The Emotion Regulation Mechanism in Neurotic Individuals: The Potential Role of Mindfulness and Cognitive Bias
Abstract
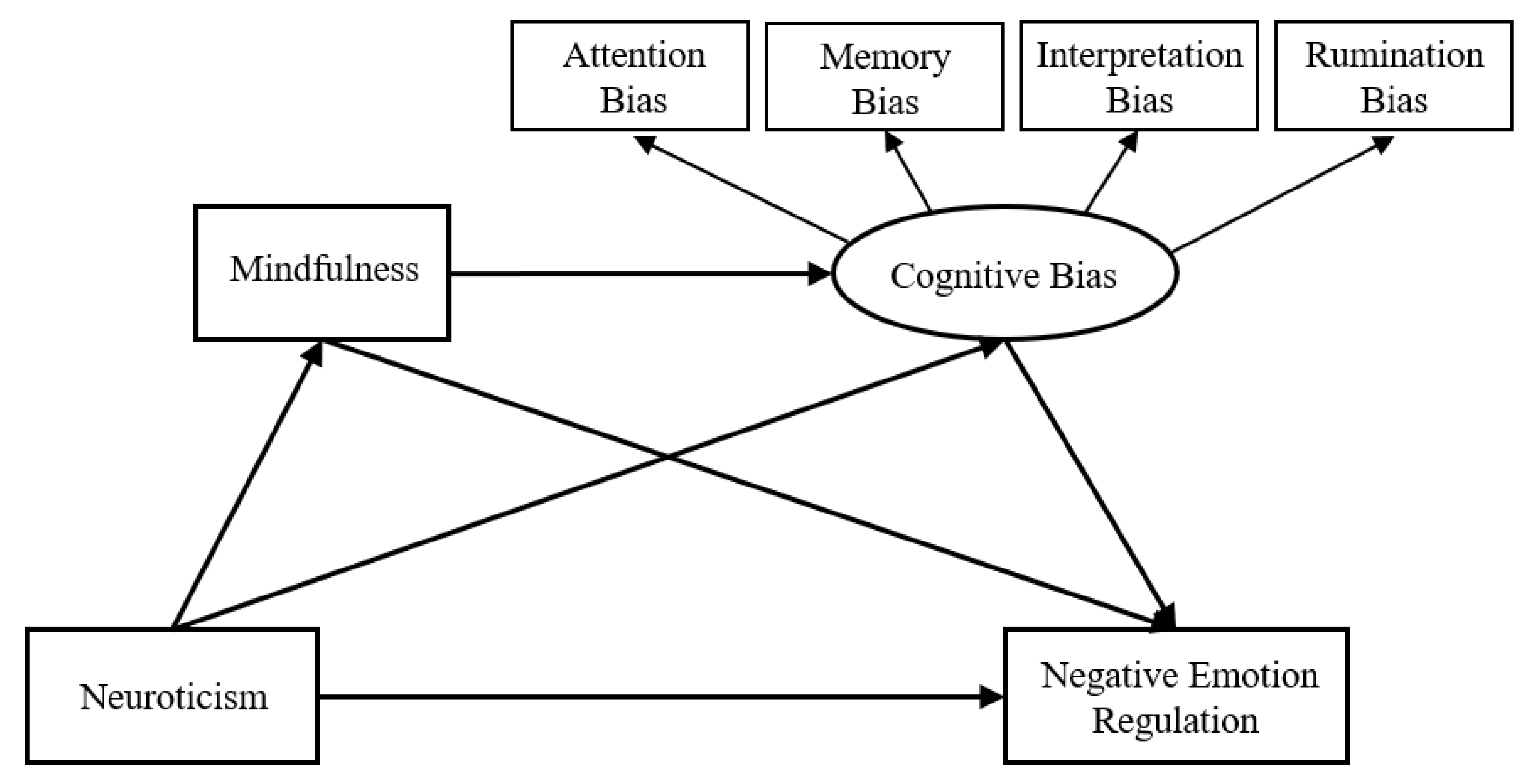
:1. Introduction
2. Study 1: Scale Study of the Emotion Regulation Mechanisms in Neurotic Individuals
2.1. Method
2.1.1. Participants
2.1.2. Procedure
2.1.3. Measures
2.1.4. Analysis
2.2. Results
2.2.1. Common Method Bias
2.2.2. Correlation Analysis
2.2.3. Test of the Chain Mediation Model
2.3. Discussion
3. Study 2: The Experimental Study of Cognitive Bias in Neurotic Individuals
3.1. Method
3.1.1. Participants
3.1.2. Design
3.1.3. Materials
3.1.4. Procedure
3.1.5. Analysis
3.2. Results
3.2.1. Attention Bias
3.2.2. Memory Bias
3.2.3. Interpretation Bias
3.3. Discussion
4. General Discussion
4.1. Emotion Regulation Mechanisms of Neurotic Individuals
4.2. Cognitive Bias of Neurotic Individuals
4.3. Limitations and Future Possibilities
4.4. Implications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barlow, D.H.; Curreri, A.J.; Woodard, L.S. Neuroticism and disorders of emotion: A new synthesis. Curr. Dir. Psychol. Sci. 2021, 30, 410–417. [Google Scholar] [CrossRef]
- Klein, D.N.; Kotov, R.; Bufferd, S.J. Personality and depression: Explanatory models and review of the evidence. Annu. Rev. Clin. Psychol. 2011, 7, 269–295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Segal, D.L.; Marty, M.A.; Meyer, W.J.; Coolidge, F.L. Personality, suicidal ideation, and reasons for living among older adults. J. Gerontol. Ser. B 2012, 67, 159–166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomez, R.; Gomez, A.; Cooper, A. Neuroticism and extraversion as predictors of negative and positive emotional information processing: Comparing Eysenck’s, Gray’s, and Newman’s theories. Eur. J. Pers. 2002, 16, 333–350. [Google Scholar] [CrossRef]
- Andrés, M.L.; de Minzi, M.C.R.; Castañeiras, C.; Canet-Juric, L.; Rodríguez-Carvajal, R. Neuroticism and depression in children: The role of cognitive emotion regulation strategies. J. Genet. Psychol. 2016, 177, 55–71. [Google Scholar] [CrossRef]
- Liu, W.; Liu, F.; Chen, L. The mediating role of personality traits between childhood psychological maltreatment and maladaptive cognitive emotion regulation strategies. J. Psychol. Sci. 2018, 41, 64–70. [Google Scholar] [CrossRef]
- Thompson, R.A. Emotion regulation: A theme in search of definition. Monogr. Soc. Res. Child Dev. 1994, 59, 25–52. [Google Scholar] [CrossRef]
- Haga, S.M.; Kraft, P.; Corby, E.K. Emotion regulation: Antecedents and well-being outcomes of cognitive reappraisal and expressive suppression in cross-cultural samples. J. Happiness Stud. 2009, 10, 271–291. [Google Scholar] [CrossRef]
- Ghafur, R.D.; Suri, G.; Gross, J.J. Emotion regulation choice: The role of orienting attention and action readiness. Curr. Opin. Behav. Sci. 2018, 19, 31–35. [Google Scholar] [CrossRef]
