Regulation Effectiveness and Mechanism of Biotransformation Pathway on the Toxicity of Microcystin-LR Target to Protein Phosphatase 2A
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
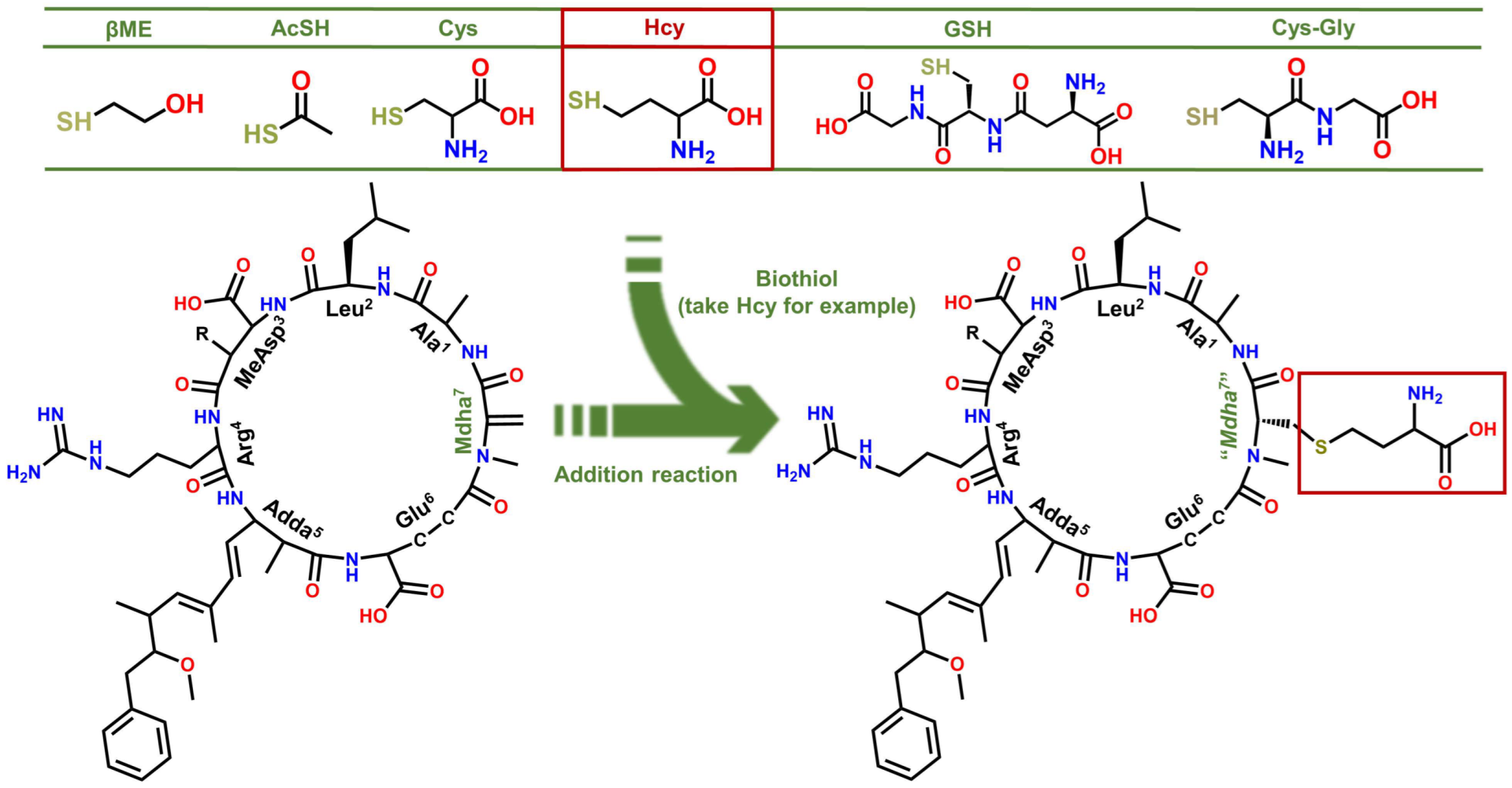
2.2. Addition Reaction of Biothiols to MCLR
2.3. Identification and Preparation of MCLR-BTPs
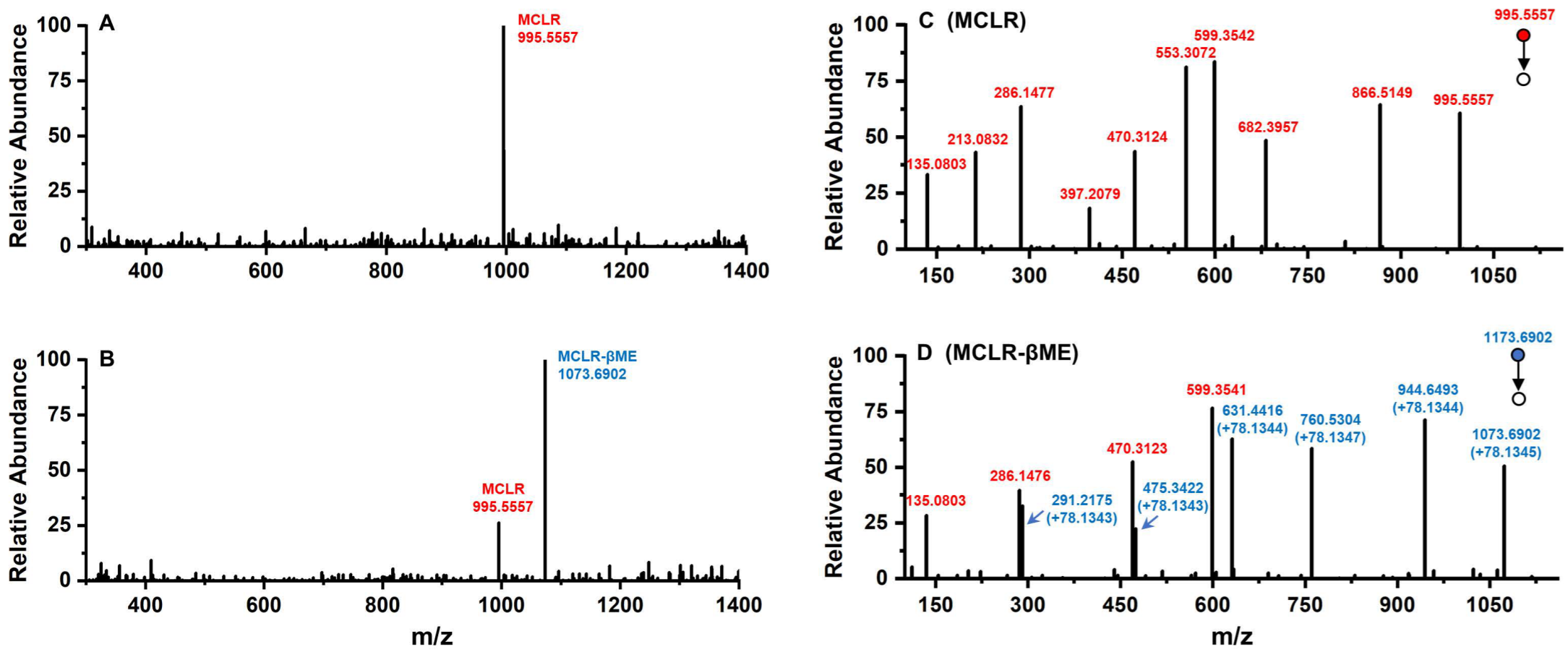
2.3.1. MS and MS/MS Analysis of MCLR-BTPs
2.3.2. Preparation of MCLR-BTPs
2.4. PP2A-Inhibition Assay for MCLR and MCLR-BTPs
2.5. Molecular Simulation for the Interactions between MCLR/MCLR-BTPs and PP2A
2.6. Statistical Analysis
3. Results and Discussion
3.1. MCLR-BTP Identification and Preparation
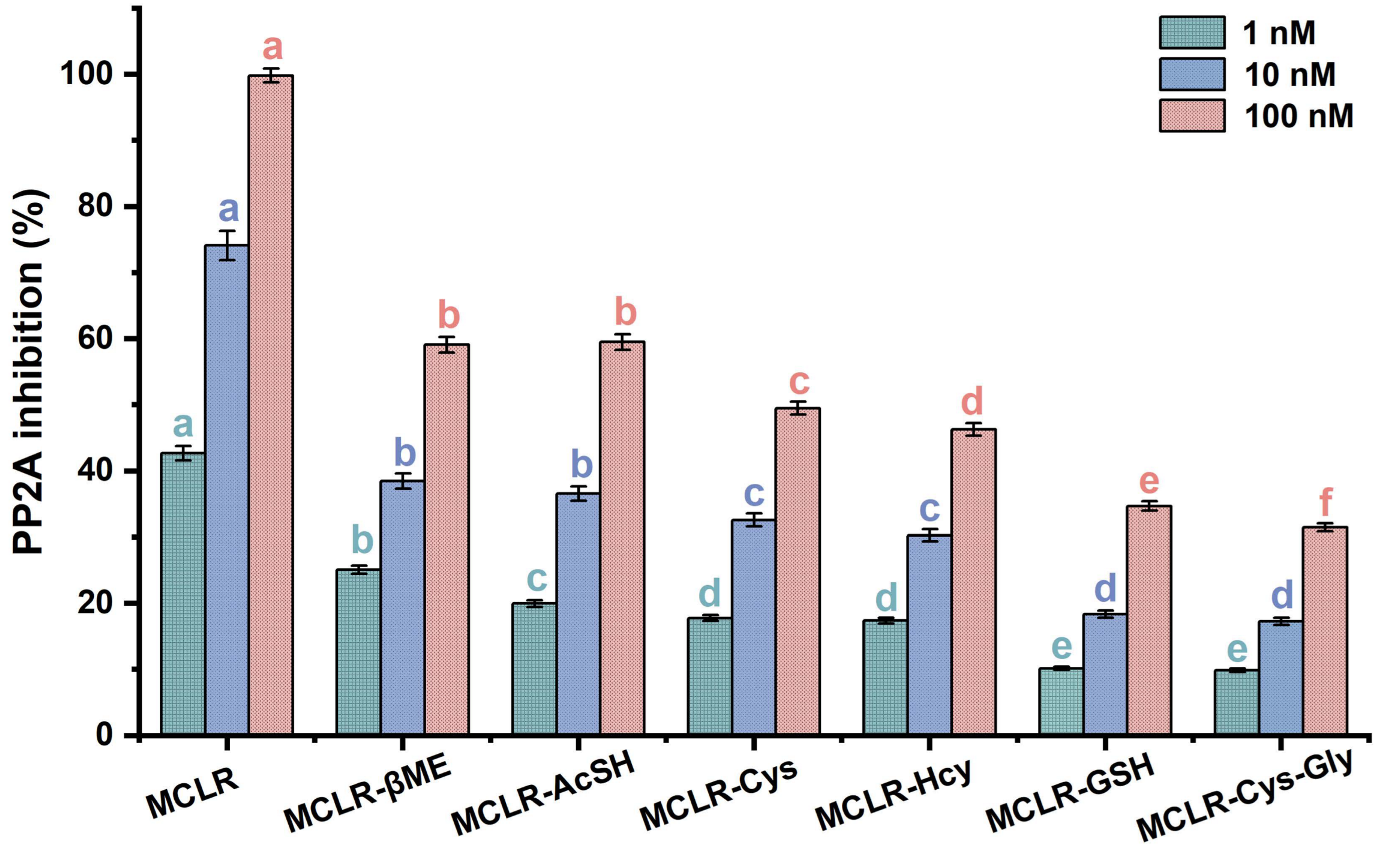
3.2. Evaluation of the Inhibition Effects of MCLR/MCLR-BTPs Targeted to PP2A
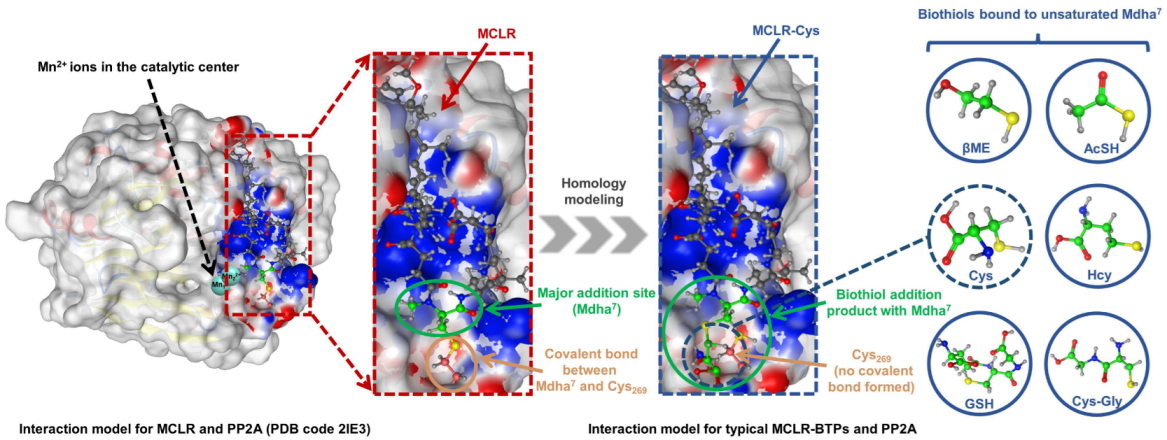
3.3. Simulation of the Interactions between MCLR-BTPs and PP2A Based on Homology Modeling and Molecular Simulation
3.4. Pearson Correlation Analysis for the Candidate Interaction Parameters and Inhibition Data
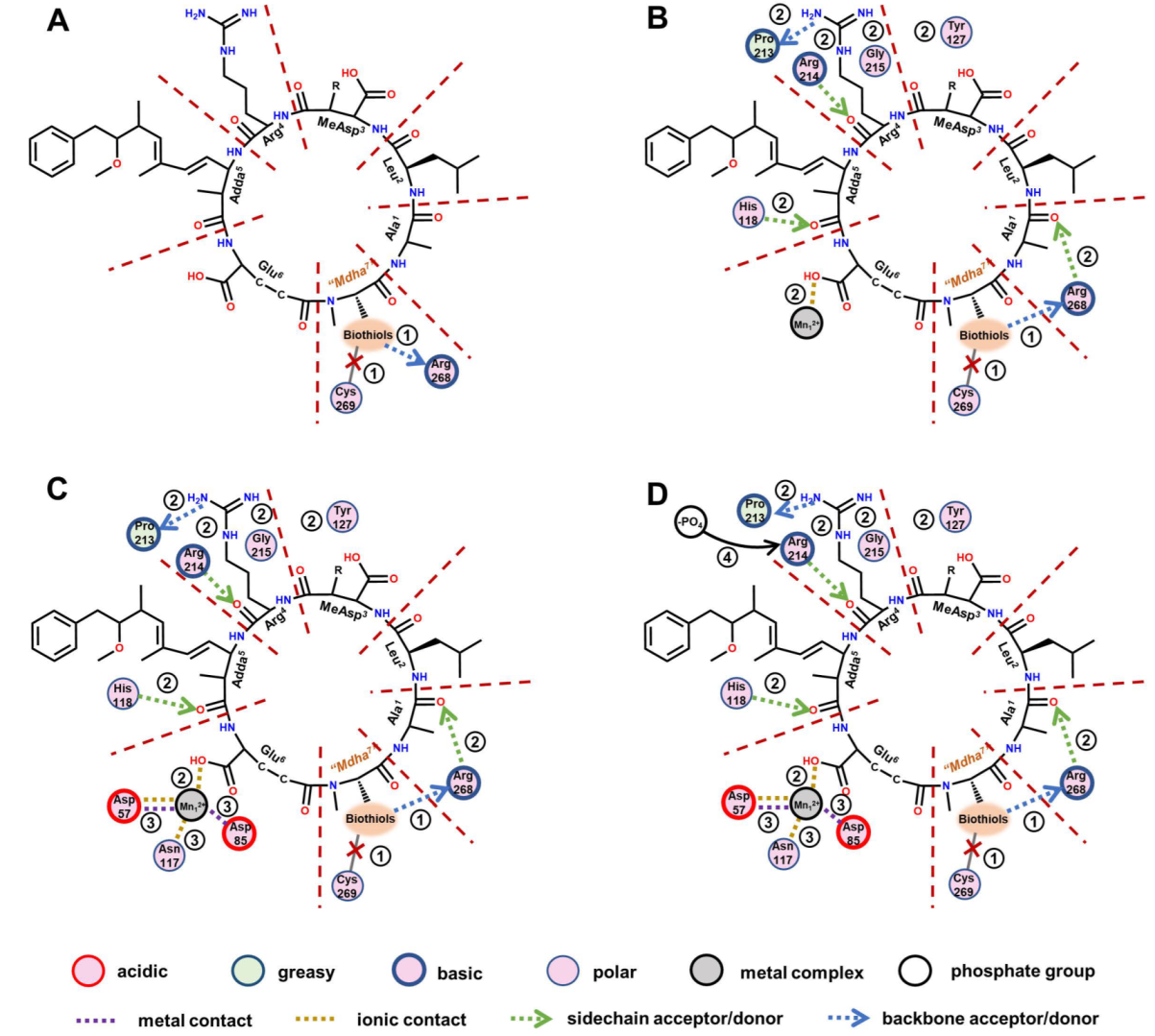
3.5. Molecular Mechanism behind Biothiols’ Regulation of the Inhibition Effects of MCLR-BTPs on PP2A
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Buratti, F.M.; Manganelli, M.; Vichi, S.; Stefanelli, M.; Scardala, S.; Testai, E.; Funari, E. Cyanotoxins: Producing organisms, occurrence, toxicity, mechanism of action and human health toxicological risk evaluation. Arch. Toxicol. 2017, 91, 1049–1130. [Google Scholar] [CrossRef]
