Enhancing the Biological Oxidation of H2S in a Sewer Pipe with Highly Conductive Concrete and Electricity-Producing Bacteria
Abstract
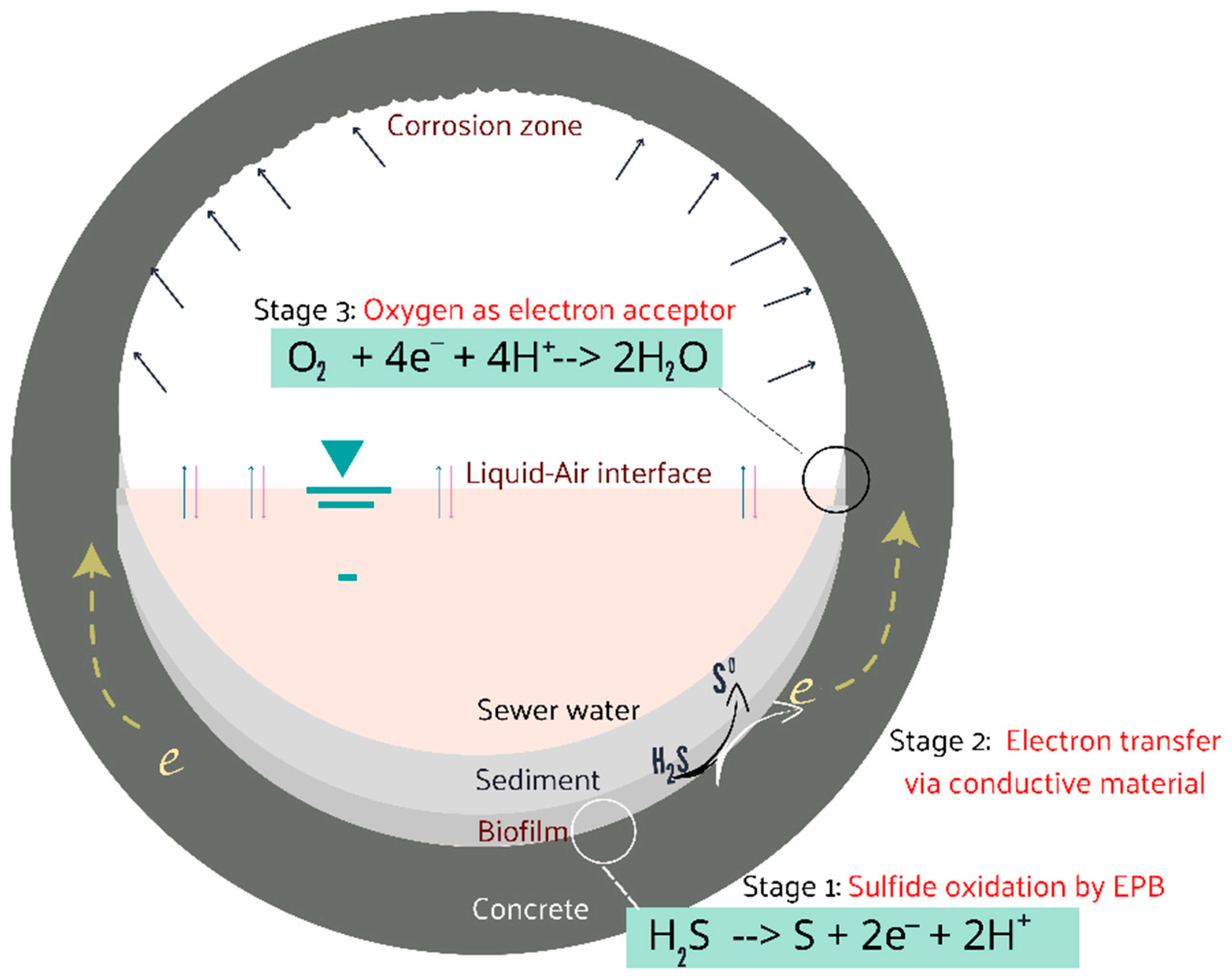
:1. Introduction
2. Materials and Methods
2.1. Oxidation of Sulfide by the Electron-Emitting Bacteria
2.2. Experimental Models and Materials
- -
- San-earth M5C, commercially manufactured by Sankosha Co., Ltd., Tokyo, Japan (abbreviated as S), contained amorphous carbon produced from oil refining as a conductive component, with a maximum size of 0.3 mm (a specific gravity of approximately 1.285 g/cm3 and surface area of 1.9 m2/g). Its content was approximately 50 wt.% (amounts of conductive substance in the total powder) (S50), with a water-to-powder ratio of 42%.
- -
- Denka Black (trade name of Denka Acetylene Black) was manufactured by Denka Co., Ltd., Tokyo, Japan (abbreviated as D), with a mean size of 35 nm (a specific gravity of approximately 0.04 g/cm3 and surface area of 50–68 m2/g). Its contents were approximately 5 wt.% D5 and 10 wt.% D10, with water-to-powder ratios of 50% D5 and 62% D10.
- -
- Magnetite MTB-30 (trade name Magnetite) was manufactured by Morishita Bengara Kogyo Co., Ltd., Iga City, Japan (abbreviated as M), with a mean size of 0.5 µm (surface area of 13.3 m2/g). Its contents were approximately 5 wt.% M5, 25 wt.% M25, and 50 wt.% M50, with water-to-powder ratios of 38% M25 and 40% M50.
