Risk Factors Associated with Multimorbidity among Children Aged Under-Five Years in Sub-Saharan African Countries: A Scoping Review
Abstract
1. Introduction
The Aim of the Scoping Review
2. Materials and Methods
2.1. Design
2.2. Identification of the Research Questions
- (i)
- What types of statistical analyses are employed in the existing evidence to determine the risk factors associated with multimorbidity among children under five?
- (ii)
- What are the various childhood disease combinations that formed the multimorbidity structures examined in the eligible studies?
- (iii)
- What are the multiple overlaps in the risk factors across the selected studies?
- (iv)
- What are the possible gaps in knowledge identified from the selected literature?
2.3. Eligibility Criteria
2.3.1. Inclusion Criteria
- (i)
- Population (P): The studies included male and female children under five years of age who resided in any Sub-Saharan Africa (SSA) country. The review also includes studies involving adults (or/and above five years children) and under five years children, provided data for under five were reported separately from any other age-groups.
- (ii)
- Intervention (I): Studies that focused on predictors or risk factors or determinants of multimorbidity among under five or preschool children in SSA that covered both individual and contextual exposures using classical frequentist statistical regression methods only.
- (iii)
- Comparator (C): The presence of two or more diseases versus no diseases was the focus of the comparators in the studies.
- (iv)
- Outcomes (O): Studies that involved two or more childhood diseases that were evaluated jointly rather than independently such that the outcomes reflect the interactions of the diseases.
- (v)
- Timing (T): The publication period for the article is between 1 January 1990 and 19 March 2022, to capture recent publications.
- (vi)
- Settings/Design (S): Observational studies, such as cross-sectional and longitudinal studies that focused on risk factors as exposures.
2.3.2. Exclusion Criteria
- (i)
