Syphilis, the Great Imitator—Clinical and Dermoscopic Features of a Rare Presentation of Secondary Syphilis
Abstract
:1. Introduction
1.1. Sexually Transmitted Infections
1.2. Syphilis
1.2.1. Epidemiology
1.2.2. Pathogenesis
1.2.3. Clinical Presentation
1.2.4. Diagnosis
1.2.5. Differential Diagnosis
1.2.6. Treatment
1.3. Dermoscopy
Dermoscopy of Syphilis
1.4. Aim of the Study
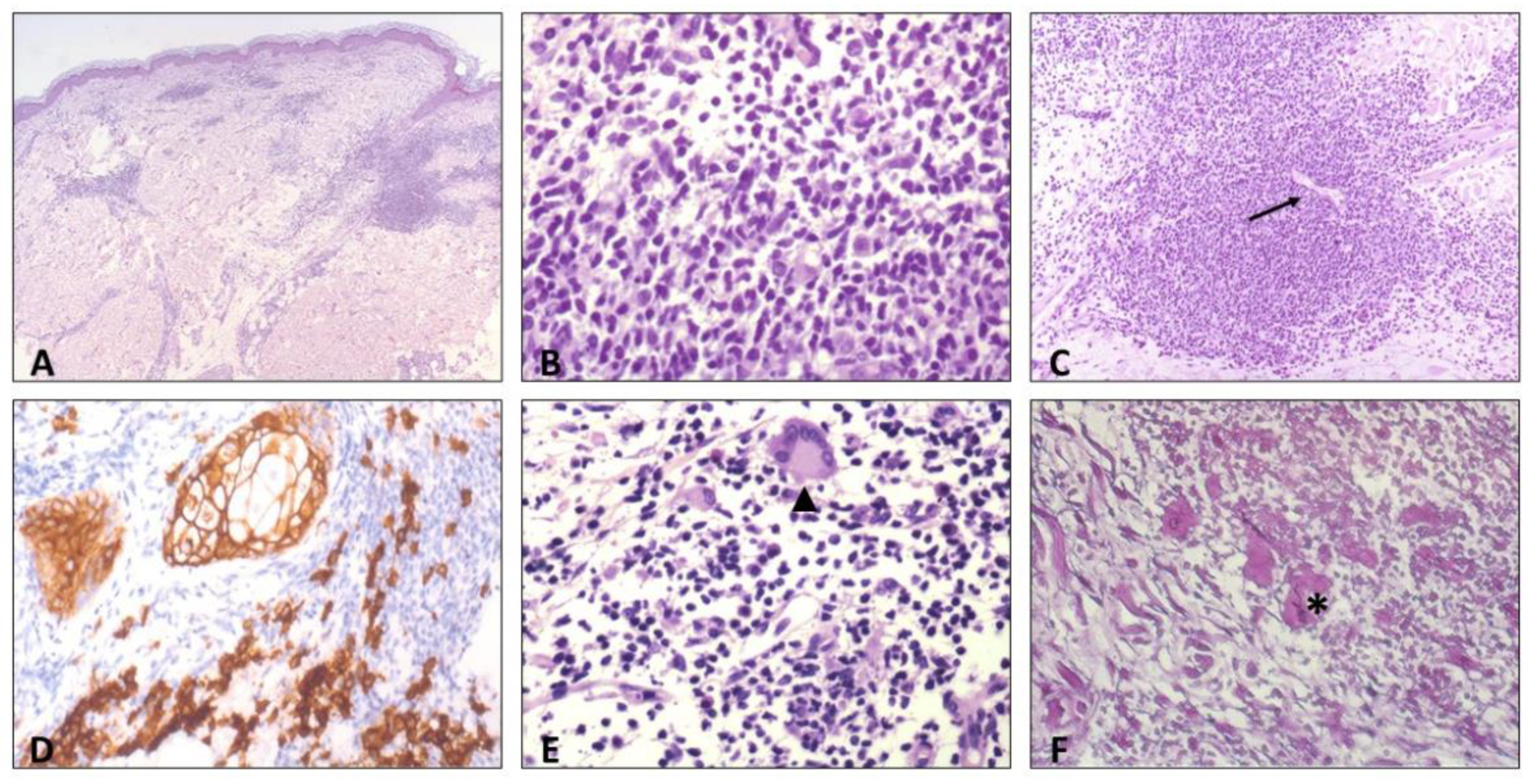
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- De Schryver, A.; Meheus, A. Epidemiology of sexually transmitted diseases: The global picture. Bull. World Health Organ. 1990, 68, 639–654. [Google Scholar] [PubMed]
- Kassie, B.A.; Yenus, H.; Berhe, R.; Kassahun, E.A. Prevalence of sexually transmitted infections and associated factors among the University of Gondar students, Northwest Ethiopia: A cross-sectional study. Reprod. Health 2019, 16, 163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wagenlehner, F.M.; Brockmeyer, N.H.; Discher, T.; Friese, K.; Wichelhaus, T.A. The Presentation, Diagnosis, and Treatment of Sexually Transmitted Infections. Dtsch. Arztebl. Int. 2016, 113, 11–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brunham, R.C.; Gottlieb, S.L.; Paavonen, J. Pelvic inflammatory disease. N. Engl. J. Med. 2015, 372, 2039–2048. [Google Scholar] [CrossRef]
- Olusola, P.; Banerjee, H.N.; Philley, J.V.; Dasgupta, S. Human Papilloma Virus-Associated Cervical Cancer and Health Disparities. Cells 2019, 8, 622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keat, A. Sexually transmitted arthritis syndromes. Med. Clin. N. Am. 1990, 74, 1617–1631. [Google Scholar] [CrossRef] [PubMed]
- Sampath, A.; Maduro, G.; Schillinger, J.A. Infant Deaths Due To Herpes Simplex Virus, Congenital Syphilis, and HIV in New York City. Pediatrics 2016, 137, e20152387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baggaley, R.F.; Owen, B.N.; Silhol, R.; Elmes, J.; Anton, P.; McGowan, I.; van der Straten, A.; Shacklett, B.; Dang, Q.; Swann, E.M.; et al. Does per-act HIV-1 transmission risk through anal sex vary by gender? An updated systematic review and meta-analysis. Am. J. Reprod. Immunol. 2018, 80, e13039. [Google Scholar] [CrossRef]