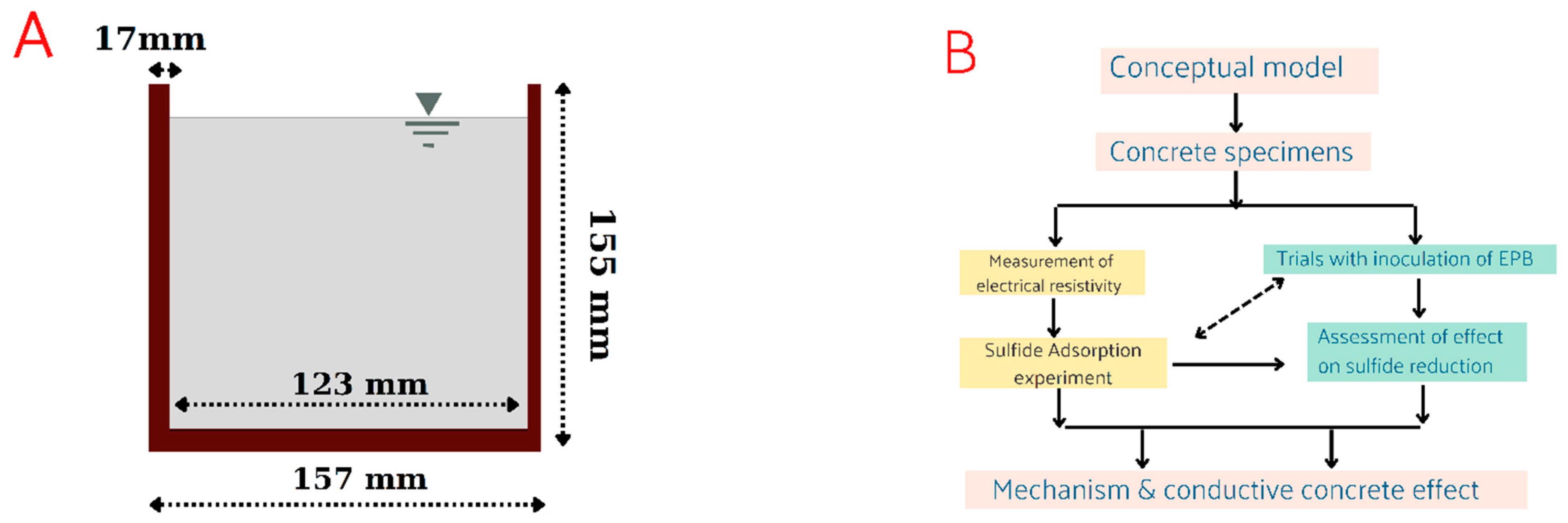
2.3. Preparation of the Cup-Shaped Concrete Specimens
2.4. Measurement of the Electrical Resistivity
2.5. Sulfide Adsorption Experiments
2.6. Preparation and Cultivation of EPB for the Trials of the Sulfide Reduction with Conductive Concrete