- Studies that involved older children, but no separate data involving under five years were reported.
- (ii)
- Studies that do not meet the definition of ‘multimorbidity’ as the “cooccurrence of two or diseases among children under five years without reference to an index disease”.
- (iii)
- Studies not written in the English language.
2.3.3. Steps Involved in the Inclusion and Exclusion Processes
- If the study is multimorbidity, then
- Are children aged under five years the unit of analysis? then
- Is the country of analysis from SSA? then
- Does the study utilise a nationally representative survey in data collection?
- Does the study utilise classical frequentist statistical regression methods in the analysis?
2.4. Identify the Relevant Sources of Evidence
2.4.1. Information Sources
2.4.2. Search Strategy
2.4.3. Selection Process
2.4.4. Data Charting Management
3. Results
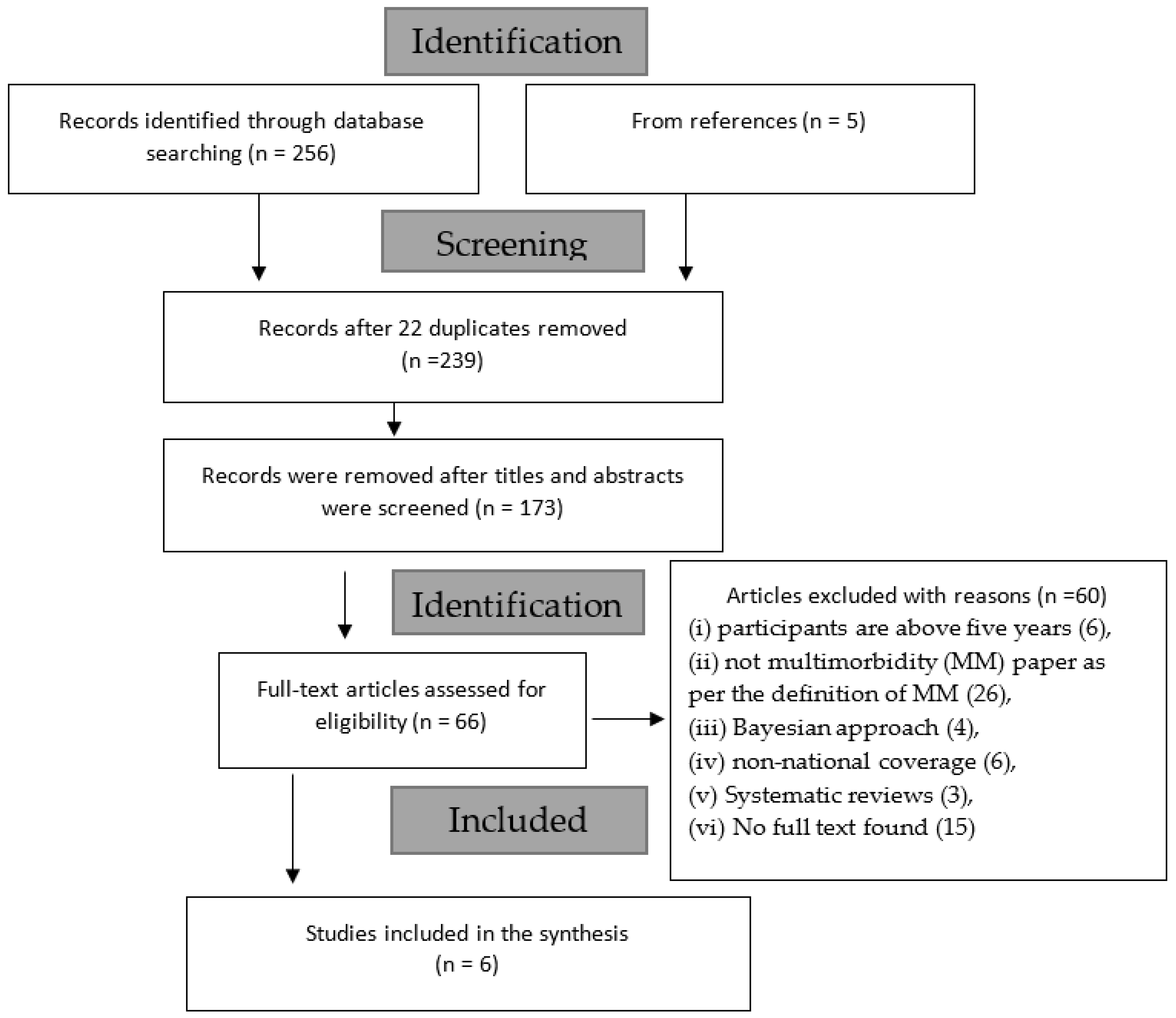
3.1. Selection of Sources of Evidence
3.2. Characteristics of Sources of Evidence
3.3. Study Characteristics
Distribution of the Extracted Risk Factors of Multimorbidity
3.4. Child-Related Characteristics
3.4.1. Child’s Age
3.4.2. Child’s Sex
3.5. Parental- and Household-Related Characteristics
3.6. Community- and Area-Related Characteristics
3.7. Risk Factors with Directions by Authors
3.7.1. Significant Effects-Related Characteristics by Authors
3.7.2. No Significant Effects-Related Characteristics by Authors
3.8. Distribution of Common Risk Factors across the Studies
4. Discussion
4.1. Some Identified Study Gaps
4.2. Strength and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Nguyen, P.H.; Scott, S.; Avula, R.; Tran, L.M.; Menon, P. Trends and drivers of change in the prevalence of anaemia among 1 million women and children in India, 2006 to 2016. BMJ Glob. Health 2018, 3, e001010. [Google Scholar] [CrossRef] [PubMed]
- Bramley, D.; Moody, D. NHS England Multimorbidity—The Biggest Clinical Challenge Facing the NHS? Available online: https://www.england.nhs.uk/blog/dawn-moody-david-bramley/ (accessed on 20 August 2020).