- Hou, L.; Chang, L.; Chen, L.; Fei, J.; Zhou, R. Exploring the roles of dispositional mindfulness and cognitive reappraisal in the relationship between neuroticism and depression among postgraduate students in China. Int. J. Public Health 2022, 67, 1605074. [Google Scholar] [CrossRef]
- Grisham, J.R.; Becker, L.; Williams, A.D.; Whitton, A.E.; Makkar, S.R. Using cognitive bias modification to deflate responsibility in compulsive checkers. Cognit. Ther. Res. 2014, 38, 505–517. [Google Scholar] [CrossRef]
- Gsa, B.; Kmlac, D.; Gpa, E.; Mbb, A.; Mla, C. Efficacy of psychological interventions targeting cognitive biases in schizophrenia: A systematic review and meta-analysis. Clin. Psychol. Rev. 2020, 78, 108054. [Google Scholar] [CrossRef]
- Lester, K.J.; Mathews, A.; Davison, P.S.; Burgess, J.L.; Yiend, J. Modifying cognitive errors promotes cognitive well being: A new approach to bias modification. J. Behav. Ther. Exp. Psychiatry 2011, 42, 298–308. [Google Scholar] [CrossRef] [PubMed]
- Disner, S.G.; Beevers, C.G.; Haigh, E.; Beck, A.T. Neural mechanisms of the cognitive model of depression. Nat. Rev. Neurosci. 2011, 12, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Barnhofer, T.; Chittka, T. Cognitive reactivity mediates the relationship between neuroticism and depression. Behav. Res. Ther. 2010, 48, 275–281. [Google Scholar] [CrossRef] [Green Version]
- Chaby, L.E.; Cavigelli, S.A.; White, W.; Braithwaite, V.A. Long-term changes in cognitive bias and coping response as a result of chronic unpredictable stress during adolescence. Front. Hum. Neurosci. 2013, 7, 328. [Google Scholar] [CrossRef] [Green Version]
- Maack, D.J.; Tull, M.T.; Gratz, K.L. Experiential avoidance mediates the association between behavioral inhibition and posttraumatic stress disorder. Cognit. Ther. Res. 2012, 36, 407–416. [Google Scholar] [CrossRef]
- Hasenkamp, W.; Wilson-Mendenhall, C.D.; Duncan, E.; Barsalou, L.W. Mind wandering and attention during focused meditation: A fine-grained temporal analysis of fluctuating cognitive states. Neuroimage 2012, 59, 750–760. [Google Scholar] [CrossRef] [Green Version]
- Kabat-Zinn, J. Mindfulness-based interventions in context: Past, present, and future. Clin. Psychol. 2003, 10, 144–156. [Google Scholar] [CrossRef]
- Ding, X.; Wang, X.; Yang, Z.; Tang, R.; Tang, Y. Relationship between trait mindfulness and sleep quality in college students: A conditional process model. Front. Psychol. 2020, 11, 576319. [Google Scholar] [CrossRef]
- Brown, K.W.; Ryan, R.M. The benefits of being present: Mindfulness and its role in psychological well-being. J. Pers. Soc. Psychol. 2003, 84, 822–848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giluk, T.L. Mindfulness, Big Five personality, and affect: A meta-analysis. Pers. Individ. Differ. 2009, 47, 805–811. [Google Scholar] [CrossRef]
- Kostanski, M. The role of mindfulness in reducing stress for pre-service students. In Proceedings of the Annual Conference of the Australian Association for Research in Education, Fremantle, Australia, 4 November 2007. [Google Scholar]
