. Inst. Mech. Eng. P J. Sport. Eng. Technol. 2021, 1–8. [Google Scholar] [CrossRef]
- Rodriguez-Perea, A.; Jerez-Mayorga, D.; García-Ramos, A.; Martínez-García, D.; Chirosa Rios, L.J. Reliability and concurrent validity of a functional electromechanical dynamometer device for the assessment of movement velocity. Proc. Inst. Mech. Eng. Part P J. Sport. Eng. Technol. 2021, 235, 176–181. [Google Scholar] [CrossRef]
- Reyes-Ferrada, W.; Barboza-Gonzalez, P.; Jerez-Mayorga, D.; Chirosa-Ríos, L.; Chirosa-Ríos, I.; Martínez-García, D.; Ulloa-Díaz, D.; RodríGuez-Perea, A. A new reliable device to assess trunk extensors strength. Acta Bioeng. Biomech. 2022, 24, 49–57. [Google Scholar] [CrossRef]
- Sánchez-Sánchez, A.J.; Chirosa-Ríos, L.J.; Chirosa-Ríos, I.J.; García-Vega, A.J.; Jerez-Mayorga, D. Test-retest reliability of a functional electromechanical dynamometer on swing eccentric hamstring exercise measures in soccer players. PeerJ 2021, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Garcia, D.; Rodriguez-Perea, A.; Barboza, P.; Ulloa-Díaz, D.; Jerez-Mayorga, D.; Chirosa, I.; Ríos, L.J.C. reliability of a standing isokinetic shoulder rotators strength test using a functional electromechanical dynamometer: Effects of velocity. PeerJ 2020, 8, e9951. [Google Scholar] [CrossRef]
- Çetin, O.; Akyildiz, Z.; Demirtaş, B.; Sungur, Y.; Clemente, F.M.; Cazan, F.; Ardigò, L.P. Reliability and validity of the multi-point method and the 2-point method’s variations of estimating the one-repetition maximum for deadlift and back squat exercises. PeerJ 2022, 10, e13013. [Google Scholar] [CrossRef]
- Pérez-Castilla, A.; Janicijevic, D.; Akyildiz, Z.; Senturk, D.; García-Ramos, A. Assessment of back-squat performance at submaximal loads: Is the reliability affected by the variable, exercise technique, or repetition criterion? Int. J. Environ. Res. Public Health 2021, 18, 4626. [Google Scholar] [CrossRef]
- Association, W.M. World medical association declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar]
- Hopkins, W.G. Spreadsheets for analysis of validity and reliability. Sportscience 2017, 21, 36–44. [Google Scholar]
- Weir, J.P. Quantifying test-retest reliability using the intraclass correlation coefficient and the SEM. J. Strength Cond. Res. 2005, 19, 231–240. [Google Scholar]
- Boehringer, S.; Whyte, D.G. Validity and test-retest reliability of the 1080 quantum system for bench press exercise. J. Strength Cond. Res. 2019, 33, 3242–3251. [Google Scholar] [CrossRef] [PubMed]
