Effects of Mindfulness-Based Cognitive Therapy on Major Depressive Disorder with Multiple Episodes: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
1.1. Impact of Major Depressive Disorder on Mental Health
1.2. Mindfulness-Based Cognitive Therapy and Major Depressive Disorder
1.3. Study Aim
2. Methods
2.1. Identification of Relevant Literature
2.2. Inclusion Criteria (Selection Criteria)
2.3. Search Outcome
2.4. Assessment of Study Quality
2.5. Quality Appraisal
2.5.1. Data Extraction and Review Process
2.5.2. Data Abstraction and Synthesis
2.6. Statistical Analysis
3. Results
3.1. Characteristics of Included Studies
3.2. Study Quality
3.3. Effectiveness of MBCT Using Meta-Analysis
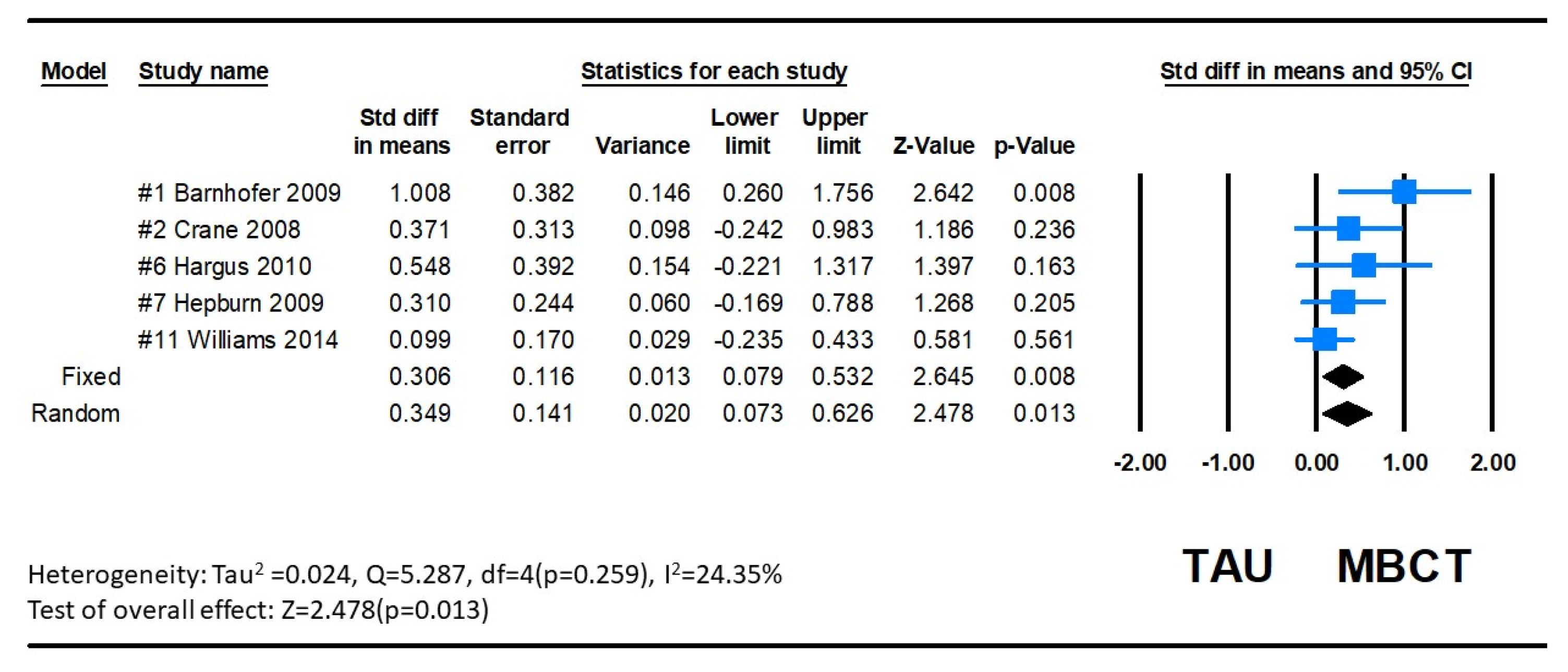
3.3.1. Depression
3.3.2. Suicidal Ideation
4. Discussion
4.1. Implications
4.2. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- World Health Organization. Depression (Fact Sheet); WHO: Geneva, Switzerland, 2020; Available online: https://www.who.int/news-room/fact-sheets/detail/depression (accessed on 1 December 2022).