- O’Loughlin, R.; Zuckerman, M. Mindfulness as a moderator of the relationship between dehydroepiandrosterone and reported physical symptoms. Pers. Individ. Differ. 2008, 44, 1193–1202. [Google Scholar] [CrossRef]
- Plaut, V.C.; Bartlett, R.P. Blind Consent? A social psychological investigation of Non-readership of Click-through agreements. Law Hum. Behav. 2012, 36, 293–311. [Google Scholar] [CrossRef]
- Watson, D.; Clark, L.A. Negative affectivity: The disposition to experience aversive emotional states. Psychol. Bull. 1984, 96, 465–490. [Google Scholar] [CrossRef]
- Denovan, A.; Dagnall, N.; Lofthouse, G. Neuroticism and somatic complaints: Concomitant effects of rumination and worry. Behav. Cogn. Psychother. 2019, 47, 431–445. [Google Scholar] [CrossRef]
- Borkovec, T.D.; Ray, W.J.; Stober, J. Worry: A cognitive phenomenon intimately linked to affective, physiological, and interpersonal behavioral processes. Cognit. Ther. Res. 1998, 22, 561–576. [Google Scholar] [CrossRef]
- Matthews, G.; Wells, A. Attention and Emotion: A Clinical Perspective, Classic ed.; Psychology: New York, NY, USA, 2014; ISBN 9781138814837. [Google Scholar]
- Beck, A.T. Cognitive models of depression. J. Cogn. Psychother. 1987, 1, 5–37. [Google Scholar]
- Beck, A.T. Depression: Clinical, Experimental, and Theoretical Aspects; University of Pennsylvania: Philadelphia, PA, USA, 1967. [Google Scholar]
- Beck, A.T. The evolution of the cognitive model of depression and its neurobiological correlates. Am. J. Psychiatry 2008, 165, 969–977. [Google Scholar] [CrossRef]
- Shapiro, S.L.; Carlson, L.E.; Astin, J.A.; Freedman, B. Mechanisms of mindfulness. J. Clin. Psychol. 2010, 62, 373–386. [Google Scholar] [CrossRef] [Green Version]
- Baer, R.A. Assessment of mindfulness by self-report: The Kentucky inventory of mindfulness skills. Assessment 2004, 11, 191–206. [Google Scholar] [CrossRef] [Green Version]
- Hill, C.L.M.; Updegraff, J.A. Mindfulness and its relationship to emotional regulation. Emotion 2012, 12, 81–90. [Google Scholar] [CrossRef]
- Qian, M.; Wu, T.; Zhu, R. Development of the revised Eysenck Personality Questionnaire short scale for Chinese (EPQ-RSC). Acta Polym. Sin. 2000, 32, 317–323. [Google Scholar] [CrossRef]
- Baer, R.A. Using self-report assessment methods to explore facets of mindfulness. Assessment 2006, 13, 27–45. [Google Scholar] [CrossRef] [Green Version]
- Deng, Y. Effect of Mental Awareness Training on Mental Health of College Students. Master’s Thesis, Capital Normal University, Beijing, China, 2009. [Google Scholar]
- Wei, Y.; Liu, Y. Reliability and validity of the Cognitive Emotion Regulation Questionnaire (CERQ) in Chinese college students. Ment. Health Phys. Act. 2008, 22, 281–283. [Google Scholar] [CrossRef]
- Zhang, R. The Compilation and Application of Negative Cognitive Processing Bias Questionnaire. Master’s Thesis, The Third Military Medical University, Chongqing, China, 2015. [Google Scholar]