- Baggaley, R.F.; White, R.G.; Boily, M.C. HIV transmission risk through anal intercourse: Systematic review, meta-analysis and implications for HIV prevention. Int. J. Epidemiol. 2010, 39, 1048–1063. [Google Scholar] [CrossRef] [Green Version]
- Cohen, M.S.; Council, O.D.; Chen, J.S. Sexually transmitted infections and HIV in the era of antiretroviral treatment and prevention: The biologic basis for epidemiologic synergy. J. Int. AIDS Soc. 2019, 22, e25355. [Google Scholar] [CrossRef]
- Forhan, S.E.; Gottlieb, S.L.; Sternberg, M.R.; Xu, F.; Datta, S.D.; McQuillan, G.M.; Berman, S.M.; Markowitz, L.E. Prevalence of sexually transmitted infections among female adolescents aged 14 to 19 in the United States. Pediatrics 2009, 124, 1505–1512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Workowski, K.A.; Bolan, G.A. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm. Rep. 2015, 64, 1–137. [Google Scholar] [PubMed]
- Herbst, J.H.; Jacobs, E.D.; Finlayson, T.J.; McKleroy, V.S.; Neumann, M.S.; Crepaz, N. Estimating HIV prevalence and risk behaviors of transgender persons in the United States: A systematic review. AIDS Behav. 2008, 12, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Volk, J.E.; Marcus, J.L.; Phengrasamy, T.; Blechinger, D.; Nguyen, D.P.; Follansbee, S.; Hare, C.B. No New HIV Infections With Increasing Use of HIV Preexposure Prophylaxis in a Clinical Practice Setting. Clin. Infect. Dis. 2015, 61, 1601–1603. [Google Scholar] [CrossRef] [PubMed]
- Mayer, K.H.; de Vries, H. HIV and sexually transmitted infections: Responding to the “newest normal”. J. Int. AIDS Soc. 2018, 21, e25164. [Google Scholar] [CrossRef] [PubMed]