- Nock, M.K.; Hwang, I.; Sampson, N.; Kessler, R.C.; Angermeyer, M.; Beautrais, A.; Borges, G.; Bromet, E.; Bruffaerts, R.; de Girolamo, G.; et al. Cross-national analysis of the associations among mental disorders and suicidal behavior: Findings from the WHO world mental health surveys. PLoS Med. 2009, 6, e1000123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steinert, C.; Hofmann, M.; Kruse, J.; Leichsenring, F. Relapse rates after psychotherapy for depression—Stable long-term effects? A meta-analysis. J. Affect. Disord. 2014, 168, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Park, E.-H.; Jung, M.H. The impact of major depressive disorder on adaptive function. Medicine 2019, 98, e18515. [Google Scholar] [CrossRef] [PubMed]
- Tomažič, T.; Čelofiga, A.K. The Role of Different Behavioral and Psychosocial Factors in the Context of Pharmaceutical Cognitive Enhancers’ Misuse. Healthcare 2022, 10, 972. [Google Scholar] [CrossRef]
- Blumberg, M.J.; Vaccarino, S.R.; McInerney, S.J. Procognitive Effects of Antidepressants and Other Therapeutic Agents in Major Depressive Disorder. J. Clin. Psychiatry 2020, 81, 19r13200. [Google Scholar] [CrossRef]
- van der Velden, A.M.; Kuyken, W.; Wattar, U.; Crane, C.; Pallesen, K.J.; Dahlgaard, J.; Fjorback, L.O.; Piet, J. A systematic review of mechanisms of change in mindfulness-based cognitive therapy in the treatment of recurrent major depressive disorder. Clin. Psychol. Rev. 2015, 37, 26–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teasdale, J.D.; Segal, Z.V.; Williams, J.M.G.; Ridgeway, V.A.; Soulsby, J.M.; Lau, M.A. Prevention of relapse/recurrence in major depression by mindfulness-based cognitive therapy. J. Consult. Clin. Psychol. 2000, 68, 615–623. [Google Scholar] [CrossRef]
- Williams, J.M.G.; Russell, I.T.; Crane, C.; Russell, D.; Whitaker, C.J.; Duggan, D.S.; Barnhofer, T.; Fennell, M.J.; Crane, R.; Silverton, S. Staying well after depression: Trial design and protocol. BMC Psychiatry 2010, 10, 23. [Google Scholar] [CrossRef] [Green Version]
- Segal, Z.V.; Williams, J.M.G.; Teasdale, J.D. Mindfulness-Based Cognitive Therapy for Depression, 2nd ed.; The Guilford Press: New York, NY, USA, 2013. [Google Scholar]
- National Institute of Health and Clinical Excellence. Depression: The treatment and Management of Depression in Adults. 2018. Available online: https://www.nice.org.uk/guidance/ng222/documents/short-version-of-draft-guideline (accessed on 1 December 2022).