- Huisman, J.; Codd, G.A.; Paerl, H.W.; Ibelings, B.W.; Verspagen, J.M.H.; Visser, P.M. Cyanobacterial blooms. Nat. Rev. Genet. 2018, 16, 471–483. [Google Scholar] [CrossRef] [PubMed]
- Campos, A.; Vasconcelos, V. Molecular Mechanisms of Microcystin Toxicity in Animal Cells. Int. J. Mol. Sci. 2010, 11, 268–287. [Google Scholar] [CrossRef] [Green Version]
- de Figueiredo, D.R.; Azeiteiro, U.M.; Esteves, S.M.; Gonçalves, F.J.; Pereira, M.J. Microcystin-producing blooms—A serious global public health issue. Ecotoxicol. Environ. Saf. 2004, 59, 151–163. [Google Scholar] [CrossRef]
- Tachi, M.; Imanishi, S.Y.; Harada, K.-I. Phosphoprotein analysis for investigation ofin vivo relationship between protein phosphatase inhibitory activities and acute hepatotoxicity of microcystin-LR. Environ. Toxicol. 2007, 22, 620–629. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Rosso, L.; Sedan, D.; Giannuzzi, L.; Andrinolo, D.; Volmer, D.A. Seven new microcystin variants discovered from a native Microcystis aeruginosa strain–unambiguous assignment of product ions by tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2015, 29, 220–224. [Google Scholar] [CrossRef]