2.6.1. Culture of EPB Isolated from the Sewer Sludge
2.6.2. Preparation of the Samples of EPB for the Sulfide Suppression by Conductive Concrete
2.7. H2S Suppression Experiment by Conductive Concrete
3. Results
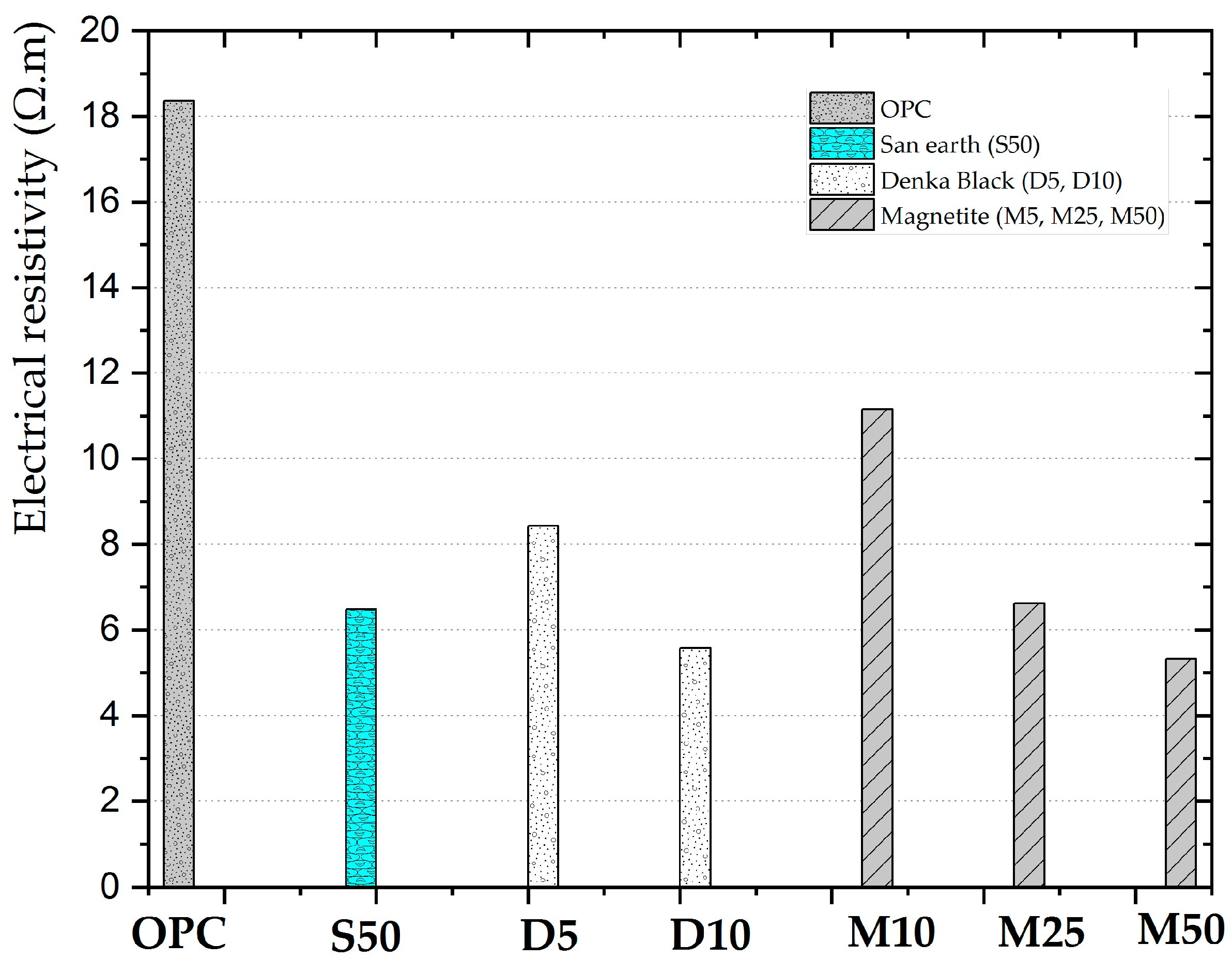
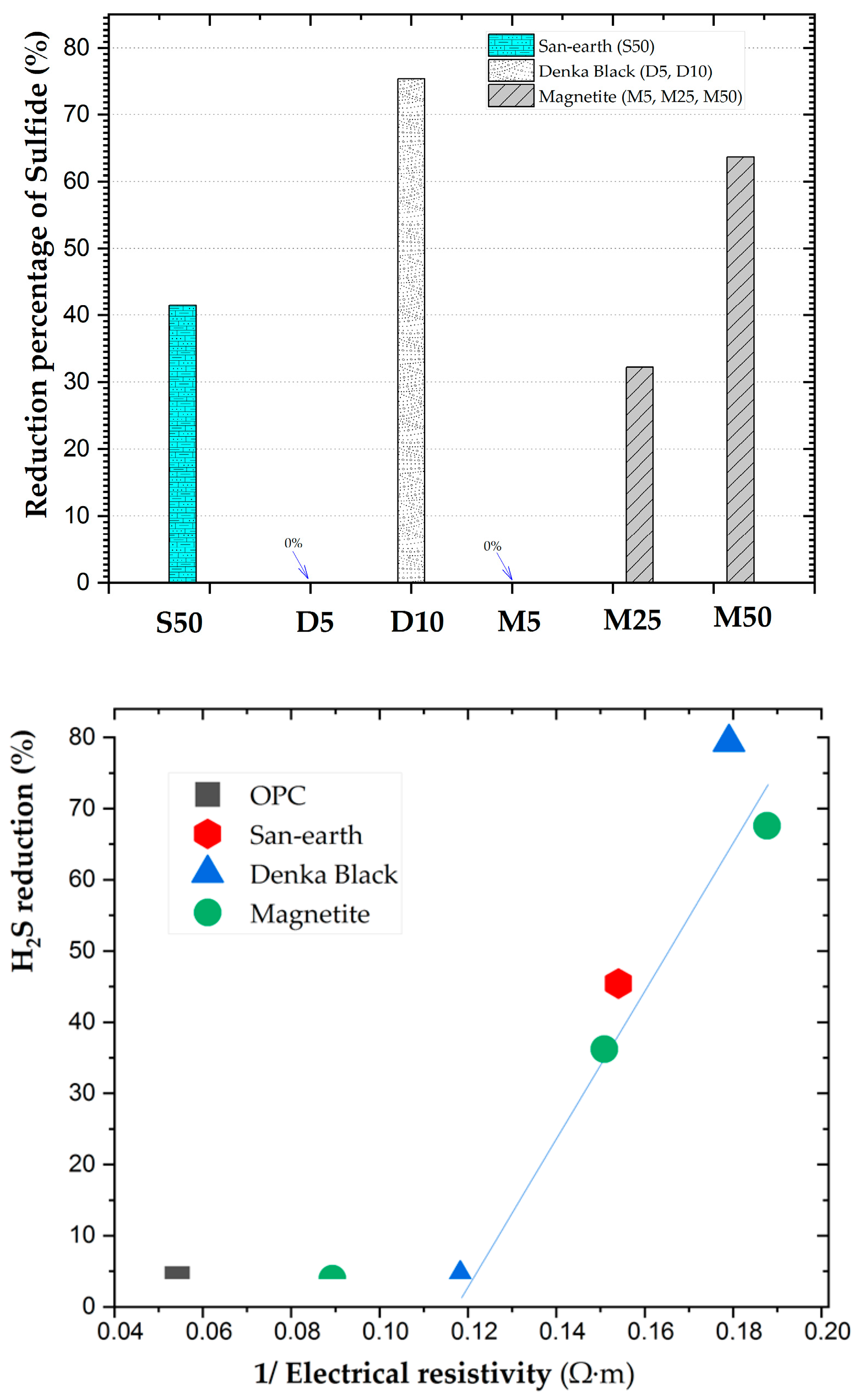
3.1. Measurement of the Electrical Resistivity
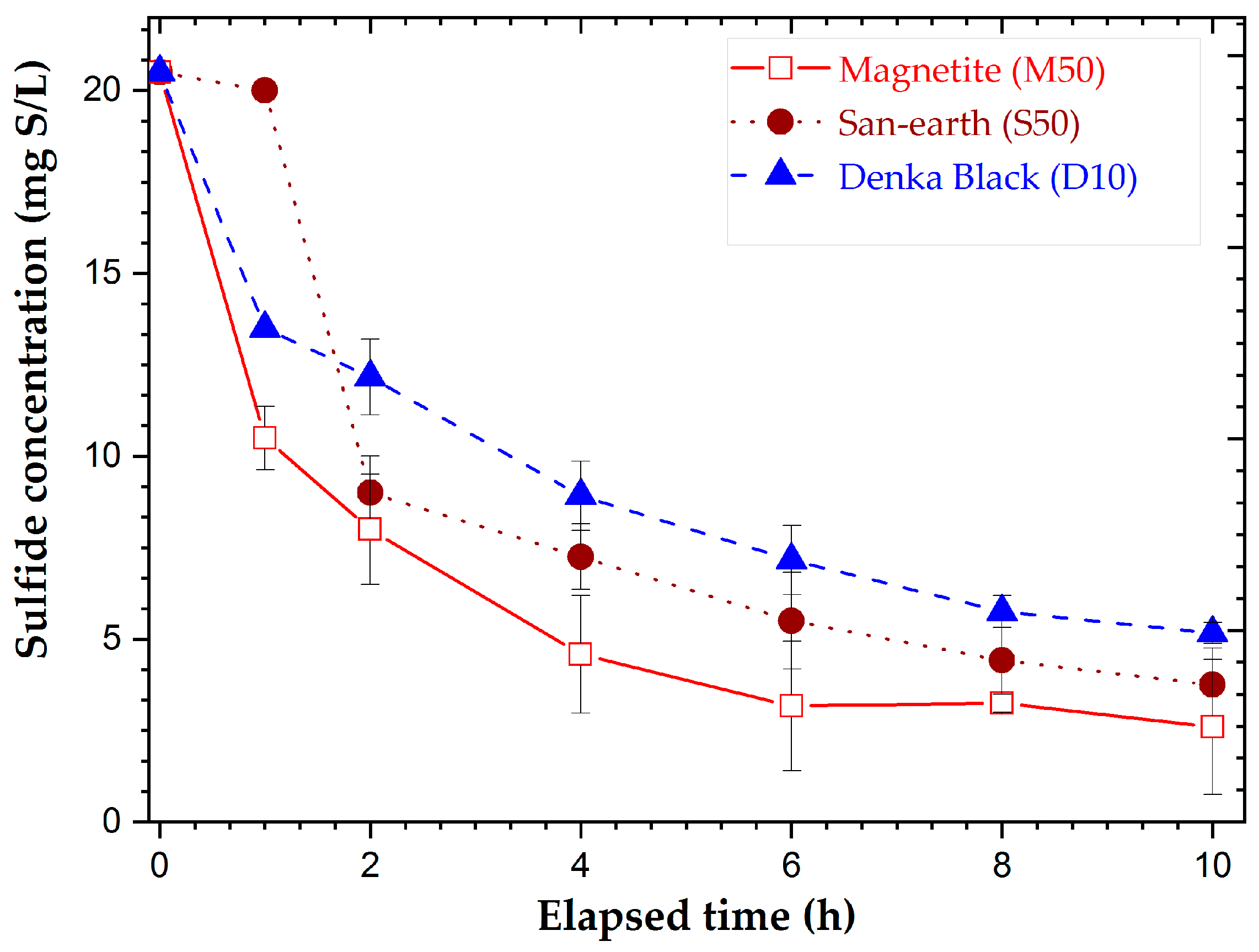
3.2. Results of the Adsorption Experiment of H2S
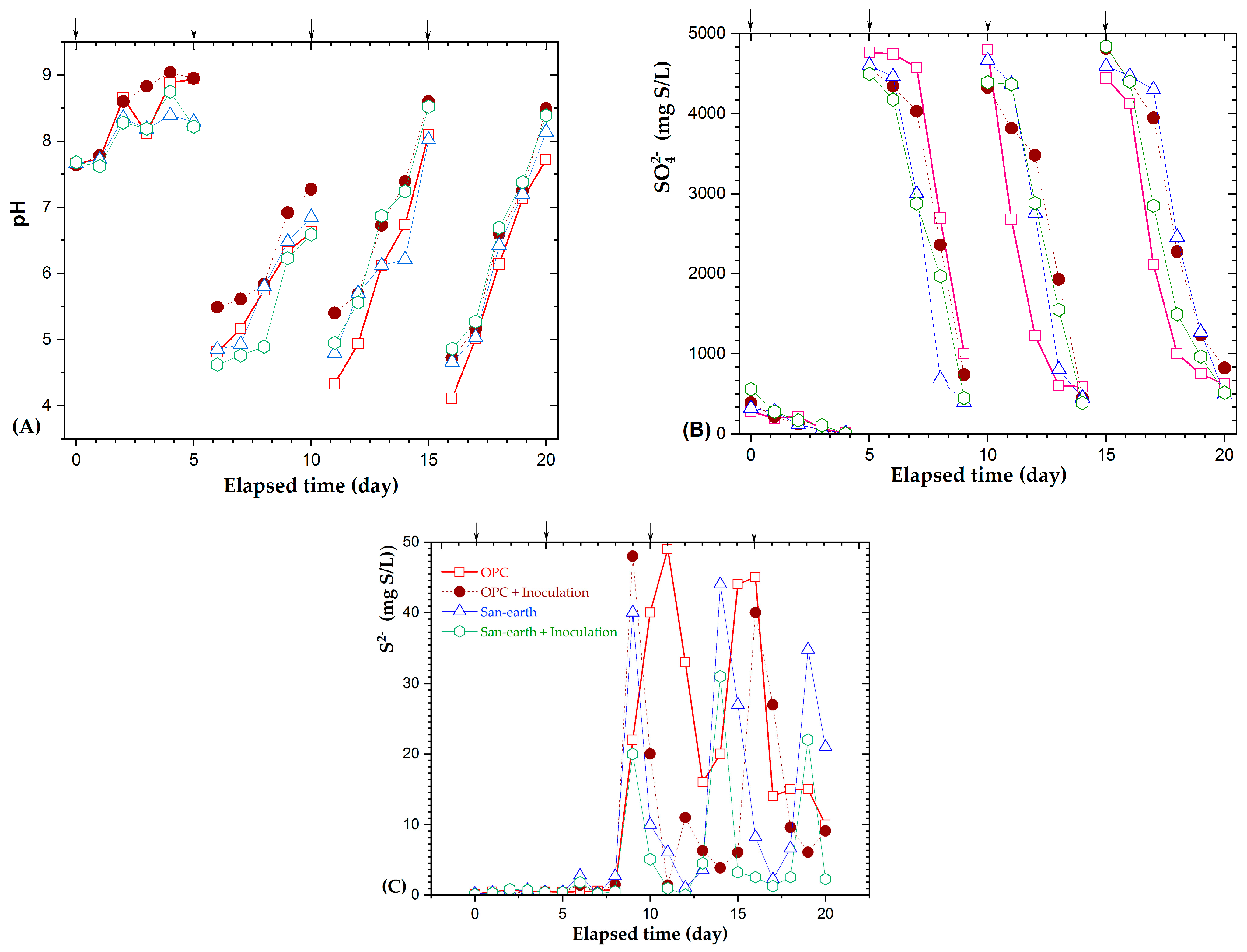