- Lőrincz, K.; Meznerics, F.A.; Jobbágy, A.; Kiss, N.; Madarász, M.; Belvon, L.; Tóth, B.; Tamási, B.; Wikonkál, N.M.; Marschalkó, M.; et al. STIs during the COVID-19 Pandemic in Hungary: Gonorrhea as a Potential Indicator of Sexual Behavior. Int. J. Environ. Res Public Health 2022, 19, 9627. [Google Scholar] [CrossRef]
- Mäki-Koivisto, V.; Sinikumpu, S.P.; Jokelainen, J.; Aho-Laukkanen, E.; Junttila, I.S.; Huilaja, L. Impact of COVID-19 Pandemic on the Incidence of Sexually Transmitted Infections in Northern Finland in 2019 to 2022. Acta Dermatol. Venereol. 2022, 102, adv00795. [Google Scholar] [CrossRef]
- Balestri, R.; Magnano, M.; Rizzoli, L.; Infusino, S.D.; Urbani, F.; Rech, G. STIs and the COVID-19 pandemic: The lockdown does not stop sexual infections. J. Eur. Acad. Dermatol. Venereol. 2020, 34, e766–e768. [Google Scholar] [CrossRef]
- Hook, E.W., 3rd. Syphilis. Lancet 2017, 389, 1550–1557. [Google Scholar] [CrossRef]
- Leichliter, J.S.; Aral, S.O. Scientific and Programmatic Gaps in STD Prevention: A 2020 Assessment. Sex. Transm. Dis. 2021, 48, S88–S89. [Google Scholar] [CrossRef]
- Jobbágy, A.; Kiss, N.; Meznerics, F.A.; Farkas, K.; Plázár, D.; Bozsányi, S.; Fésűs, L.; Bartha, Á.; Szabó, E.; Lőrincz, K.; et al. Emergency Use and Efficacy of an Asynchronous Teledermatology System as a Novel Tool for Early Diagnosis of Skin Cancer during the First Wave of COVID-19 Pandemic. Int. J. Environ. Res Public Health 2022, 19, 2699. [Google Scholar] [CrossRef] [PubMed]
- Bhargava, S.; McKeever, C.; Kroumpouzos, G. Impact of COVID-19 pandemic on dermatology practices: Results of a web-based, global survey. Int. J. Womens Dermatol. 2021, 7, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Moscarella, E.; Pasquali, P.; Cinotti, E.; Tognetti, L.; Argenziano, G.; Rubegni, P. A survey on teledermatology use and doctors’ perception in times of COVID-19. J. Eur. Acad. Dermatol. Venereol. 2020, 34, e772–e773. [Google Scholar] [CrossRef]
- Sexually Transmitted Disease Surveillance 2020; US Department of Health and Human Services: Atlanta, GA, USA, 2022.