- McGeorge, S. Morbidity—Comorbidity and Multimorbidity. What Do They Mean? Available online: https://www.bgs.org.uk/resources/morbidity-comorbidity-and-multimorbidity-what-do-they-mean (accessed on 20 August 2020).
- Ferro, M.A.; Lipman, E.L.; Van Lieshout, R.J.; Gorter, J.W.; Shanahan, L.; Boyle, M.; Georgiades, K.; Timmons, B. Multimorbidity in Children and Youth Across the Life-course (MY LIFE): Protocol of a Canadian prospective study. BMJ Open 2019, 9, e034544. [Google Scholar] [CrossRef] [PubMed]
- Eyowas, F.A.; Schneider, M.; Yirdaw, B.A.; Getahun, F.A. Multimorbidity of chronic non-communicable diseases and its models of care in low- and middle-income countries: A scoping review protocol. BMJ Open 2019, 9, e033320. [Google Scholar] [CrossRef] [PubMed]
- Khatab, K.; Kandala, N.-B. Latent variable modelling of risk factors associated with childhood diseases: Case study for Nigeria. Asian Pac. J. Trop. Dis. 2011, 1, 169–176. [Google Scholar] [CrossRef]
- Pathirana, T.I.; Jackson, C.A. Socioeconomic status and multimorbidity: A systematic review and meta-analysis. Aust. N. Z. J. Public Health 2018, 42, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Arksey, H.; O’Malley, L. Scoping studies: Towards a methodological framework. Int. J. Soc. Res. Methodol. 2005, 8, 19–32. [Google Scholar] [CrossRef]
- Levac, D.; Colquhoun, H.; O’Brien, K.K. Scoping studies: Advancing the methodology. Implement. Sci. 2010, 5, 69. [Google Scholar] [CrossRef] [PubMed]
- FDA Media. Using the PICOTS Framework to Strengthen Evidence Gathered in Clinical Trials—Guidance from the AHRQ’s Evidence-Based Practice Centers Program. Available online: https://www.fda.gov/media/109448/download (accessed on 29 July 2020).
- Maphosa, T.P.; Mulqueeny, D.M.; Osei, E.; Kuupiel, D.; Mashamba-Thompson, T.P. Mapping evidence on malnutrition screening tools for children under 5 years in sub-Saharan Africa: A scoping review protocol. Syst. Rev. 2020, 9, 52. [Google Scholar] [CrossRef] [PubMed]
- Larissa Shamseer, D.M. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: Elaboration and explanation. BMJ 2015, 349, g7647. [Google Scholar] [CrossRef] [PubMed]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Adedokun, S.T. Correlates of childhood morbidity in Nigeria: Evidence from ordinal analysis of cross-sectional data. PLoS ONE 2020, 15, e0233259. [Google Scholar] [CrossRef] [PubMed]
- Atsu, B.K.; Guure, C.; Laar, A.K. Determinants of overweight with concurrent stunting among Ghanaian children. BMC Pediatr. 2017, 17, 177. [Google Scholar] [CrossRef] [PubMed]
- Duah, H.O.; Amankwa, C.E.; Adomako, I.; Owusu, B.; Agbadi, P. Comorbid patterns of anaemia and diarrhoea among children aged under 5 years in Ghana: A multivariate complex sample logistic regression analysis and spatial mapping visualisation. Int. Health 2020, 13, 562–572. [Google Scholar] [CrossRef] [PubMed]
- Mulatya, D.M.; Mutuku, F.W. Assessing Comorbidity of Diarrhea and Acute Respiratory Infections in Children Under 5 Years: Evidence From Kenya’s Demographic Health Survey 2014. J. Prim. Care Community Health 2020, 11, 2150132720925190. [Google Scholar] [CrossRef] [PubMed]
- Geda, N.R.; Feng, C.X.; Henry, C.J.; Lepnurm, R.; Janzen, B.; Whiting, S.J. Multiple anthropometric and nutritional deficiencies in young children in Ethiopia: A multi-level analysis based on a nationally representative data. BMC Pediatr. 2021, 21, 11. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.D.; Biggs, B.-A.; Holton, S.; Nguyen, H.T.M.; Hanieh, S.; Fisher, J. Co-morbid anaemia and stunting among children of pre-school age in low- and middle-income countries: A syndemic. Public Health Nutr. 2019, 22, 35–43. [Google Scholar] [CrossRef] [PubMed]
- CDC. What Is Sickle Cell Disease? Available online: https://www.cdc.gov/ncbddd/sicklecell/facts.html (accessed on 26 March 2022).