- Shen, Q.; Feng, J.; Wang, J.; Li, S.; Wang, Y.; Ma, J.; Wang, H. Laser irradiation desorption of microcystins from protein complex in fish tissue and liquid chromatography—Tandem mass spectrometry analysis. Electrophoresis 2019, 40, 1805–1811. [Google Scholar] [CrossRef] [PubMed]
- Craig, M.; Luu, H.A.; McCready, T.L.; Holmes, C.F.B.; Williams, D.; Andersen, R.J. Molecular mechanisms underlying the interaction of motuporin and microcystins with type-1 and type-2A protein phosphatases. Biochem. Cell Biol. 1996, 74, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Fontanillo, M.; Köhn, M. Microcystins: Synthesis and structure–activity relationship studies toward PP1 and PP2A. Bioorg. Med. Chem. 2018, 26, 1118–1126. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Fan, Z.; Zhang, L.; You, Q.; Wang, L. Strategies for Targeting Serine/Threonine Protein Phosphatases with Small Molecules in Cancer. J. Med. Chem. 2021, 64, 8916–8938. [Google Scholar] [CrossRef]
- Ren, L.; Hu, Z.; Wang, Q.; Du, Y.; Zong, W. Regulation Efficacy and Mechanism of the Toxicity of Microcystin-LR Targeting Protein Phosphatase 1 via the Biodegradation Pathway. Toxins 2020, 12, 790. [Google Scholar] [CrossRef]
- Zhang, Y.; Shao, Y.; Gao, N.; Chu, W.; Sun, Z. Removal of microcystin-LR by free chlorine: Identify of transformation products and disinfection by-products formation. Chem. Eng. J. 2016, 287, 189–195. [Google Scholar] [CrossRef]