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gotzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Healthcare Interventions: Explanation and Elaboration. BMJ 2009, 339, b2700. [Google Scholar] [CrossRef] [Green Version]
- Williams, J.M.; Crane, C.; Barnhofer, T.; Brennan, K.; Duggan, D.S.; Fennell, M.J.; Hackmann, A.; Krusche, A.; Muse, K.; Von Rohr, I.R.; et al. Mindfulness-based cognitive therapy for preventing relapse in recurrent depression: A randomized dismantling trial. J. Consult. Clin. Psychol. 2014, 82, 275–286. [Google Scholar] [CrossRef]
- Kuyken, W.; Warren, F.C.; Taylor, R.S.; Whalley, B.; Crane, C.; Bondolfi, G.; Hayes, R.; Huijbers, M.; Ma, H.; Schweizer, S.; et al. Efficacy of Mindfulness-Based Cognitive Therapy in Prevention of Depressive Relapse: An individual patient data meta-analysis from randomized trials. JAMA Psychiatry 2016, 73, 565–574. [Google Scholar] [CrossRef]
- Zhang, B.; Fu, W.; Guo, Y.; Chen, Y.; Jiang, C.; Li, X.; He, K. Effectiveness of mindfulness-based cognitive therapy against suicidal ideation in patients with depression: A systematic review and meta-analysis. J. Affect. Disord. 2022, 319, 655–662. [Google Scholar] [CrossRef] [PubMed]
- Oremus, M.; Wolfson, C.; Perrault, A.; Demers, L.; Momoli, F.; Moride, Y. Interrater reliability of the modified Jadad quality scale for systematic reviews of Alzheimer’s disease drug trials. Dement. Geriatr. Cogn. Disord. 2001, 12, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ Br. Med. J. 2003, 327, 557–560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borenstein, M.; Hedges, L.V.; Higgins, J.P.; Rothstein, H.R. Introduction to Meta-Analysis, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2021. [Google Scholar]
- Hedges, L.V.; Vevea, J.L. Fixed- and random-effects models in meta-analysis. Psychol. Methods 1998, 3, 486–504. [Google Scholar] [CrossRef]
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. Br. Med. J. 1997, 315, 629–634. [Google Scholar] [CrossRef] [Green Version]
- Fragkos, K.; Tsagris, M.; Frangos, C.C. Publication Bias in Meta-Analysis: Confidence Intervals for Rosenthal’s Fail-Safe Number. Int. Sch. Res. Not. 2014, 2014, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Barnhofer, T.; Crane, C.; Hargus, E.; Amarasinghe, M.; Winder, R.; Williams, J.M.G. Mindfulness-based cognitive therapy as a treatment for chronic depression: A preliminary study. Behav. Res. Ther. 2009, 47, 366–373. [Google Scholar] [CrossRef] [Green Version]
- Forkmann, T.; Wichers, M.; Geschwind, N.; Peeters, F.; van Os, J.; Mainz, V.; Collip, D. Effects of mindfulness-based cognitive therapy on self-reported suicidal ideation: Results from a randomised controlled trial in patients with residual depressive symptoms. Compr. Psychiatry 2014, 55, 1883–1890. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Ren, X.; Zhan, J.; Dong, Y.; Yan, W. Effect of mindfulness treatment on improving negative emotion and passive response in patients with depression. China Modern Dr. 2018, 56, 93–96. [Google Scholar]
- Feng, N. The effect of mindfulness training on depression and self-esteem in patients with recurrent depression. Chin. Nurs. Res. 2016, 30, 2682–2683. [Google Scholar] [CrossRef]