| S/N | Terms and Keywords | Results |
|---|---|---|
| 1 | Sub-Saharan Africa OR SSA OR low-and middle-income countries | 37,452 |
| 2 | Socioeconomic OR demographic OR contextual OR environmental OR community OR determinants OR risk factor OR predictor OR Association | 3,298,141 |
| 3 | comorbidity OR comorbidity OR multimorbidity OR multimorbidity OR multiple chronic conditions OR multi-diseases | 451,419 |
| 4 | Logistic regression OR multilevel regression OR multinomial logistic OR random-effects OR hierarchical OR fixed effects OR mixed-effects | 55,708 |
| 5 | 1 AND 2 AND 3 AND 4 | 12 |
| 6 | Limit 6 to human and English language and infant <to one year> OR preschool child <1 to 6 years> | |
| Limit to last 30 years (1990 to 2020) | 12 |
| Authors & Dates | Title | Objectives | Outcome Variables (Prevalence) | Sample Size (Participant’s Age) | Methods of Analysis | Country/Survey |
|---|---|---|---|---|---|---|
| Adedokun 2020 [14] | Correlates of childhood morbidity in Nigeria: Evidence from ordinal analysis of cross-sectional data | Correlates of childhood morbidity in Nigeria: Evidence from ordinal analysis of cross-sectional data | Diarrhoea, malaria and Pneumonia (9.0%) | 27,571 (Under-5) | Generalised ordinal logistic regression mode | Nigeria/ Nigeria Demographic and Health Survey (NDHS 2013) |
| Atsu et al. 2017 [15] | Determinants of overweight with concurrent stunting among Ghanaian children | This paper presents the burden, the individual-level, and contextual determinants of overweight with concurrent stunting among Ghanaian children. | overweight with concurrent stunting (1.2%) | 7550 (0–5 years) | A multivariable Poison regression model | Ghana/ Multiple Indicator Cluster Survey (MICS 2011) |
| Duah et al. 2020 [16] | Comorbid patterns of anaemia and diarrhoea among children aged under five years in Ghana: a multivariate complex sample logistic regression analysis and spatial mapping visualisation | To investigate the prevalence and independent predictors of comorbid patterns of anaemia and diarrhoea in children aged < 5 years in Ghana. | Anaemia and Diarrhoea (9.28%) | 2343 (under-5 years) | multivariate logistic regression | Ghana/ Ghana Demographic and Health Survey (GDHS 2014). |
| Mulatya & Mutuku 2014 [17] | Assessing Comorbidity of Diarrheal and Acute Respiratory Infections in Children Under 5 Years: Evidence from Kenya’s Demographic Health Survey 2014. J. Prim. Care Community Health | This study seeks to assess the prevalence of comorbidity of pneumonia and diarrheal in children under-5 years, and to identify risk factors associated with comorbidity of pneumonia and diarrheal in children | Acute respiratory infection (ARI) and Diarrhoea (2.2%) | 18,702 (under-5 years) | Multivariate logistic regression | Kenya/ Kenya Demographic Health Survey (KDHS 2014) |
| Geda et al. 2021 [18] | Multiple anthropometric and nutritional deficiencies in young children in Ethiopia: a multilevel analysis based on a nationally representative data | To examine the risk factors of cooccurrence of undernutrition and anaemia among children of age 6–59 months in Ethiopia based on nationally representative data | Anaemia and concurrent stunting (24.8%) | 9218 (6–59 months) | Mixed effect logistic regression | Ethiopia/ Ethiopian Demographic and Health Survey (EDHS 2016) |