- Wang, H.; Ho, L.; Lewis, D.M.; Brookes, J.D.; Newcombe, G. Discriminating and assessing adsorption and biodegradation removal mechanisms during granular activated carbon filtration of microcystin toxins. Water Res. 2007, 41, 4262–4270. [Google Scholar] [CrossRef] [PubMed]
- Zong, W.; Sun, F.; Pei, H.; Hu, W.; Pei, R. Microcystin-associated disinfection by-products: The real and non-negligible risk to drinking water subject to chlorination. Chem. Eng. J. 2015, 279, 498–506. [Google Scholar] [CrossRef]
- Hayes, J.D.; Flanagan, J.U.; Jowsey, I.R. Glutathione Transferases. Annu. Rev. Pharmacol. Toxicol. 2005, 45, 51–88. [Google Scholar] [CrossRef]
- Metcalf, J.; Beattie, K.A.; Ressler, J.; Gerbersdorf, S.; Pflugmacher, S.; Codd, G.A. Cross-reactivity and performance assessment of four microcystin immunoassays with detoxication products of the cyanobacterial toxin, microcystin-LR. J. Water Supply Res. Technol. 2002, 51, 145–151. [Google Scholar] [CrossRef]
- Zong, W.-S.; Zhang, S.-H.; Wang, Q.; Teng, Y.; Liu, Y.-Z.; Du, Y.-G. Evaluation of the Direct and Indirect Regulation Pathways of Glutathione Target to the Hepatotoxicity of Microcystin-LR. BioMed Res. Int. 2018, 2018, 5672637. [Google Scholar] [CrossRef] [PubMed]
- Zong, W.; Wang, X.; Du, Y.; Zhang, S.; Zhang, Y.; Teng, Y. Molecular Mechanism for the Regulation of Microcystin Toxicity to Protein Phosphatase 1 by Glutathione Conjugation Pathway. BioMed Res. Int. 2017, 2017, 9676504. [Google Scholar] [CrossRef] [Green Version]