- Hepburn, S.R.; Crane, C.; Barnhofer, T.; Duggan, D.S.; Fennell, M.J.; Williams, J.M. Mindfulness-based cognitive therapy may reduce thought suppression in previously suicidal participants: Findings from a preliminary study. Br. J. Clin. Psychol. 2009, 48 Pt 2, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Crane, C.; Barnhofer, T.; Duggan, D.S.; Hepburn, S.; Fennell, M.V.; Williams, J.M.G. Mindfulness-Based Cognitive Therapy and Self-Discrepancy in Recovered Depressed Patients with a History of Depression and Suicidality. Cogn. Ther. Res. 2008, 32, 775–787. [Google Scholar] [CrossRef]
- Hargus, E.; Crane, C.; Barnhofer, T.; Williams, J.M.G. Effects of mindfulness on meta-awareness and specificity of describing prodromal symptoms in suicidal depression. Emotion 2010, 10, 34–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.; Zhao, H.; Ji, C.; Zhu, P.; Lu, X.; Kong, F. A comparative study of mindfulness-based cognitive therapy to treat patients with depression. J. Psychiatry 2017, 30, 334–336. [Google Scholar] [CrossRef]
- Mu, Y. Effects of intervention by mindfulness-based cognitive therapy on mental status and suicide intervention in patients with depression. J. Navy Med. 2017, 38, 530–533. [Google Scholar]
- Peng, F.R.; Pang, R. Effect of Mindfulness-Based Cognitive Therapy on symptoms of depression and quality of life in patients with depression. J. Clin. Res. 2018, 35, 1133–1135. [Google Scholar] [CrossRef]
- Zhang, P.; Bai, X. The effect of Mindfulness-based cognitive therapy on depression and coping styles of young and middle-aged depression patients. Psychol. Dr. 2018, 24, 354–355. [Google Scholar]
- Crane, C.; Williams, J.M.G. Factors Associated with Attrition from Mindfulness-Based Cognitive Therapy in Patients with a History of Suicidal Depression. Mindfulness 2010, 1, 10–20. [Google Scholar] [CrossRef] [Green Version]
- Braboszcz, C.; Hahusseau, S.; Delorme, A. Meditation and neuroscience: From basic research to clinical practice. In Integrative Clinical Psychology, Psychiatry and Behavioral Medicine: Perspectives, Practices and Research; Carlstedt, R.A., Ed.; Springer Publishing: New York, NY, USA, 2010. [Google Scholar]
- Kong, L.; Chen, K.; Womer, F.; Jiang, W.; Luo, X.; Driesen, N.; Liu, J.; Blumberg, H.; Tang, Y.; Xu, K.; et al. Sex differences of gray matter morphology in cortico-limbic-striatal neural system in major depressive disorder. J. Psychiatr. Res. 2013, 47, 733–739. [Google Scholar] [CrossRef]







| First Author, Year/Country | Diagnosis/ Assessed with | Sample Size; Attrition Rate (%); Gender (% Female) | Treatment Conditions/Age (Mean ± SD) | Treatment concept/Duration (Weeks)/Number of Group Participants | Treatment Classes (Hour/Day/Week)/Homework (Hour/Week) | Comparison Condition | Instruments/ Follow-up Duration | Main Result (Mean ± SD; p Value) | Modified Jadad Scale |
|---|---|---|---|---|---|---|---|---|---|
| #1 Barnhofer, 2009/England | Currently remitted recurrent MDD (DSM-IV-TR)/ history of at least 3 episodes of major depression/SCID | 31; 28 analyzed completers; 67.86% | (1) MBCT+TAU (n = 16/14)/42.07 ± 11.34 (2) TAU (n = 15/14)/41.79 ± 9.52 | MBCT/8 wks/NA | 2/1/wk/6/wk | Naturalistic care, i.e., standard treatment | BDI-II BSS/post-treatment | 1. Pre-assessments I 29.36 ± 9.66 vs. C: 31.32 ± 10.79/post-assessments I: 17.62 ± 10.94 vs. C: 28.86 ± 12.97; p = 0.03. 2. Pre-assessments E 2.21 ± 2.45 vs. C: 2.78 ± 2.08/ post-assessments I: 1.14 ± 1.79 vs. C: 2.42 ± 2.53. | 8/8 |