| Tran et al. 2019 [19] | Comorbid anaemia and stunting among children of preschool age in low- and middle-income countries: a syndemic | To determine the prevalence of comorbidity of anaemia and stunting, among children aged 6–59 months in low- and middle-income countries | Anaemia and concurrent stunting (21.5%) | 193,065 (6–59 months) | Multinomial logistic models | Multi-countries Demographic and Health Surveys (DHS 2005–2015) |
| Child-Related Variables | |
|---|---|
| Child’s age | Protective effects <1 year (ref); 3 years and above, aOR = 0.43 (0.34–0.55) [14]. 0–11 months (ref), 12–23 months, aPR = 0.991 (0.982–0.999) [15]. 0.5–1 year (ref), 1–2 years, aRRR = 0.59 (0.55–0.64), 2–3 years, aRRR = 0.87 (0.80–0.94) [19] ‡. Harmful effects 24–59 months (ref), 6–23 months, OR = 2.17 (1.42 to 3.33) [16]. <6 months (ref), 6–11 months, aOR = 3.48 (2.02–5.99), 24–35 months, aOR = 2.84 (1.71–4.70) [17]. 0–23 months (ref), 24–35 months, aOR = 6.55 (5.26–8.15), 36–59 months, aOR = 4.29 (3.43–5.36) [18]. 0.5–1 year (ref), 3–4 years, aRRR = 1.27 (1.18–1.37). 4–5 years, aRRR = 1.86 (1.72–2.01) [19]. |
| Child’s sex | Protective effects Male (ref), Female, aOR = 0.84 (0.74–0.93) [18]. Female (ref), Male, aRRR = 0.78 (0.75–0.81) [19]. Harmful effects Female (ref), Male, OR = 1.50 (1.04 to 2.16) [16]. |
| Child’s birth size | Protective effects Large (ref) Average size at birth, aOR = 0.68 (0.57–0.82) [14]. |
| Diarrheal status | Harmful effects No (ref), Had diarrhoea (Yes), aPR = 1.019 (1.006–1.032) [15]. |
| Fever status | Harmful effects No (ref), Had fever (Yes), OR = 4.37 (2.94 to 6.50) [16]. |
| Vaccination status | Protective effects No (ref), Ever been vaccinated, aPR = 0.997 (0.960–0.995) [15]. |
| Breastfeeding status | Protective effects No (ref), Ever been breastfed, aPR = 0.995 (0.984–1.006) [15]. |
| Parental-related variables | |
| Maternal education status | Protective effects No education (ref), Secondary education and above, aOR = 0.64 (0.48–0.86) [18]. Secondary or higher (ref), Primary education, aRRR = 0.43 (0.41–0.46) No formal education, aRRR = 0.20 (0.19–0.21) [19]. Harmful effects No education (ref), Incomplete primary education, aOR = 1.66 (1.11–2.50) [17]. Primary education, aOR = 1.29 (1.13–1.46), secondary/higher, aOR = 1.42 (1.23–1.65) [14] |
| Paternal education status | Protective effects No formal education (ref), Secondary or higher education, OR = 0.57 (0.33 to 0.97) [16]. No education (ref), Secondary or higher education, aOR = 0.81 (0.65–1.00) [18]. |
| Caregiver’s age | Protective effects 15–19 years (ref), 30–34 years, aOR = 0.49 (0.28–0.85), 40–44 years, aOR = 0.47 (0.23–0.95) [17]. |
| Maternal exposure to media | Protective effects Never exposed (ref), Exposed to media, aOR = 0.82 (0.67–0.99) [14]. |
| Household-related variables | |
| Wealth status | Protective effects Poorest (ref), Richer wealth households, aOR = 0.83 (0.70–0.99) [14]. Poorest (ref), Richer household, OR = 0.38 (0.16 to 0.89) [16]. Poorest (ref), Middle wealth quintile, aOR = 0.58 (0.39–0.85), Highest wealth quintile aOR = 0.43 (0.24–0.77) [17]. Poorer/poorest (ref), Middle wealth quintile, aOR = 0.73 (0.61–0.87) Richer/richest, aOR = 0.64 (0.54–0.75) [18]. Richest (ref), 4th wealth quintile, (Richer) aRRR = 0.71 (0.65–0.77), Middle wealth quintile, aRRR = 0.62 (0.57–0.68), 2nd quintile (poorer), aRRR = 0.55 (0.50–0.60), Poorest wealth quintile, aRRR = 0.49 (0.45–0.53) [19]. Harmful effects Poorest (ref), 4th wealth quintile, aPR = 1.011 (1.001–1.021) [15]. |