- Pereira, S.R.; Vasconcelos, V.M.; Antunes, A. Computational study of the covalent bonding of microcystins to cysteine residues–a reaction involved in the inhibition of the PPP family of protein phosphatases. FEBS J. 2013, 280, 674–680. [Google Scholar] [CrossRef] [Green Version]
- Yang, M.; Lam, P.K.; Huang, M.; Wong, B.S. Effects of microcystins on phosphorylase-a binding to phosphatase-2A: Kinetic analysis by surface plasmon resonance biosensor. Biochim. Biophys. Acta (BBA) Gen. Subj. 1999, 1427, 62–73. [Google Scholar] [CrossRef]
- Guo, X.; Chen, L.; Chen, J.; Xie, P.; Li, S.; He, J.; Li, W.; Fan, H.; Yu, D.; Zeng, C. Quantitatively evaluating detoxification of the hepatotoxic microcystin-LR through the glutathione (GSH) pathway in SD rats. Environ. Sci. Pollut. Res. 2015, 22, 19273–19284. [Google Scholar] [CrossRef]
- Zong, W.; Wang, Q.; Zhang, S.; Teng, Y.; Du, Y. Regulation on the toxicity of microcystin-LR target to protein phosphatase 1 by biotransformation pathway: Effectiveness and mechanism. Environ. Sci. Pollut. Res. 2018, 25, 26020–26029. [Google Scholar] [CrossRef]
- Li, C.-M.; Chu, R.Y.-Y.; Hsieh, D.P.H. An enhanced LC-MS/MS method for microcystin-LR in lake water. J. Mass Spectrom. 2006, 41, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Barco, M.; Flores, C.; Rivera, J.; Caixach, J. Determination of microcystin variants and related peptides present in a water bloom of Planktothrix (Oscillatoria) rubescens in a Spanish drinking water reservoir by LC/ESI-MS. Toxicon 2004, 44, 881–886. [Google Scholar] [CrossRef]