| #2 Crane, 2008/USA | Currently remitted recurrent MDD (DSM-IV-TR)/ history of at least 3 episodes of major depression | 68; 68 analyzed completers; 67.86% | (1) MBCT (n = 33)/49.75 ± 8.11 (2) waitlist (n = 35)/40.44 ± 9.09 | MBCT/8 wks/NA | 2/1/wk/NA | Naturalistic care, i.e., standard treatment | BDI-II /post-treatment | 1. Pre-assessments I: 16.58 ± 14.23 vs. C: 12.78 ± 9.83/post-assessments I: 8.40 ± 12.59 vs. C: 12.90 ± 11.76/, p = 0.004. | 8/8 |
| #3 Crane, 2010/USA | Currently remitted recurrent MDD (DSM-IV-TR)/ history of at least 3 episodes of major depression | 91; 35 analyzed completers; NA | (1) MBCT (n = 35)/49.75 ± 8.11 (2) waitlist (n = 36)/40.44 ± 9.09 | MBCT/8 wks/NA | 2.5/1/wk/NA | Naturalistic care, i.e., standard treatment | BDI-II /post-treatment | 1. Pre-assessments I: 12.85 ± 10.89, p > 0.05. | 7/8 |
| #4 Feng, 2016/China | Currently remitted recurrent MDD | 69; 69 analyzed completers; 55.07% | (1) MBCT+TAU (n = 34) (2) TAU (n = 35) | MBCT/8 wks/NA | 2/1/wk/NA | Naturalistic care, i.e., standard treatment | SDS HAMD /post-treatment | 1. Pre-assessments I50.92 ± 11.29 vs. C: 51.48 ± 10.94/post-assessments I: 40.65 ± 9.17 vs. C: 45.59 ± 10.21; p < 0.05 2. Pre-assessments I: 26.56 ± 10.45 vs. C: 27.07 ± 1019/post-assessments I: 8.83 ± 6.14 vs. C: 12.37 ± 7.32; p < 0.05. | 1/8 |
| #5 Forkmann, 2014/USA | Currently remitted recurrent MDD (DSM-IV)/SCID/HDRS > 7 | 130; 130 analyzed completers; MBCT79.0%/TAU73.0% | (1) MBCT+TAU (n = 64)/44.6 ± 9.7 (2) TAU (n = 66)/43.2 ± 9.5 | MBCT/8 wks/10–15 | 2.5/1/wk/3–6/wk (in the weekly group sessions, participants received CDs with guided exercises and were assigned daily) | Naturalistic care, i.e., standard treatment | HDRS/ post-treatment | 1. Pre-assessments I: 10.27 ± 3.69 vs. C: 10.21 ± 3.55/ post-assessments I: 7.14 ± 4.81 vs. C: 9.68 ± 4.04; p = 0.00. | 7/8 |
| #6 Hargus, 2010/USA | Currently remitted recurrent MDD (DSM-IV-TR) | 90; 68 analyzed completers; MBCT75%/TAU62.5% | (1) MBCT (n = 33)/42.07 ± 11.04 (2) TAU (n = 35)/41.69 ± 9.90 | MBCT/9 wks/NA | 2/1/wk | Naturalistic care, i.e., standard treatment | BDI-II /post-treatment, 3, months | 1. I: 30.35 ± 9.93 vs. C: 32.37 ± 11.18; p > 0.05. | 7/8 |
| #7 Hepburn, 2009/USA | Currently remitted recurrent MDD (DSM-IV-TR) | 125; 68 analyzed completers;; 73.53% | (1) MBCT+TAU (n = 33)/48.77 ± 9.04 (2) TAU (n = 35)/41.24 ± 9.00 | MBCT/8 wks | 2/1/wk; 360/wk | Naturalistic care, i.e., standard treatment | HAMD-17 /post-treatment, 3, 6, 9, 12 months | 1. Pre-assessments I: 15.62 ± 13.84 vs. C: 12.83 ± 9.59/post-assessments I: 8.67 ± 12.00 vs. C: 12.25 ± 11.14, p < 0.01 | 8/8 |
| #8 Li, 2017/China | Currently remitted recurrent MDD (DSM-5)/ HAMD > 7 | 126; 126 analyzed completers, 53.96% | (1) MBCT+TAU (n = 61)/39.95 ± 12.50 (2) TAU (n = 65)/41.05 ± 15.41 | MBCT/8 wks/10–12 | 2/1/wk/6/wk (total 12 weeks) | Naturalistic care, i.e., standard treatment | HAMD-17 /post-treatment | 1. Pre-assessments I: 17.39 ± 5.70 vs. C: 18.10 ± 5.57/post-assessments I: 5.83 ± 4.74 vs. C: 11.19 ± 4.18; p < 0.01. | 4.5/8 |