- Muthén, L.K.; Muthén, B.O. Mplus Statistical Analysis with Latent Variables: User’s Guide; Version 8; Muthén & Muthén: Los Angeles, CA, USA, 2017; ISBN 0982998325. [Google Scholar]
- Wen, Z.; Ye, B. Analyses of mediating effects: The development of methods and models. Adv. Psychol. Sci. 2014, 22, 731–745. [Google Scholar] [CrossRef]
- Drake, M.M.; Morris, D.M.; Davis, T.J. Neuroticism’s susceptibility to distress: Moderated with mindfulness. Pers. Individ. Dif. 2016, 106, 248–252. [Google Scholar] [CrossRef]
- Rebecca, L.; Nicholas, R.; Maital, N. Interpersonal emotion regulation mitigates the link between trait neuroticism and a more negative valence bias. Pers. Individ. Dif. 2022, 196, 111726. [Google Scholar] [CrossRef]
- Wang, Y.; Luo, Y.J. Standardization and assessment of college students’ facial expression of emotion. Chin. J. Clin. Oncol. 2005, 13, 396–398. [Google Scholar] [CrossRef]
- Dearing, K.; Gotlib, I. Interpretation of ambiguous information in girls at risk for depression. J. Abnorm. Child Psychol. 2008, 37, 79–91. [Google Scholar] [CrossRef] [Green Version]
- Koster, E.; Crombez, G.; Verschuere, B.; Damme, S.V.; Wiersema, J.R. Components of attentional bias to threat in high trait anxiety: Facilitated engagement, impaired disengagement, and attentional avoidance. Behav. Res. Ther. 2006, 44, 1757–1771. [Google Scholar] [CrossRef]
- Norris, C.J. The negativity bias revisited: Evidence from neuroscience measures and an individual differences approach. Soc. Neurosci. 2019, 16, 68–82. [Google Scholar] [CrossRef]
- Horstmann, G.; Borgstedt, K.; Heumann, M. Flanker effects with faces may depend on perceptual as well as emotional differences. Emotion 2006, 6, 28–39. [Google Scholar] [CrossRef]
- Mitte, K. Memory bias for threatening information in anxiety and anxiety disorders: A meta-analytic review. Psychol. Bull. 2008, 134, 886–911. [Google Scholar] [CrossRef]
- Stopa, L.; Clark, D.M. Social phobia and interpretation of social events. Behav. Res. Ther. 2000, 38, 273–283. [Google Scholar] [CrossRef]
- Orchard, F.; Pass, L.; Reynolds, S. ‘It was all my fault’; Negative interpretation bias in depressed adolescents. J. Abnorm. Child Psychol. 2016, 44, 991–998. [Google Scholar] [CrossRef]
- Cheung, G.W.; Rensvold, R.B. Evaluating goodness-of-fit indexes for testing measurement invariance. Struct. Equ. Model. 2002, 9, 233–255. [Google Scholar] [CrossRef]
- McCrae, R.R.; Costa, P.T. The Five-Factor Theory of Personality. In Handbook of Personality: Theory and Research: North Carolina, 3rd ed.; John, O.P., Robins, R.W., Pervin, L.A., Eds.; Guilford Press: New York, NY, USA, 2008; pp. 159–181. [Google Scholar]
- Chen, X.; Pu, J.; Shi, W.; Zhou, Y. The impact of neuroticism on symptoms of anxiety and depression in elderly adults: The mediating role of rumination. Curr. Psychol. 2020, 39, 42–50. [Google Scholar] [CrossRef]
- Tonarely, N.A.; Sherman, J.A.; Grossman, R.A.; Shaw, A.M.; Ehrenreich-May, J. Neuroticism as an underlying construct in youth emotional disorders. Bull. Menninger Clin. 2020, 84, 214–236. [Google Scholar] [CrossRef]