- Xu, Y.; Cui, J.; Yu, H.; Zong, W. Insight into the Molecular Mechanism for the Discrepant Inhibition of Microcystins (MCLR, LA, LF, LW, LY) on Protein Phosphatase 2A. Toxins 2022, 14, 390. [Google Scholar] [CrossRef] [PubMed]
- Zong, W.; Sun, F.; Sun, X. Oxidation by-products formation of microcystin-LR exposed to UV/H2O2: Toward the generative mechanism and biological toxicity. Water Res. 2013, 47, 3211–3219. [Google Scholar] [CrossRef] [PubMed]
- Prieto-Martínez, F.D.; Medina-Franco, J.L. Current advances on the development of BET inhibitors: Insights from computational methods. Adv. Protein Chem. Struct. Biol. 2020, 122, 127–180. [Google Scholar] [CrossRef]
- Pflugmacher, S.; Codd, G.A.; Steinberg, C.E.W. Effects of the cyanobacterial toxin microcystin-LR on detoxication enzymes in aquatic plants. Environ. Toxicol. 1999, 14, 111–115. [Google Scholar] [CrossRef]
- Pflugmacher, S.; Wiegand, C.; Oberemm, A.; Beattie, K.A.; Krause, E.; Codd, G.A.; Steinberg, C.E. Identification of an enzymatically formed glutathione conjugate of the cyanobacterial hepatotoxin microcystin-LR: The first step of detoxication. Biochim. Et Biophys. Acta BBA Gen. Subj. 1998, 1425, 527–533. [Google Scholar] [CrossRef]