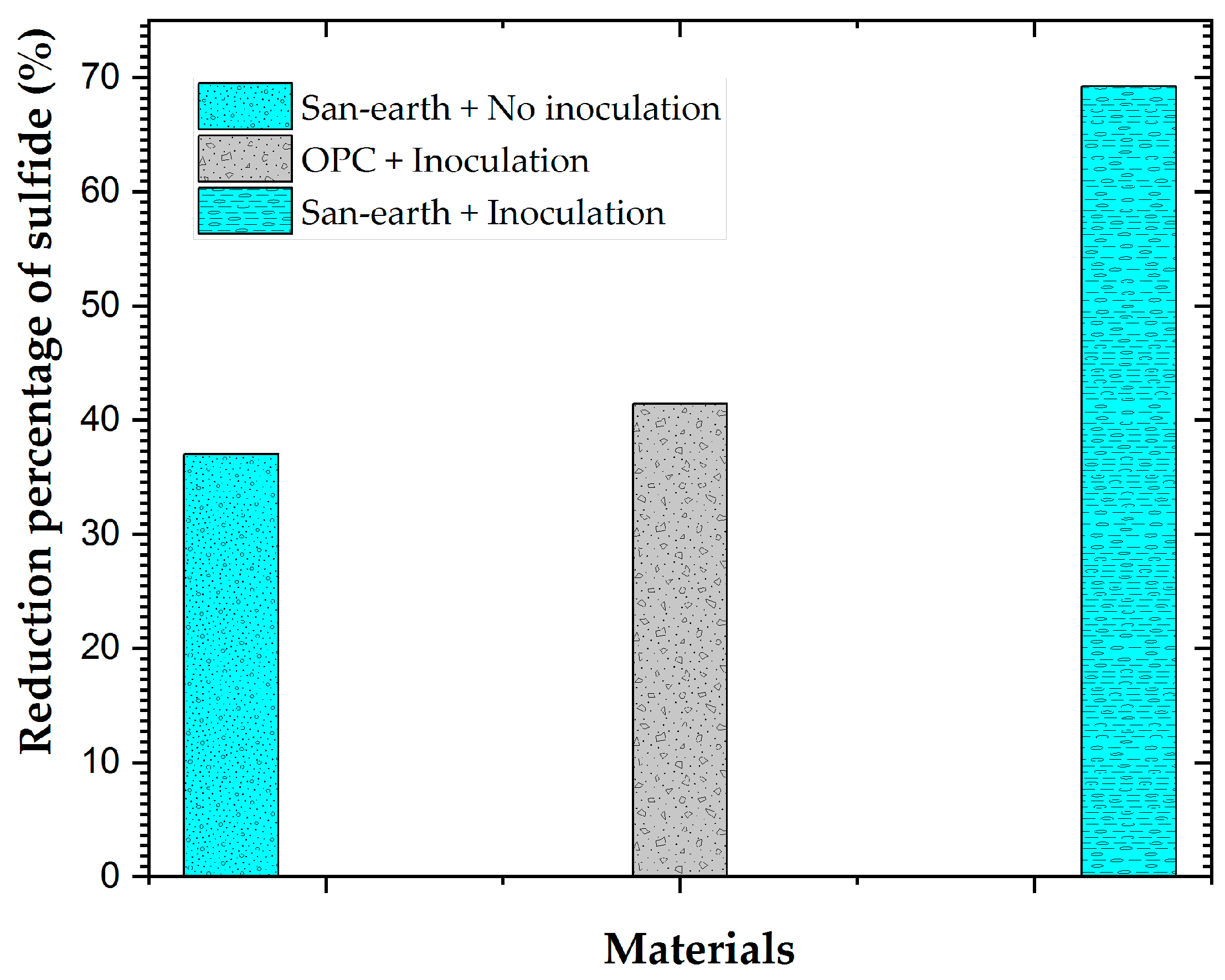
3.3. Effect of the EPB Inoculation on the H2S Reduction
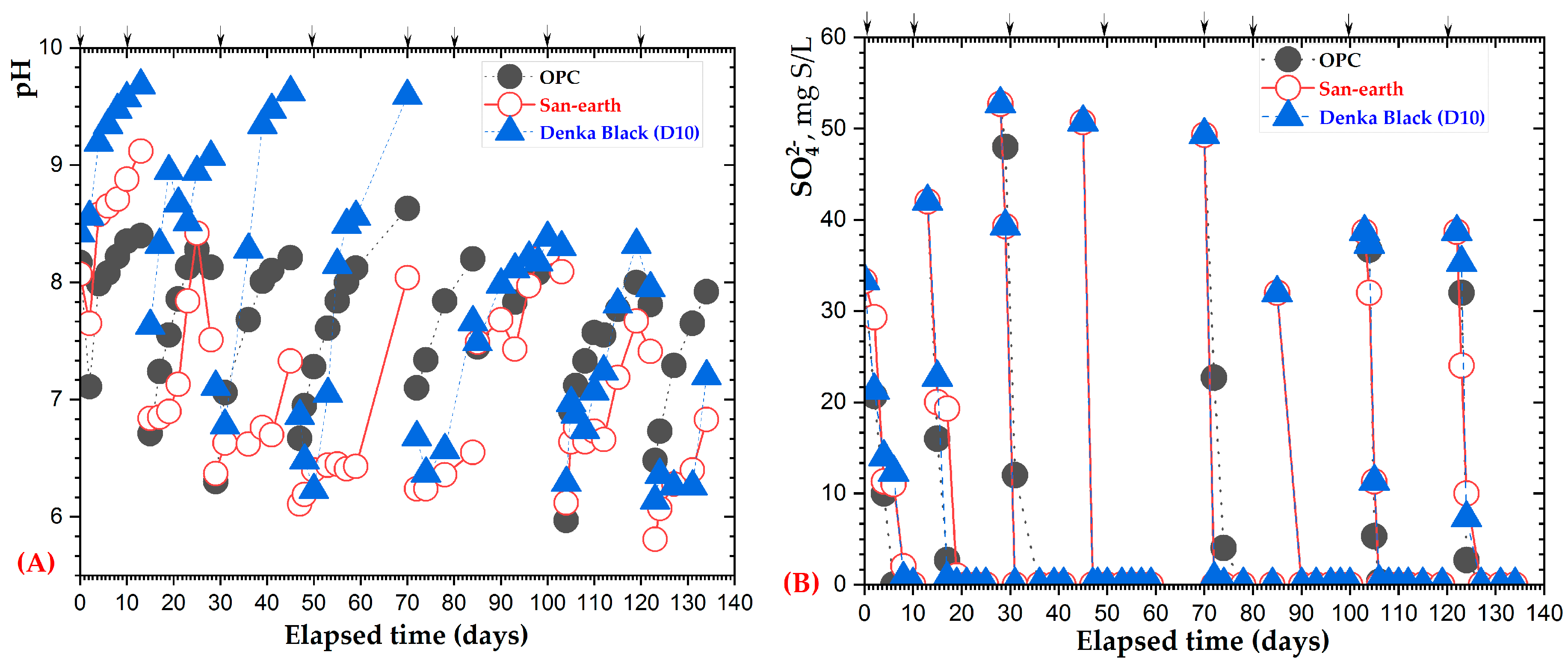
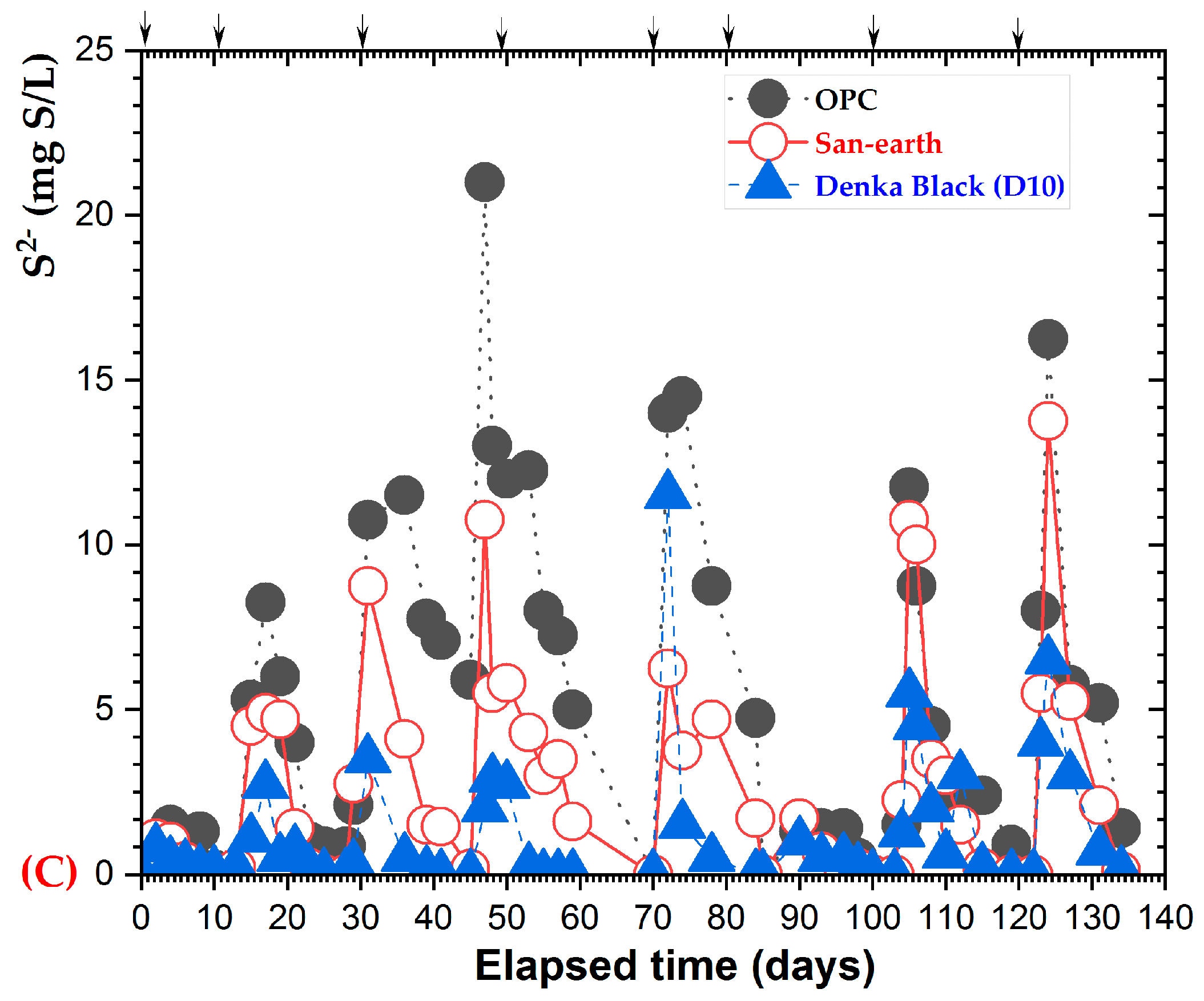
3.4. H2S Suppression Using Highly Conductive Concrete
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- US EPA. Process Design Manual for Sulfide Control in Sanitary Sewerage SystemsNo Title. 1974. Available online: http://cfpub.epa.gov/si/si_public_comments.cfm (accessed on 8 January 2023).