| #9 Mu, 2017/China | WHO criteria of major depression | 80; 45 analyzed completers; 55% | (1) MBCT+TAU (n = 40)/37.69 ± 7.06 (2) TAU (n = 40)/36.41 ± 8.62) y | MBCT/8 wks | NA/NA | HAMD-17 | 1. Post-assessments; p < 0.005 | 1/8 | |
| #10 Peng, 2018/China | Currently remitted recurrent MDD (CCMD-3))/ HAMD > 17 | 116; 116 analyzed completers; 51.72% | (1) MBCT+TAU (n = 58)/37.86 ± 5.12 (2) TAU (n = 58)/38.12 ± 5.24 | NA | 1.5–2/1/wk/NA | Naturalistic care, i.e., standard treatment | HAMD-17 /post-treatment | 1. Pre-assessments I: 25.86 ± 3.85 vs. C: 26.54 ± 4.12/post-assessments I: 19.45 ± 3.67 vs. C: 14.12 ± 3.24; p < 0.05. | 5/8 |
| #11 Williams, 2014/USA | Currently remitted recurrent MDD (DSM-IV-TR) | 257; 257 analyzed completers; NA | (1) MBCT+TAU (n = 99)/NA (2) CPE+TAU (n = 103)/NA (3) TAU (n = 55)/NA | MBCT/8 wks/NA | 2/1/wk/NA | Naturalistic care, i.e., standard treatment | BDI-II HAMD-17 SCID /post-treatment, 3, 6, 9, 12 months | 1. Measure of depressive symptomatology: BDI-II: no significance 2. Residual depressive symptomatology: HAMD: no significance 3. Suicidality questions of the SCID: no significance | 5/8 |
| #12 Zhang, 2018/China | Currently remitted recurrent MDD (CCMD-3)) | 86; 86 analyzed completers; 56.97% | (1) MBCT+TAU (n = 43)/35.74 ± 3.98 (2) TAU (n = 43)/4.92 ± 4.33 | MBCT/6 wks | 0.33–0.5/5/wk/NA | Naturalistic care, i.e., standard treatment | HAMD /post-treatment | 1. Pre-assessments I: 28.11 ± 8.84 vs. C: 27.65 ± 8.69/ post-assessments I: 11.45 ± 5.71 vs. C: 17.29 ± 5.36; p < 0.05. | 1/8 |
| #13 Zhao, 2018/China | Currently remitted recurrent MDD (ICD-10)/HAMD > 17 | 80; 80 analyzed completers; 51.25% | (1) MBCT+TAU (n = 41)/42.93 ± 1.77 (2) TAU (n = 39)/43.45 ± 1.82 | MBCT/8 wks/8 | NA/NA | Naturalistic care, i.e., standard treatment | HAMD-17 /post-treatment | 1. Pre-assessments I: 26.16 ± 5.19 vs. C: 26.22 ± 5.08/post-assessments I: 14.01 ± 1.70 vs. C: 14.22 ± 1.91; p < 0.05. | 5/8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tseng, H.-W.; Chou, F.-H.; Chen, C.-H.; Chang, Y.-P. Effects of Mindfulness-Based Cognitive Therapy on Major Depressive Disorder with Multiple Episodes: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2023, 20, 1555. https://doi.org/10.3390/ijerph20021555
Tseng H-W, Chou F-H, Chen C-H, Chang Y-P. Effects of Mindfulness-Based Cognitive Therapy on Major Depressive Disorder with Multiple Episodes: A Systematic Review and Meta-Analysis. International Journal of Environmental Research and Public Health. 2023; 20(2):1555. https://doi.org/10.3390/ijerph20021555
Chicago/Turabian StyleTseng, Hui-Wen, Fan-Hao Chou, Ching-Hsiu Chen, and Yu-Ping Chang. 2023. "Effects of Mindfulness-Based Cognitive Therapy on Major Depressive Disorder with Multiple Episodes: A Systematic Review and Meta-Analysis" International Journal of Environmental Research and Public Health 20, no. 2: 1555. https://doi.org/10.3390/ijerph20021555
APA StyleTseng, H.-W., Chou, F.-H., Chen, C.-H., & Chang, Y.-P. (2023). Effects of Mindfulness-Based Cognitive Therapy on Major Depressive Disorder with Multiple Episodes: A Systematic Review and Meta-Analysis. International Journal of Environmental Research and Public Health, 20(2), 1555. https://doi.org/10.3390/ijerph20021555