- Cohen, S.E.; Chew Ng, R.A.; Katz, K.A.; Bernstein, K.T.; Samuel, M.C.; Kerndt, P.R.; Bolan, G. Repeat syphilis among men who have sex with men in California, 2002-2006: Implications for syphilis elimination efforts. Am. J. Public Health 2012, 102, e1–e8. [Google Scholar] [CrossRef] [PubMed]
- Forrestel, A.K.; Kovarik, C.L.; Katz, K.A. Sexually acquired syphilis: Historical aspects, microbiology, epidemiology, and clinical manifestations. J. Am. Acad. Dermatol. 2020, 82, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Landovitz, R.J.; Tseng, C.H.; Weissman, M.; Haymer, M.; Mendenhall, B.; Rogers, K.; Veniegas, R.; Gorbach, P.M.; Reback, C.J.; Shoptaw, S. Epidemiology, sexual risk behavior, and HIV prevention practices of men who have sex with men using GRINDR in Los Angeles, California. J. Urban Health 2013, 90, 729–739. [Google Scholar] [CrossRef] [Green Version]
- Wong, W.; Chaw, J.K.; Kent, C.K.; Klausner, J.D. Risk factors for early syphilis among gay and bisexual men seen in an STD clinic: San Francisco, 2002–2003. Sex. Transm. Dis. 2005, 32, 458–463. [Google Scholar] [CrossRef] [Green Version]
- Radolf, J.D.; Deka, R.K.; Anand, A.; Šmajs, D.; Norgard, M.V.; Yang, X.F. Treponema pallidum, the syphilis spirochete: Making a living as a stealth pathogen. Nat. Rev. Microbiol. 2016, 14, 744–759. [Google Scholar] [CrossRef] [Green Version]
- Fraser, C.M.; Norris, S.J.; Weinstock, G.M.; White, O.; Sutton, G.G.; Dodson, R.; Gwinn, M.; Hickey, E.K.; Clayton, R.; Ketchum, K.A.; et al. Complete genome sequence of Treponema pallidum, the syphilis spirochete. Science 1998, 281, 375–388. [Google Scholar] [CrossRef]
- Forrestel, A.K.; Kovarik, C.L.; Katz, K.A. Sexually acquired syphilis: Laboratory diagnosis, management, and prevention. J. Am. Acad. Dermatol. 2020, 82, 17–28. [Google Scholar] [CrossRef]
- Peeling, R.W.; Mabey, D.; Kamb, M.L.; Chen, X.S.; Radolf, J.D.; Benzaken, A.S. Syphilis. Nat. Rev. Dis. Prim. 2017, 3, 17073. [Google Scholar] [CrossRef]
- Singh, A.E.; Romanowski, B. Syphilis: Review with emphasis on clinical, epidemiologic, and some biologic features. Clin. Microbiol. Rev. 1999, 12, 187–209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dourmishev, L.A.; Dourmishev, A.L. Syphilis: Uncommon presentations in adults. Clin. Dermatol. 2005, 23, 555–564. [Google Scholar] [CrossRef]
- Balagula, Y.; Mattei, P.L.; Wisco, O.J.; Erdag, G.; Chien, A.L. The great imitator revisited: The spectrum of atypical cutaneous manifestations of secondary syphilis. Int. J. Dermatol. 2014, 53, 1434–1441. [Google Scholar] [CrossRef] [PubMed]
- Pournaras, C.C.; Masouye, I.; Piletta, P.; Piguet, V.; Saurat, J.H.; French, L.E. Extensive annular verrucous late secondary syphilis. Br. J. Dermatol. 2005, 152, 1343–1345. [Google Scholar] [CrossRef]
- Battistella, M.; Le Cleach, L.; Lacert, A.; Perrin, P. Extensive nodular secondary syphilis with prozone phenomenon. Arch. Dermatol. 2008, 144, 1078–1079. [Google Scholar] [CrossRef]
- Noppakun, N.; Dinehart, S.M.; Solomon, A.R. Pustular secondary syphilis. Int. J. Dermatol. 1987, 26, 112–114. [Google Scholar] [CrossRef]
- Tham, S.N.; Ng, S.K. Secondary syphilis with framboesiform lesions. Sex. Transm. Infect. 1990, 66, 99–100. [Google Scholar] [CrossRef]