- Chu, Y.; Lee, E.Y.; Schlender, K.K. Activation of Protein Phosphatase 1. J. Biol. Chem. 1996, 271, 2574–2577. [Google Scholar] [CrossRef] [PubMed]
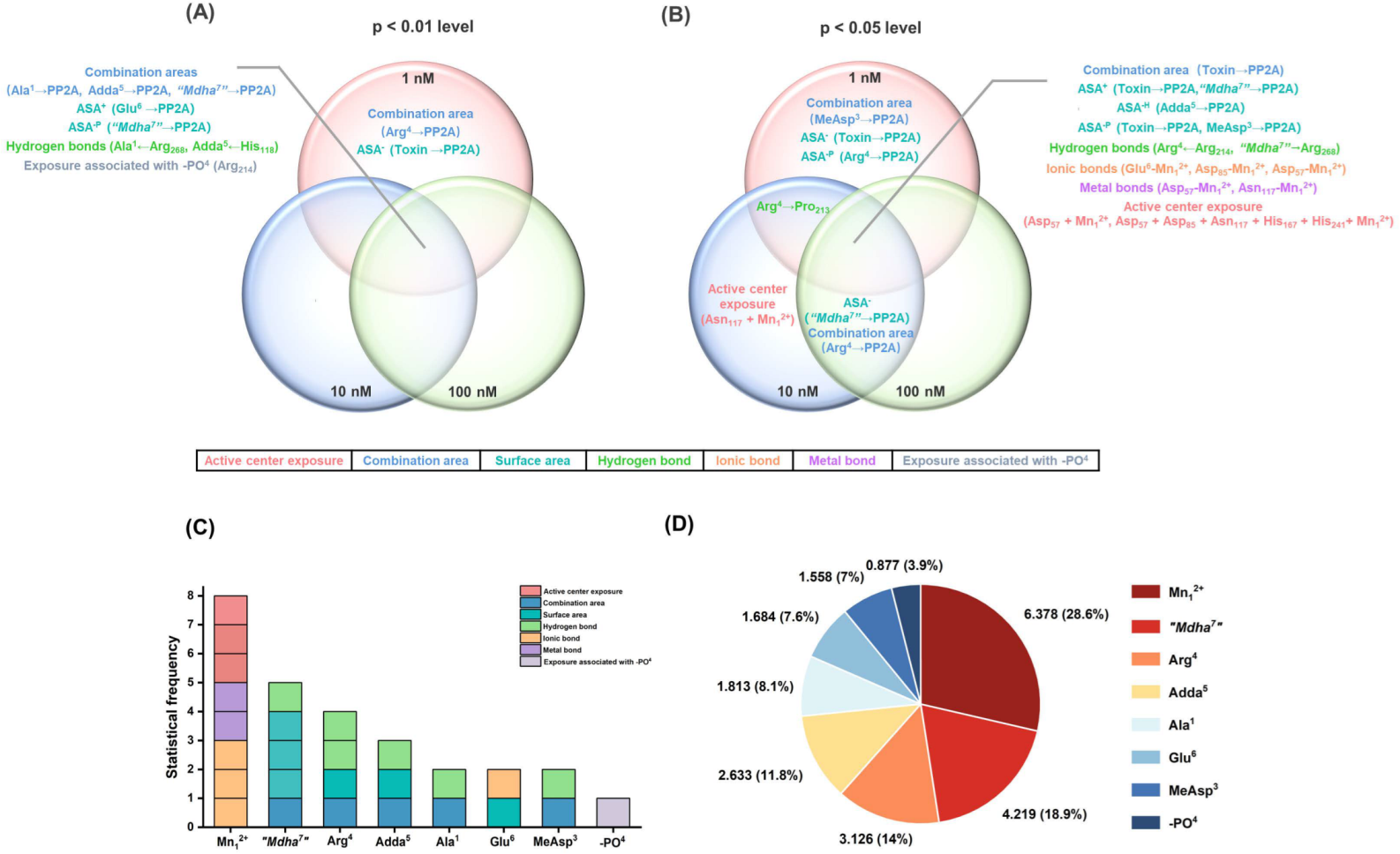
 ,
,  ,
,  mean that the interaction parameters are extremely significantly correlated with the inhibition data at the levels of 1, 10, and 100 nM, respectively (p < 0.01). The symbols
mean that the interaction parameters are extremely significantly correlated with the inhibition data at the levels of 1, 10, and 100 nM, respectively (p < 0.01). The symbols  ,
,  ,
,  mean that the interaction parameters are significantly correlated with the inhibition data at the levels of 1, 10, and 100 nM, respectively (p < 0.05).
mean that the interaction parameters are significantly correlated with the inhibition data at the levels of 1, 10, and 100 nM, respectively (p < 0.05).
 ,
,  ,
,  mean that the interaction parameters are extremely significantly correlated with the inhibition data at the levels of 1, 10, and 100 nM, respectively (p < 0.01). The symbols
mean that the interaction parameters are extremely significantly correlated with the inhibition data at the levels of 1, 10, and 100 nM, respectively (p < 0.01). The symbols  ,
,  ,
,  mean that the interaction parameters are significantly correlated with the inhibition data at the levels of 1, 10, and 100 nM, respectively (p < 0.05).