| Number of under-5 years | Harmful effects 0–1 (ref), Had two children aged < 5 years, OR = 1.80 (1.14 to 2.84) [16]. |
| Household size | Protective effects 1–5 (ref), Had ≥ 6 members, OR = 0.46 (0.28 to 0.75) [16]. |
| Ethnicity of household head | Harmful effects Akan (ref), Head is of the Ewe tribes, aPR = 1.023 (1.000–1.046) [15] |
| The religion of household head | Orthodox (ref), Religion (others, besides being Orthodox) aOR = 1.37 (1.17–1.61) [18]. |
| Sanitation | Median (ref) Sanitation score aOR = 1.12 (1.01–1.24) [18]. |
| Community-related variables | |
| Maternal education status | Protective effects Mean (ref), maternal education at cluster level, aOR = 0.94 (0.90–0.98) [18]. |
| State-related variables | |
| Region of residence | Harmful effects North-Central (ref), North-East, aOR = 5.34 (3.86–7.39) South-East, aOR = 3.17 (2.15–4.66) [14]. |
| Place of residence | Protective effects Urban (ref), Rural, aRRR = 0.72 (0.67–0.77) [19]. |
| Authors and Date | Harmful Effects (Increased Likelihood) | Protective Effects (Decreased Likelihood) | No Significant Effects |
|---|---|---|---|
| Adedokun. 2020 [14] | Child is from middle wealth households; Child’s region of residence is: North-East and South-East. | Child is 3 years and above, Mothers are exposed to media, Child’s average size at birth. | Mother’s age difference, Maternal education, being in the poorer, richer, and richest household, North-West, South-South, child’s age is 1–2 years, child is born small, Birth order, delivered in health facility, improved source of drinking water and cooking method. |
| Atsu et al. 2017 [15] | Ethnicity of household head is of the Ewe tribes, fourth wealth quintile, child is with diarrhoea, child ever been vaccinated. | Child’s age is 12–23 months, child ever been breastfed | Child’s age is 24–35, 36–47, 48–59 months; sex; religion of household head, maternal education status, wealth index is second, middle and richest, area of residence, mosquito net utilization malaria rapid test, child had cough |
| Duah et al. 2021 [16] | Child’s age between 6 and 23 months, Child is male gender, History of fever, and living in a household with two children aged < 5 years. | Father having secondary or higher education, Living in a household with ≥6 members, and Living in a richer household. | Number of children aged < 5 years; household quintile is poorer, middle, richest; improved source of drinking water, main floor material, locality of residence is rural, region of residence. |
| Mulatya & Mutuku 2014 [17] | Child’s age is 6–11 months, and caregivers have incomplete primary education. | High wealth quintile, and older caregivers. | Nutritional status of a child, sex, residence, exclusive breastfeeding between 0 and 6 months, and combined morbidity from diarrheal and ARI, caregivers had Primary complete, and secondary and above |
| Geda et al. 2021 [18] | Child’s age is 24–35 months, 36–59 months, religion (others), sanitation score | Child’s sex is female, child’s mother has secondary education and above, child’s father has secondary education and above, wealth index is middle, and richer/richest, and mean maternal education at cluster level. | Mother’s age, mother has primary education level, father has primary education level, child never breastfed, and diet diversity score. |