- Haslund, A. Syphilis maligna. Arch. Dermatol. Syph. 1897, 38, 345–392. [Google Scholar] [CrossRef] [Green Version]
- Neisser, A. Malignant syphilis. Br. J. Dermatol. 1897, 9, 11–26. [Google Scholar]
- Larsen, S.A.; Steiner, B.M.; Rudolph, A.H. Laboratory diagnosis and interpretation of tests for syphilis. Clin. Microbiol. Rev. 1995, 8, 1–21. [Google Scholar] [CrossRef]
- Satyaputra, F.; Hendry, S.; Braddick, M.; Sivabalan, P.; Norton, R. The Laboratory Diagnosis of Syphilis. J. Clin. Microbiol. 2021, 59, e0010021. [Google Scholar] [CrossRef]
- Park, I.U.; Fakile, Y.F.; Chow, J.M.; Gustafson, K.J.; Jost, H.; Schapiro, J.M.; Novak-Weekley, S.; Tran, A.; Nomura, J.H.; Chen, V.; et al. Performance of Treponemal Tests for the Diagnosis of Syphilis. Clin. Infect. Dis. 2019, 68, 913–918. [Google Scholar] [CrossRef] [Green Version]
- Janier, M.; Unemo, M.; Dupin, N.; Tiplica, G.S.; Potočnik, M.; Patel, R. 2020 European guideline on the management of syphilis. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 574–588. [Google Scholar] [CrossRef]
- Engelkens, H.J.; ten Kate, F.J.; Vuzevski, V.D.; van der Sluis, J.J.; Stolz, E. Primary and secondary syphilis: A histopathological study. Int. J. STD AIDS 1991, 2, 280–284. [Google Scholar] [CrossRef]
- Alessi, E.; Innocenti, M.; Ragusa, G. Secondary syphilis. Clinical morphology and histopathology. Am. J. Dermatol. 1983, 5, 11–17. [Google Scholar] [CrossRef]
- Jordaan, H.F. Secondary syphilis. A clinicopathological study. Am. J. Dermatol. 1988, 10, 399–409. [Google Scholar]
- Jeerapaet, P.; Ackerman, A.B. Histologic patterns of secondary syphilis. Arch. Dermatol. 1973, 107, 373–377. [Google Scholar] [CrossRef]
- Hoang, M.P.; High, W.A.; Molberg, K.H. Secondary syphilis: A histologic and immunohistochemical evaluation. J. Cutan. Pathol. 2004, 31, 595–599. [Google Scholar] [CrossRef]
- Domantay-Apostol, G.P.; Handog, E.B.; Gabriel, M.T. Syphilis: The international challenge of the great imitator. Dermatol. Clin. 2008, 26, 191–202. [Google Scholar] [CrossRef]
- Dylewski, J.; Duong, M. The rash of secondary syphilis. Cmaj 2007, 176, 33–35. [Google Scholar] [CrossRef] [Green Version]
- Tognetti, L.; Sbano, P.; Fimiani, M.; Rubegni, P. Dermoscopy of Biett’s sign and differential diagnosis with annular maculo-papular rashes with scaling. Indian J. Dermatol. Venereol. Leprol. 2017, 83, 270–273. [Google Scholar] [CrossRef]
- Clement, M.E.; Okeke, N.L.; Hicks, C.B. Treatment of syphilis: A systematic review. Jama 2014, 312, 1905–1917. [Google Scholar] [CrossRef]
- Workowski, K.A.; Berman, S. Sexually transmitted diseases treatment guidelines, 2010. MMWR Recomm. Rep. 2010, 59, 1–110. [Google Scholar]
- Yélamos, O.; Braun, R.P.; Liopyris, K.; Wolner, Z.J.; Kerl, K.; Gerami, P.; Marghoob, A.A. Dermoscopy and dermatopathology correlates of cutaneous neoplasms. J. Am. Acad. Dermatol. 2019, 80, 341–363. [Google Scholar] [CrossRef]
- Friedman, R.J.; Rigel, D.S.; Kopf, A.W. Early detection of malignant melanoma: The role of physician examination and self-examination of the skin. CA Cancer J. Clin. 1985, 35, 130–151. [Google Scholar] [CrossRef]