- Noeiaghaei, T.; Mukherjee, A.; Dhami, N.; Chae, S.R. Biogenic deterioration of concrete and its mitigation technologies. Constr. Build. Mater. 2017, 149, 575–586. [Google Scholar] [CrossRef]
- Anwar, A.; Liu, X.; Zhang, L. Biogenic corrosion of cementitious composite in wastewater sewerage system—A review. Process Saf. Environ. Prot. 2022, 165, 545–585. [Google Scholar] [CrossRef]
- Zuo, Z.; Chang, J.; Lu, Z.; Wang, M.; Lin, Y.; Zheng, M.; Zhu, D.Z.; Yu, T.; Huang, X.; Liu, Y. Environmental Science Environmental Science Hydrogen sulfide generation and emission in urban sanitary sewer in China: What factor plays the critical role? Environ. Sci. Water Res. Technol 2019, 5, 839. [Google Scholar] [CrossRef]
- Wu, M.; Wang, T.; Wu, K.; Kan, L. Microbiologically induced corrosion of concrete in sewer structures: A review of the mechanisms and phenomena. Constr. Build. Mater. 2020, 239, 117813. [Google Scholar] [CrossRef]
- De Belie, N.; Monteny, J.; Beeldens, A.; Vincke, E.; Van Gemert, D.; Verstraete, W. Experimental research and prediction of the effect of chemical and biogenic sulfuric acid on different types of commercially produced concrete sewer pipes. Cem. Concr. Res. 2004, 34, 2223–2236. [Google Scholar] [CrossRef]
- Eštokova, A.; Harbuláková, V.O.; Luptáková, A.; Števulová, N. Study of the Deterioration of Concrete Influenced by Biogenic Sulphate Attack. Procedia Eng. 2012, 42, 1731–1738. [Google Scholar] [CrossRef] [Green Version]
- Fytianos, G.; Baltikas, V.; Loukovitis, D.; Banti, D.; Sfikas, A.; Papastergiadis, E.; Samaras, P. Biocorrosion of concrete sewers in Greece: Current practices and challenges. Sustainability 2020, 12, 2638. [Google Scholar] [CrossRef] [Green Version]
- Foorginezhad, S.; Mohseni-Dargah, M.; Firoozirad, K.; Aryai, V.; Razmjou, A.; Abbassi, R.; Garaniya, V.; Beheshti, A.; Asadnia, M. Recent Advances in Sensing and Assessment of Corrosion in Sewage Pipelines. Process Saf. Environ. Prot. 2021, 147, 192–213. [Google Scholar] [CrossRef]
- Madraszewski, S.; Dehn, F.; Gerlach, J.; Stephan, D. Experimentally driven evaluation methods of concrete sewers biodeterioration on laboratory-scale: A critical review. Constr. Build. Mater. 2022, 320, 126236. [Google Scholar] [CrossRef]
- AlSalhi, M.S.; Devanesan, S.; Rajasekar, A.; Kokilaramani, S. Characterization of plants and seaweeds based corrosion inhibitors against microbially influenced corrosion in a cooling tower water environment. Arab. J. Chem. 2023, 16, 104513. [Google Scholar] [CrossRef]
- Gutierrez, O.; Mohanakrishnan, J.; Sharma, K.R.; Meyer, R.L.; Keller, J.; Yuan, Z. Evaluation of oxygen injection as a means of controlling sulfide production in a sewer system. Water Res. 2008, 42, 4549–4561. [Google Scholar] [CrossRef] [PubMed]
- Mohanakrishnan, J.; Gutierrez, O.; Meyer, R.L.; Yuan, Z. Nitrite effectively inhibits sulfide and methane production in a laboratory scale sewer reactor. Water Res. 2008, 42, 3961–3971. [Google Scholar] [CrossRef] [PubMed]
- Thorkild Hvitved-Jacobsen, J.V.A.H.N. Sewer Processes: Microbial and Chemical Process Engineering of Sewer Networks, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar]
- Tanaka, N.; Takenaka, K. Control of hydrogen sulfide and degradation of organic matter by air injection into a wastewater force main. Water Sci. Technol. 1995, 31, 273–282. [Google Scholar] [CrossRef]
- García, J.T.; Vigueras-Rodriguez, A.; Castillo, L.G.; Carrillo, J.M. Evaluation of Sulfide Control by Air-Injection in Sewer Force Mains: Field and Laboratory Study. Sustainability 2017, 9, 402. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Chang, N.; Wang, S.; Lu, J.; Li, K.; Zheng, C. Enhancing sulfide mitigation via the sustainable supply of oxygen from air-nanobubbles in gravity sewers. Sci. Total Environ. 2022, 808, 152203. [Google Scholar] [CrossRef] [PubMed]
- Devai, I.; Delaune, R.D. Effectiveness of selected chemicals for controlling emission of malodorous sulfur gases in sewage sludge. Environ. Technol. 2002, 23, 319–329. [Google Scholar] [CrossRef]
- Can-Dogan, E.; Turker, M.; Dagasan, L.; Arslan, A. Sulfide removal from industrial wastewaters by lithotrophic denitrification using nitrate as an electron acceptor. Water Sci. Technol. 2010, 62, 2286–2293. [Google Scholar] [CrossRef] [Green Version]
- Gutierrez, O.; Sudarjanto, G.; Ren, G.; Ganigué, R.; Jiang, G.; Yuan, Z. Assessment of pH shock as a method for controlling sulfide and methane formation in pressure main sewer systems. Water Res. 2014, 48, 569–578. [Google Scholar] [CrossRef]
- Zhang, L.; De Schryver, P.; De Gusseme, B.; De Muynck, W.; Boon, N.; Verstraete, W. Chemical and biological technologies for hydrogen sulfide emission control in sewer systems: A review. Water Res. 2008, 42, 1–12. [Google Scholar] [CrossRef]
- Nemati, M.; Jenneman, G.E.; Voordouw, G. Mechanistic study of microbial control of hydrogen sulfide production in oil reservoirs. Biotechnol. Bioeng. 2001, 74, 424–434. [Google Scholar] [CrossRef]
- Jiang, G.; Yuan, Z. Synergistic inactivation of anaerobic wastewater biofilm by free nitrous acid and hydrogen peroxide. J. Hazard. Mater. 2013, 250–251, 91–98. [Google Scholar] [CrossRef]