mean that the interaction parameters are significantly correlated with the inhibition data at the levels of 1, 10, and 100 nM, respectively (p < 0.05).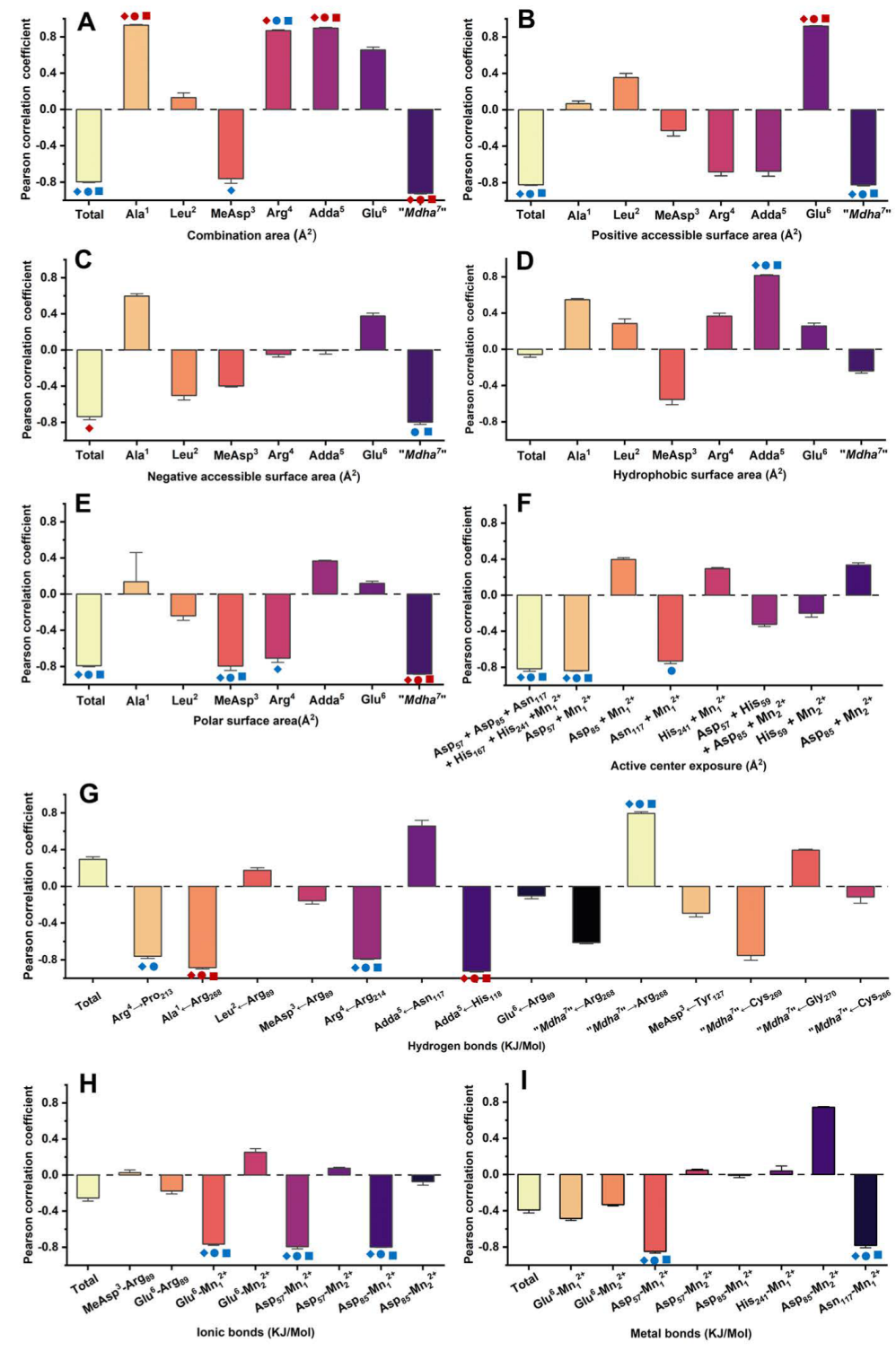
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, H.; Cui, J.; Xu, Y.; Feng, L.-J.; Zong, W. Regulation Effectiveness and Mechanism of Biotransformation Pathway on the Toxicity of Microcystin-LR Target to Protein Phosphatase 2A. Int. J. Environ. Res. Public Health 2023, 20, 964. https://doi.org/10.3390/ijerph20020964
Yu H, Cui J, Xu Y, Feng L-J, Zong W. Regulation Effectiveness and Mechanism of Biotransformation Pathway on the Toxicity of Microcystin-LR Target to Protein Phosphatase 2A. International Journal of Environmental Research and Public Health. 2023; 20(2):964. https://doi.org/10.3390/ijerph20020964
Chicago/Turabian StyleYu, Huiqun, Jiyuan Cui, Yixue Xu, Li-Juan Feng, and Wansong Zong. 2023. "Regulation Effectiveness and Mechanism of Biotransformation Pathway on the Toxicity of Microcystin-LR Target to Protein Phosphatase 2A" International Journal of Environmental Research and Public Health 20, no. 2: 964. https://doi.org/10.3390/ijerph20020964