- Whited, J.D.; Grichnik, J.M. The rational clinical examination. Does this patient have a mole or a melanoma? Jama 1998, 279, 696–701. [Google Scholar] [CrossRef]
- Zalaudek, I.; Argenziano, G.; Soyer, H.P.; Corona, R.; Sera, F.; Blum, A.; Braun, R.P.; Cabo, H.; Ferrara, G.; Kopf, A.W.; et al. Three-point checklist of dermoscopy: An open internet study. Br. J. Dermatol. 2006, 154, 431–437. [Google Scholar] [CrossRef]
- Errichetti, E. Dermoscopy of Inflammatory Dermatoses (Inflammoscopy): An Up-to-Date Overview. Dermatol. Pract. Concept. 2019, 9, 169–180. [Google Scholar] [CrossRef] [Green Version]
- Errichetti, E.; Stinco, G. Dermoscopy in General Dermatology: A Practical Overview. Dermatol. Ther. 2016, 6, 471–507. [Google Scholar] [CrossRef] [Green Version]
- Errichetti, E.; Zalaudek, I.; Kittler, H.; Apalla, Z.; Argenziano, G.; Bakos, R.; Blum, A.; Braun, R.P.; Ioannides, D.; Lacarrubba, F.; et al. Standardization of dermoscopic terminology and basic dermoscopic parameters to evaluate in general dermatology (non-neoplastic dermatoses): An expert consensus on behalf of the International Dermoscopy Society. Br. J. Dermatol. 2020, 182, 454–467. [Google Scholar] [CrossRef] [PubMed]
- Lallas, A.; Giacomel, J.; Argenziano, G.; García-García, B.; González-Fernández, D.; Zalaudek, I.; Vázquez-López, F. Dermoscopy in general dermatology: Practical tips for the clinician. Br. J. Dermatol. 2014, 170, 514–526. [Google Scholar] [CrossRef] [PubMed]
- Lallas, A.; Zalaudek, I.; Argenziano, G.; Longo, C.; Moscarella, E.; Di Lernia, V.; Al Jalbout, S.; Apalla, Z. Dermoscopy in general dermatology. Dermatol. Clin. 2013, 31, 679–694. [Google Scholar] [CrossRef] [PubMed]
- Micali, G.; Lacarrubba, F.; Massimino, D.; Schwartz, R.A. Dermatoscopy: Alternative uses in daily clinical practice. J. Am. Acad. Dermatol. 2011, 64, 1135–1146. [Google Scholar] [CrossRef] [PubMed]
- Mathur, M.; Acharya, P.; Karki, A.; Shah, J.; Kc, N. Dermoscopic clues in the skin lesions of secondary syphilis. Clin. Case Rep. 2019, 7, 431–434. [Google Scholar] [CrossRef] [Green Version]
- Errichetti, E.; Stinco, G. Dermoscopy in differentiating palmar syphiloderm from palmar papular psoriasis. Int. J. STD AIDS 2017, 28, 1461–1463. [Google Scholar] [CrossRef]
- Li, F.G.; Huang, W.B.; Chen, H.S.; Wang, T.; Fan, Y.M. Clinicopathological, dermoscopic, and ultrastructural observation of annular secondary syphilis on the penis. Int. J. STD AIDS 2020, 31, 699–701. [Google Scholar] [CrossRef]
- Yuylana, C.; Iswanty, M.; Paturusi, I.I. Secondary syphilis mimicking tinea cruris in an HIV infected patient: A case report. Pan. Afr. Med. J. 2021, 38, 133. [Google Scholar] [CrossRef]
- Schettini, A.P.; Sardinha, J.C.; Marques, E.A.; Massone, C. Psoriasiform syphilis: A challenge. Dermatol. Rep. 2021, 13, 8830. [Google Scholar] [CrossRef]
- Miyachi, H.; Taniguchi, T.; Matsue, H. Syphilis imitating urticarial vasculitis. Cmaj 2019, 191, E1384. [Google Scholar] [CrossRef] [Green Version]
- Gianfaldoni, S.; Tchernev, G.; Wollina, U.; Gianfaldoni, R.; Lotti, T. Secondary Syphilis Presenting As Palmoplantar Psoriasis. Open Access Maced. J. Med. Sci. 2017, 5, 445–447. [Google Scholar] [CrossRef] [Green Version]