- Whinton, A.K.; Thompson, K.M.A.; Power, G.A.; Burr, J.F. Testing a novel isokinetic dynamometer constructed using a 1080 quantum. PLoS ONE 2018, 13, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reyes-Ferrada, W.; Rodríguez-Perea, Á.; Chirosa-Ríos, L.; Martínez-García, D.; Jerez-Mayorga, D. Muscle quality and functional and conventional ratios of trunk strength in young healthy subjects: A pilot study. Int. J. Environ. Res. Public Health 2022, 19, 12673. [Google Scholar] [CrossRef] [PubMed]
- Morrow Jr, J.R.; Jackson, A.W. How “significant” is your reliability? Res. Q. Exerc. Sport 1993, 64, 352–355. [Google Scholar] [CrossRef] [PubMed]
- Cerda Vega, E.; Jerez-Mayorga, D.; Machado Payer, R.; Campos Jara, C.; Guzman-Guzman, I.; Reyes Ponce, A.; Chirosa, L.J. Validity and reliability of evaluating hip abductor strength using different normalization methods in a functional electromechanical device. PLoS ONE 2018, 13, 1–12. [Google Scholar] [CrossRef]
- Campbell, M.J.; Machin, D. Medical Statistics: A Commonsense Approach; John Wiley & Sons: Hoboken, NJ, USA, 1999; ISBN 0471987212. [Google Scholar]
- Buckthorpe, M.W.; Hannah, R.; Pain, T.G.; Folland, J.P. Reliability of neuromuscular measurements during explosive isometric contractions, with special reference to electromyography normalization techniques. Muscle Nerve 2012, 46, 566–576. [Google Scholar] [CrossRef]
- Campos Jara, C.A.; Bautista, I.; Chirosa, L.; Martin, I.; López, A.; Chirosa, I. validity and reliability of the haefni health system 1.0 device in the measurement of the isokinetic velocity range. CPD 2014, 14, 91–98. [Google Scholar] [CrossRef]
- Chamorro, C.; la Fuente, C.; Rubio, J.; Campos, C.; Chirosa, L.J. Absolute Reliability and Concurrent Validity of a Novel Electromechanical Pulley Dynamometer for Measuring Shoulder Rotation Isometric Strength in Asymptomatic Subjects. Study Conducted at Pontificia Universidad Católica, Santiago, Chile. J. Pak. Med. Assoc. 2019, 69, 1000–1005. [Google Scholar]




| Mean ± SD | p-Value | ES | ICC | SEM | CV (%) | |||
|---|---|---|---|---|---|---|---|---|
| Serie 1 | Serie 2 | (95% CI) | (95% CI) | (95% CI) | (95% CI) | |||
| ROM (cm) | 30 reps. 50% 1RM | 38.4 ± 6.0 | 38.4 ± 6.3 | 0.068 | 0.00 (−0.74, 0.74) | 0.95 (0.89, 0.98) | 0.34 (0.30, 0.39) | 3.72 (3.72, 5.07) |
| MDS (kg) | 41.4 ± 11.4 | 40.8 ± 11.4 | 0.006 | 0.06 (−0.80, 0.69) | 1.00 (1.00, 1.00) | 0.05 (0.04, 0.06) | 1.09 (0.86, 1.48) | |
| PDS (kg) | 53.6 ± 15.6 | 55.1 ± 15.8 | 0.001 | 0.09 (−0.65, 0.83) | 1.00 (0.99, 1.00) | 0.08 (0.07, 0.11) | 1.97 (1.46, 3.05) | |
| MV (cm/s) | 58.2 ± 21.1 | 64.0 ± 22.2 | 0.001 | 0.26 (−0.47, 1.01) | 0.95 (0.90, 0.98) | 0.23 (0.20, 0.26) | 7.55 (5.97, 10.28) | |
| PV (cm/s) | 118.7 ± 29.7 | 125.8 ± 30.1 | 0.000 | 0.24 (−0.50, 0.98) | 0.96 (0.90, 0.98) | 0.26 (0.21, 0.32) | 5.09 (3.76, 7.88) | |
| MP (W) | 250.2 ± 124.5 | 270.5 ± 123.5 | 0.005 | 0.16 (−0.58, 0.91) | 0.97 (0.92, 0.99) | 0.17 (0.14, 0.22) | 8.36 (6.18, 12.94) | |