| Tran et al. 2019 [19] | The child’s age is 3–4 years, and 4–5 years | Child’s sex is boy; child’s age is 1–2 years, 2–3 years, place of residence is rural; child’s mother has primary education, has no formal education; household wealth index is 4th quintile (Richer), middle, 2nd quintile (poorer), and poorest. |
| Authors | Adedokun 2020 [14] | Atsu et al. 2017 [15] | Duah et al. 2020 [16] | Mulatya & Mutuku 2014 [17] | Geda et al. 2021 [18] | Tran et al. 2019 [19] | |
|---|---|---|---|---|---|---|---|
| Conditions studied | Pneumonia, diarrhoea, and malaria | overweight with concurrent stunting | Anaemia and Diarrhoea | Diarrheal and acute respiratory infection (ARI) | Concurrent stunting & anaemia | Concurrent stunting and anaemia | |
| Child-related variables | |||||||
| Child’s age | Significant | Significant | Significant | Significant | Significant | Significant | 6 |
| Child’s sex | Significant | Significant | Significant | 3 | |||
| Child’s birth size | Significant | 1 | |||||
| Diarrheal status | Significant | 1 | |||||
| Fever status | Significant | 1 | |||||
| Vaccination status | Significant | 1 | |||||
| Breastfeeding status | Significant | 1 | |||||
| Parental-related variables | |||||||
| Maternal education status | Significant | Significant | Significant | 3 | |||
| Paternal education status | Significant | Significant | 2 | ||||
| Caregiver’s age | Significant | 1 | |||||
| Maternal exposure to media | Significant | 1 | |||||
| Household-related variables | |||||||
| Wealth status | Significant | Significant | Significant | Significant | Significant | Significant | 6 |
| Number of under-5 years | Significant | 1 | |||||
| Household size | Significant | 1 | |||||
| Ethnicity of household head | Significant | 1 | |||||
| The religion of household head | Significant | 1 | |||||
| Sanitation | Significant | 1 | |||||
| Community-related variables | |||||||
| Maternal education status | Significant | 1 | |||||
| State-related variables | |||||||
| Region of residence | Significant | 1 | |||||
| Place of residence | Significant | 1 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Obasohan, P.E.; Walters, S.J.; Jacques, R.; Khatab, K. Risk Factors Associated with Multimorbidity among Children Aged Under-Five Years in Sub-Saharan African Countries: A Scoping Review. Int. J. Environ. Res. Public Health 2023, 20, 1377. https://doi.org/10.3390/ijerph20021377
Obasohan PE, Walters SJ, Jacques R, Khatab K. Risk Factors Associated with Multimorbidity among Children Aged Under-Five Years in Sub-Saharan African Countries: A Scoping Review. International Journal of Environmental Research and Public Health. 2023; 20(2):1377. https://doi.org/10.3390/ijerph20021377
Chicago/Turabian StyleObasohan, Phillips Edomwonyi, Stephen J. Walters, Richard Jacques, and Khaled Khatab. 2023. "Risk Factors Associated with Multimorbidity among Children Aged Under-Five Years in Sub-Saharan African Countries: A Scoping Review" International Journal of Environmental Research and Public Health 20, no. 2: 1377. https://doi.org/10.3390/ijerph20021377
APA StyleObasohan, P. E., Walters, S. J., Jacques, R., & Khatab, K. (2023). Risk Factors Associated with Multimorbidity among Children Aged Under-Five Years in Sub-Saharan African Countries: A Scoping Review. International Journal of Environmental Research and Public Health, 20(2), 1377. https://doi.org/10.3390/ijerph20021377