- Gross, J.J. Emotion regulation: Past, present, future. Cogn. Emot. 1999, 13, 551–573. [Google Scholar] [CrossRef]
- Beevers, C.G. Cognitive vulnerability to depression: A dual process model. Clin. Psychol. Rev. 2005, 25, 975–1002. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Strauss, C.; Bond, R.; Cavanagh, K. How do mindfulness-based cognitive therapy and mindfulness-based stress reduction improve mental health and wellbeing? A systematic review and meta-analysis of mediation studies. Clin. Psychol. Rev. 2015, 37, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Garland, E.L.; Farb, N.A.; Goldin, P.R.; Fredrickson, B.L. The mindfulness-to-meaning theory: Extensions, applications, and challenges at the attention–appraisal–emotion interface. Psychol. Inq. 2015, 26, 377–387. [Google Scholar] [CrossRef]
- Bishop, S. Mindfulness: A proposed operational definition. Clin. Psychol. Sci. Pract. 2004, 11, 230. [Google Scholar] [CrossRef]
- Rijsdijk, F.V.; Riese, H.; Tops, M.; Snieder, H.; Brouwer, W.H.; Smid, H.G.O.M.; Ormel, J. Neuroticism, recall bias and attention bias for valenced probes: A twin study. Psychol. Med. 2009, 39, 45–54. [Google Scholar] [CrossRef] [Green Version]
- Ikeda, S.; Mizuno-Matsumoto, Y.; Canuet, L.; Ishii, R.; Aoki, Y.; Hata, M.; Katsimichas, T.; Pascual-Marqui, R.D.; Hayashi, T.; Okamoto, E.; et al. Emotion regulation of neuroticism: Emotional information processing related to psychosomatic state evaluated by electroencephalography and exact low-resolution brain electromagnetic tomography. Neuropsychobiology 2015, 71, 34–41. [Google Scholar] [CrossRef] [Green Version]
- Trafimow, D. Editorial. Basic Appl. Soc. Psychol. 2014, 36, 1–2. [Google Scholar] [CrossRef]
- Kotov, R.; Gamez, W.; Schmidt, F.; Watson, D. Linking “big” personality traits to anxiety, depressive, and substance use disorders: A meta-analysis. Psychol. Bull. 2010, 136, 768–821. [Google Scholar] [CrossRef]
- Armstrong, L.; Rimes, K.A. Mindfulness-based cognitive therapy for neuroticism (stress vulnerability): A pilot randomized study. Behav. Ther. 2016, 47, 287–298. [Google Scholar] [CrossRef]






| Variable | M | SD | 1 | 2 | 3 | 4 |
|---|---|---|---|---|---|---|
| 1. Neuroticism | 50.00 | 36.02 | 1 | |||
| 2. Mindfulness | 2.97 | 0.33 | −0.364 *** | 1 | ||
| 3. Negative cognitive bias | 4.32 | 0.74 | 0.734 *** | −0.388 *** | 1 | |
| 4. Negative emotion regulation | 3.06 | 0.52 | 0.551 *** | −0.290 *** | 0.597 *** | 1 |
| Path | Standardized Estimates | Effect Size (%) | 95% CI |
|---|---|---|---|
| Total effect | 0.553 *** | 100 | [0.445, 0.652] |
| Total indirect effect | 0.535 *** | 96.75 | [0.316, 0.783] |
| 1. Neuroticism → mindfulness → negative emotion regulation | −0.008 | 1.45 | [−0.067, 0.036] |
| 2. Neuroticism → negative cognitive bias → negative emotion regulation | 0.500 *** | 90.42 | [0.283, 0.750] |