- Imai, T.; Vo, H.T.; Fukushima, M.; Suzuki, T.; Sakuma, H.; Hitomi, T.; Hung, Y.-T. Application of Conductive Concrete as a Microbial Fuel Cell to Control H2S Emission for Mitigating Sewer Corrosion. Water 2022, 14, 3454. [Google Scholar] [CrossRef]
- Dilushani, P.D.R.; Nawodani, W.R.N.; Bambaravanage, T.; Udayakumar, K.A.C. Udayakumar soil resistivity analysis and earth electrode resistance determination. IOSR J. Electr. Electron. Eng. 2020, 15, 26–35. [Google Scholar]
- Takahashi, S.; Tomita, J.; Nishioka, K.; Hisada, T.; Nishijima, M. Development of a prokaryotic universal primer for simultaneous analysis of Bacteria and Archaea using next-generation sequencing. PLoS ONE 2014, 9, e105592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vasieva, O.; Sorokin, A.; Szydlowski, L.; Goryanin, I. Do Microbial Fuel Cells have Antipathogenic Properties? J. Comput. Sci. Syst. Biol. 2019, 12, 3. [Google Scholar]
- Ng, I.S.; Hsueh, C.C.; Chen, B.Y. Electron transport phenomena of electroactive bacteria in microbial fuel cells: A review of Proteus hauseri. Bioresour. Bioprocess. 2017, 4, 53. [Google Scholar] [CrossRef] [Green Version]
- Sugnaux, M.; Savy, C.; Cachelin, C.P.; Hugenin, G.; Fischer, F. Simulation and resolution of voltage reversal in microbial fuel cell stack. Bioresour. Technol. 2017, 238, 519–527. [Google Scholar] [CrossRef]
- Rahimnejad, M.; Najafpour, G.D.; Ghoreyshi, A.A.; Shakeri, M.; Zare, H. Methylene blue as electron promoters in microbial fuel cell. Int. J. Hydrogen Energy 2011, 36, 13335–13341. [Google Scholar] [CrossRef]
- Gunasekaran, G.; Chongdar, S.; Naragoni, S.; Rodrigues, P.V.; Bobba, R. Microbial fuel cell constructed with micro-organisms isolated from industry effluent. Int. J. Hydrogen Energy 2011, 36, 14914–14922. [Google Scholar] [CrossRef]
- Choudhury, P.; Uday, U.S.P.; Mahata, N.; Nath Tiwari, O.; Narayan Ray, R.; Kanti Bandyopadhyay, T.; Bhunia, B. Performance improvement of microbial fuel cells for waste water treatment along with value addition: A review on past achievements and recent perspectives. Renew. Sustain. Energy Rev. 2017, 79, 372–389. [Google Scholar] [CrossRef]
- Zhou, W.; Imai, T.; Ukita, M.; Sekine, M.; Higuchi, T. Triggering forces for anaerobic granulation in UASB reactors. Process Biochem. 2006, 41, 36–43. [Google Scholar] [CrossRef]
- Alzate-Gaviria, L. Microbial Fuel Cells for Wastewater Treatment. In Waste Water; Einschlag, F.S.G., Ed.; IntechOpen: Rijeka, Croatia, 2011; p. 8. [Google Scholar]
- Baranitharan, E.; Khan, M.R.; Prasad, D.M.R.; Teo, W.F.A.; Tan, G.Y.A.; Jose, R. Effect of biofilm formation on the performance of microbial fuel cell for the treatment of palm oil mill effluent. Bioprocess Biosyst. Eng. 2015, 38, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Papaharalabos, G.; Greenman, J.; Melhuish, C.; Ieropoulos, I. A novel small scale Microbial Fuel Cell design for increased electricity generation and waste water treatment. Int. J. Hydrogen Energy 2015, 40, 4263–4268. [Google Scholar] [CrossRef]
- Wrighton, K.C.; Thrash, J.C.; Melnyk, R.A.; Bigi, J.P.; Byrne-Bailey, K.G.; Remis, J.P.; Schichnes, D.; Auer, M.; Chang, C.J.; Coates, J.D. Evidence for direct electron transfer by a gram-positive bacterium isolated from a microbial fuel cell. Appl. Environ. Microbiol. 2011, 77, 7633–7639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abbas, S.Z.; Rafatullah, M.; Ismail, N.; Shakoori, F.R. Electrochemistry and microbiology of microbial fuel cells treating marine sediments polluted with heavy metals. RSC Adv. 2018, 8, 18800–18813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yaqoob, A.A.; Ibrahim, M.N.M.; Rafatullah, M.; Chua, Y.S.; Ahmad, A.; Umar, K. Recent advances in anodes for microbial fuel cells: An overview. Materials 2020, 13, 2078. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vo, H.T.; Imai, T.; Fukushima, M.; Promnuan, K.; Suzuki, T.; Sakuma, H.; Hitomi, T.; Hung, Y.-T. Enhancing the Biological Oxidation of H2S in a Sewer Pipe with Highly Conductive Concrete and Electricity-Producing Bacteria. Int. J. Environ. Res. Public Health 2023, 20, 1459. https://doi.org/10.3390/ijerph20021459
Vo HT, Imai T, Fukushima M, Promnuan K, Suzuki T, Sakuma H, Hitomi T, Hung Y-T. Enhancing the Biological Oxidation of H2S in a Sewer Pipe with Highly Conductive Concrete and Electricity-Producing Bacteria. International Journal of Environmental Research and Public Health. 2023; 20(2):1459. https://doi.org/10.3390/ijerph20021459
Chicago/Turabian StyleVo, Huy Thanh, Tsuyoshi Imai, Masato Fukushima, Kanathip Promnuan, Tasuma Suzuki, Hiraku Sakuma, Takashi Hitomi, and Yung-Tse Hung. 2023. "Enhancing the Biological Oxidation of H2S in a Sewer Pipe with Highly Conductive Concrete and Electricity-Producing Bacteria" International Journal of Environmental Research and Public Health 20, no. 2: 1459. https://doi.org/10.3390/ijerph20021459
APA StyleVo, H. T., Imai, T., Fukushima, M., Promnuan, K., Suzuki, T., Sakuma, H., Hitomi, T., & Hung, Y.-T. (2023). Enhancing the Biological Oxidation of H2S in a Sewer Pipe with Highly Conductive Concrete and Electricity-Producing Bacteria. International Journal of Environmental Research and Public Health, 20(2), 1459. https://doi.org/10.3390/ijerph20021459