| PP (W) | 545.5 ± 237.5 | 575.7 ± 222.2 | 0.000 | 0.13 (−0.61, 0.87) | 0.98 (0.95, 0.99) | 0.19 (0.16, 0.24) | 6.18 (4.57, 9.57) | |
| W (J) | 63.4 ± 61.6 | 49.1 ± 45.5 | 0.429 | 0.26 (−1.00, 0.48) | 1.00 (1.00, 1.00) | 0.21 (0.17, 0.26) | 4.69 (3.47, 7.27) | |
| I (kg·m/s) | 353.4 ± 341.3 | 313.1 ± 292.7 | 0.005 | 0.13 (−0.87, 0.62) | 0.99 (0.99, 1.00) | 0.09 (0.08, 0.11) | 8.48 (6.70, 11.54) | |
| Mean ± SD | p-Value | ES | ICC | SEM | CV (%) | |||
|---|---|---|---|---|---|---|---|---|
| Serie 1 | Serie 2 | (95% CI) | (95% CI) | (95% CI) | (95% CI) | |||
| ROM (cm) | 12 reps. 75% 1RM | 38.1 ± 6.5 | 37.7 ± 6.9 | 0.025 | 0.07 (−0.81, 0.68) | 0.95 (0.90, 0.98) | 0.19 (0.16, 0.22) | 3.90 (3.09, 5.31) |
| MDS (kg) | 61.0 ± 21.3 | 60.5 ± 21.5 | 0.796 | 0.03 (−0.76, 0.72) | 1.00 (1.00, 1.00) | 0.02 (0.02, 0.03) | 0.52 (0.38, 0.81) | |
| PDS (kg) | 72.1 ± 21.2 | 73.3 ± 21.1 | 0.138 | 0.05 (−0.69, 0.79) | 1.00 (0.99, 1.00) | 0.07 (0.06, 0.08) | 1.49 (1.09, 2.35) | |
| MV (cm/s) | 54.2 ± 21.5 | 60.7 ± 21.1 | 0.003 | 0.30 (−0.44, 1.05) | 0.95 (0.89, 0.98) | 0.25 (0.22, 0.30) | 8.97 (7.09, 12.20) | |
| PV (cm/s) | 114.2 ± 32.0 | 122.7 ± 31.2 | 0.001 | 0.27 (−0.48, 1.01) | 0.91 (0.82, 0.96) | 0.24 (0.21, 0.29) | 8.15 (6.44, 11.09) | |
| MP (W) | 335.1 ± 172.3 | 376.7 ± 176.1 | 0.002 | 0.23 (−0.50, 0.98) | 0.97 (0.93, 0.99) | 0.18 (0.15, 0.21) | 8.84 (6.99, 12.03) | |
| PP (W) | 757.7 ± 318.3 | 826.5 ± 322.6 | 0.001 | 0.21 (−0.53, 0.96) | 0.94 (0.88, 0.97) | 0.18 (0.16, 0.22) | 9.91 (7.83, 13.49) | |
| W (J) | 85.0 ± 63.8 | 78.3 ± 69.7 | 0.124 | 0.10 (−0.84, 0.64) | 1.00 (0.99, 1.00) | 0.13 (0.12, 0.16) | 4.14 (3.27, 5.63) | |
| I (kg·m/s) | 564.2 ± 524.2 | 480.3 ± 447.1 | 0.067 | 0.17 (−0.91, 0.57) | 0.98 (0.96, 0.99) | 0.12 (0.11, 0.14) | 14.06 (11.13, 19.16) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
del-Cuerpo, I.; Jerez-Mayorga, D.; Delgado-Floody, P.; Morenas-Aguilar, M.D.; Chirosa-Ríos, L.J. Test–Retest Reliability of the Functional Electromechanical Dynamometer for Squat Exercise. Int. J. Environ. Res. Public Health 2023, 20, 1289. https://doi.org/10.3390/ijerph20021289
del-Cuerpo I, Jerez-Mayorga D, Delgado-Floody P, Morenas-Aguilar MD, Chirosa-Ríos LJ. Test–Retest Reliability of the Functional Electromechanical Dynamometer for Squat Exercise. International Journal of Environmental Research and Public Health. 2023; 20(2):1289. https://doi.org/10.3390/ijerph20021289
Chicago/Turabian Styledel-Cuerpo, Indya, Daniel Jerez-Mayorga, Pedro Delgado-Floody, María Dolores Morenas-Aguilar, and Luis Javier Chirosa-Ríos. 2023. "Test–Retest Reliability of the Functional Electromechanical Dynamometer for Squat Exercise" International Journal of Environmental Research and Public Health 20, no. 2: 1289. https://doi.org/10.3390/ijerph20021289