| 3. Neuroticism → mindfulness → negative cognitive bias → negative emotion regulation | 0.043 * | 7.78 | [0.014, 0.096] |
| High Neuroticism Group | Low Neuroticism Group | Medium Control Group | F | p | |
|---|---|---|---|---|---|
| score | 90.00 ± 17.89 | 11.33 ± 12.30 | 48.00 ± 4.98 | 281.104 | 0.000 |
| N | 30 | 30 | 30 |
| High Neuroticism Group | Low Neuroticism Group | Medium Control Group | |
|---|---|---|---|
| positive consistent | 365.52 ± 62.92 | 339.57 ± 38.02 | 370.95 ± 69.79 |
| positive inconsistent | 357.34 ± 63.05 | 348.48 ± 40.18 | 376.43 ± 83.05 |
| negative consistent | 358.04 ± 64.43 | 346.40 ± 41.86 | 372.31 ± 67.21 |
| negative inconsistent | 364.52 ± 70.97 | 341.64 ± 39.90 | 372.39 ± 58.75 |
| neutral | 357.57 ± 60.90 | 345.63 ± 43.28 | 367.73 ± 66.92 |
| High Neuroticism Group | Low Neuroticism Group | Medium Control Group | |
|---|---|---|---|
| positive attention bias | −8.19 ± 13.33 | 8.91 ± 11.45 | 5.48 ± 26.18 |
| negative attention bias | 6.47 ± 16.18 | −4.76 ± 10.93 | 0.08 ± 22.27 |
| High Neuroticism Group | Low Neuroticism Group | Medium Control Group | |
|---|---|---|---|
| positive picture | 20.88 ± 19.55 | 20.66 ± 16.55 | 26.66 ± 19.65 |
| negative picture | 29.78 ± 18.67 | 37.33 ± 21.20 | 32.44 ± 22.27 |
| neutral picture | 16.88 ± 14.72 | 20.88 ± 16.77 | 22.00 ± 19.43 |
| High Neuroticism Group | Low Neuroticism Group | Medium Control Group | |
|---|---|---|---|
| endorse threat | 470.44 ± 195.72 | 461.59 ± 251.06 | 526.63 ± 275.89 |
| reject threat | 571.90 ± 336.21 | 510.90 ± 224.73 | 606.61 ± 449.04 |
| endorse benign | 524.04 ± 291.43 | 495.89 ± 249.09 | 569.41 ± 311.05 |
| reject benign | 556.50 ± 303.24 | 549.39 ± 311.79 | 556.20 ± 287.46 |
| High Neuroticism Group | Low Neuroticism Group | Medium Control Group | |
|---|---|---|---|
| positive interpretation bias | 31.86 ± 4.94 | 32.26 ± 5.13 | 31.59 ± 6.22 |
| negative interpretation bias | 35.86 ± 5.41 | 31.76 ± 3.94 | 32.23 ± 6.52 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.; Liu, X.; Weng, X.; Huang, M.; Weng, Y.; Zeng, H.; Li, Y.; Zheng, D.; Chen, C. The Emotion Regulation Mechanism in Neurotic Individuals: The Potential Role of Mindfulness and Cognitive Bias. Int. J. Environ. Res. Public Health 2023, 20, 896. https://doi.org/10.3390/ijerph20020896
Chen L, Liu X, Weng X, Huang M, Weng Y, Zeng H, Li Y, Zheng D, Chen C. The Emotion Regulation Mechanism in Neurotic Individuals: The Potential Role of Mindfulness and Cognitive Bias. International Journal of Environmental Research and Public Health. 2023; 20(2):896. https://doi.org/10.3390/ijerph20020896
Chicago/Turabian StyleChen, Ling, Xiqin Liu, Xiangrun Weng, Mingzhu Huang, Yuhan Weng, Haoran Zeng, Yifan Li, Danna Zheng, and Caiqi Chen. 2023. "The Emotion Regulation Mechanism in Neurotic Individuals: The Potential Role of Mindfulness and Cognitive Bias" International Journal of Environmental Research and Public Health 20, no. 2: 896. https://doi.org/10.3390/ijerph20020896