- Ramoni, S.; Boneschi, V.; Cusini, M. Syphilis as "the great imitator": A case of impetiginoid syphiloderm. Int. J. Dermatol. 2016, 55, e162–e163. [Google Scholar] [CrossRef]
- Genovese, G.; Nazzaro, G.; Coggi, A.; Gianotti, R.; Ramoni, S.; Cusini, M. Secondary syphilis masquerading as lupus vulgaris in an HIV-infected patient: A diagnosis suggested by histology. Int. J. STD AIDS 2018, 29, 1454–1456. [Google Scholar] [CrossRef]
- Nazzaro, G.; Boneschi, V.; Coggi, A.; Gianotti, R. Syphilis with a lichen planus-like pattern (hypertrophic syphilis). J. Cutan. Pathol. 2012, 39, 805–807. [Google Scholar] [CrossRef]
- Baughn, R.E.; Musher, D.M. Secondary syphilitic lesions. Clin. Microbiol. Rev. 2005, 18, 205–216. [Google Scholar] [CrossRef] [Green Version]
- Ashwini, P.K.; George, A.T.; Betkerur, J. Cluster of Flowers—The Unseen of Syphilis. Indian Dermatol. Online J. 2020, 11, 291–293. [Google Scholar] [CrossRef]
- Velasco-Tamariz, V.; Rodríguez-Peralto, J.L.; Ortiz-Romero, P. Corymbiform Lesions in a Young Healthy Man. JAMA Dermatol. 2017, 153, 1317–1318. [Google Scholar] [CrossRef]
- Knöpfel, N.; Del Pozo, L.J.; Díaz, I. Solitary Annular Plaque on the Scrotum. Am. J. Dermatol. 2016, 38, 930–931. [Google Scholar] [CrossRef]
- Wu, J.; Chen, H.; Long, F.Q. A solitary annular plaque on the jaw: Atypical presentation of secondary syphilis. Chin. Med. J. 2020, 133, 1383–1384. [Google Scholar] [CrossRef]
- Stone, C.E.; Onyekaba, N.A.; Lucas, M.; Jukic, D. Cutaneous Secondary Syphilis Resembling Non-Melanoma Skin Cancer. Cureus 2020, 12, e10774. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cantisani, C.; Rega, F.; Ambrosio, L.; Grieco, T.; Kiss, N.; Meznerics, F.A.; Bánvölgyi, A.; Vespasiani, G.; Arienzo, F.; Rossi, G.; et al. Syphilis, the Great Imitator—Clinical and Dermoscopic Features of a Rare Presentation of Secondary Syphilis. Int. J. Environ. Res. Public Health 2023, 20, 1339. https://doi.org/10.3390/ijerph20021339
Cantisani C, Rega F, Ambrosio L, Grieco T, Kiss N, Meznerics FA, Bánvölgyi A, Vespasiani G, Arienzo F, Rossi G, et al. Syphilis, the Great Imitator—Clinical and Dermoscopic Features of a Rare Presentation of Secondary Syphilis. International Journal of Environmental Research and Public Health. 2023; 20(2):1339. https://doi.org/10.3390/ijerph20021339
Chicago/Turabian StyleCantisani, Carmen, Federica Rega, Luca Ambrosio, Teresa Grieco, Norbert Kiss, Fanni Adél Meznerics, András Bánvölgyi, Giordano Vespasiani, Francesca Arienzo, Giovanni Rossi, and et al. 2023. "Syphilis, the Great Imitator—Clinical and Dermoscopic Features of a Rare Presentation of Secondary Syphilis" International Journal of Environmental Research and Public Health 20, no. 2: 1339. https://doi.org/10.3390/ijerph20021339
APA StyleCantisani, C., Rega, F., Ambrosio, L., Grieco, T., Kiss, N., Meznerics, F. A., Bánvölgyi, A., Vespasiani, G., Arienzo, F., Rossi, G., Soda, G., & Pellacani, G. (2023). Syphilis, the Great Imitator—Clinical and Dermoscopic Features of a Rare Presentation of Secondary Syphilis. International Journal of Environmental Research and Public Health, 20(2), 1339. https://doi.org/10.3390/ijerph20021339









