Effects of Standing after a Meal on Glucose Metabolism and Energy Expenditure
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Design
2.3. Statistical Analysis
3. Results
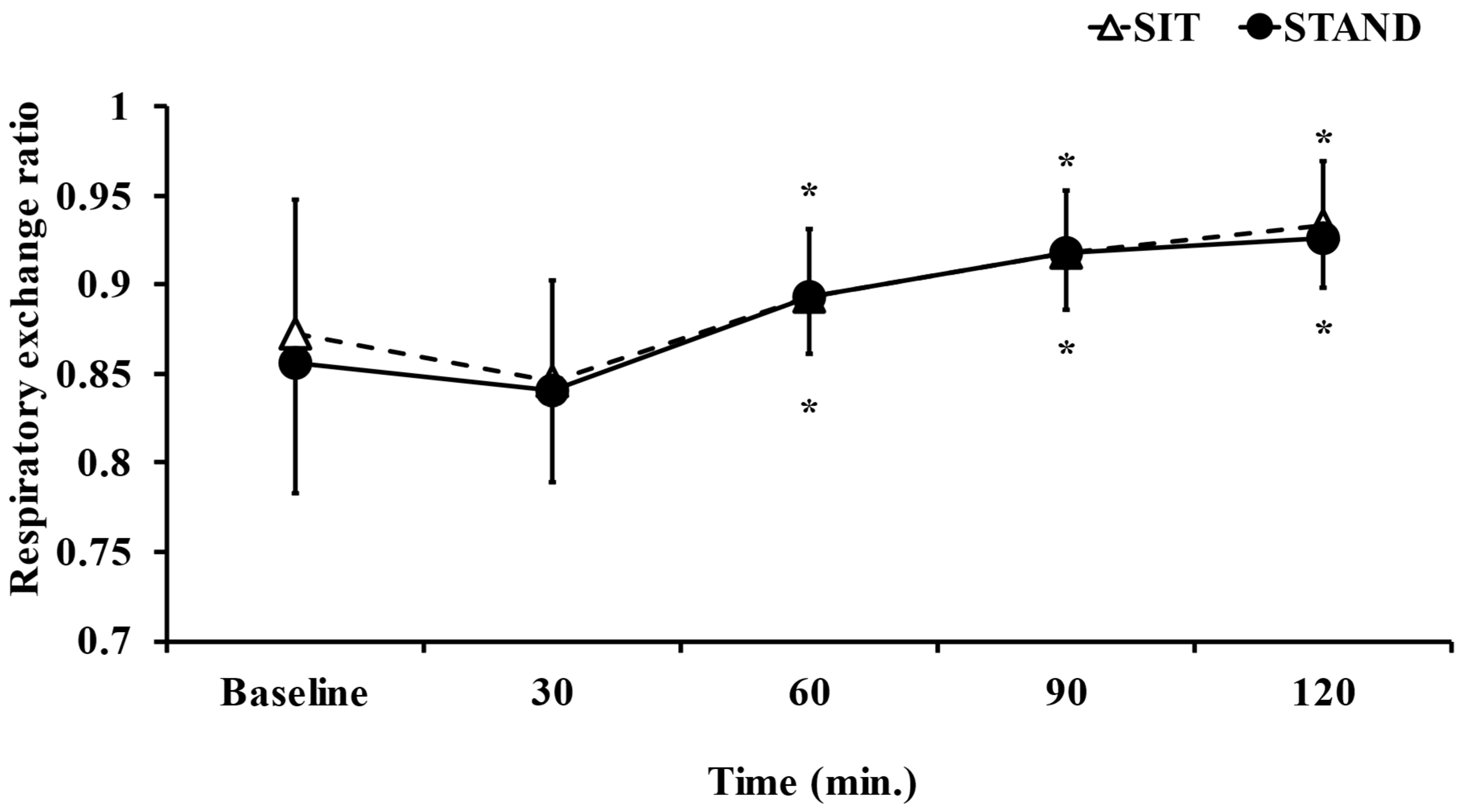
3.1. Energy Expenditure and RER
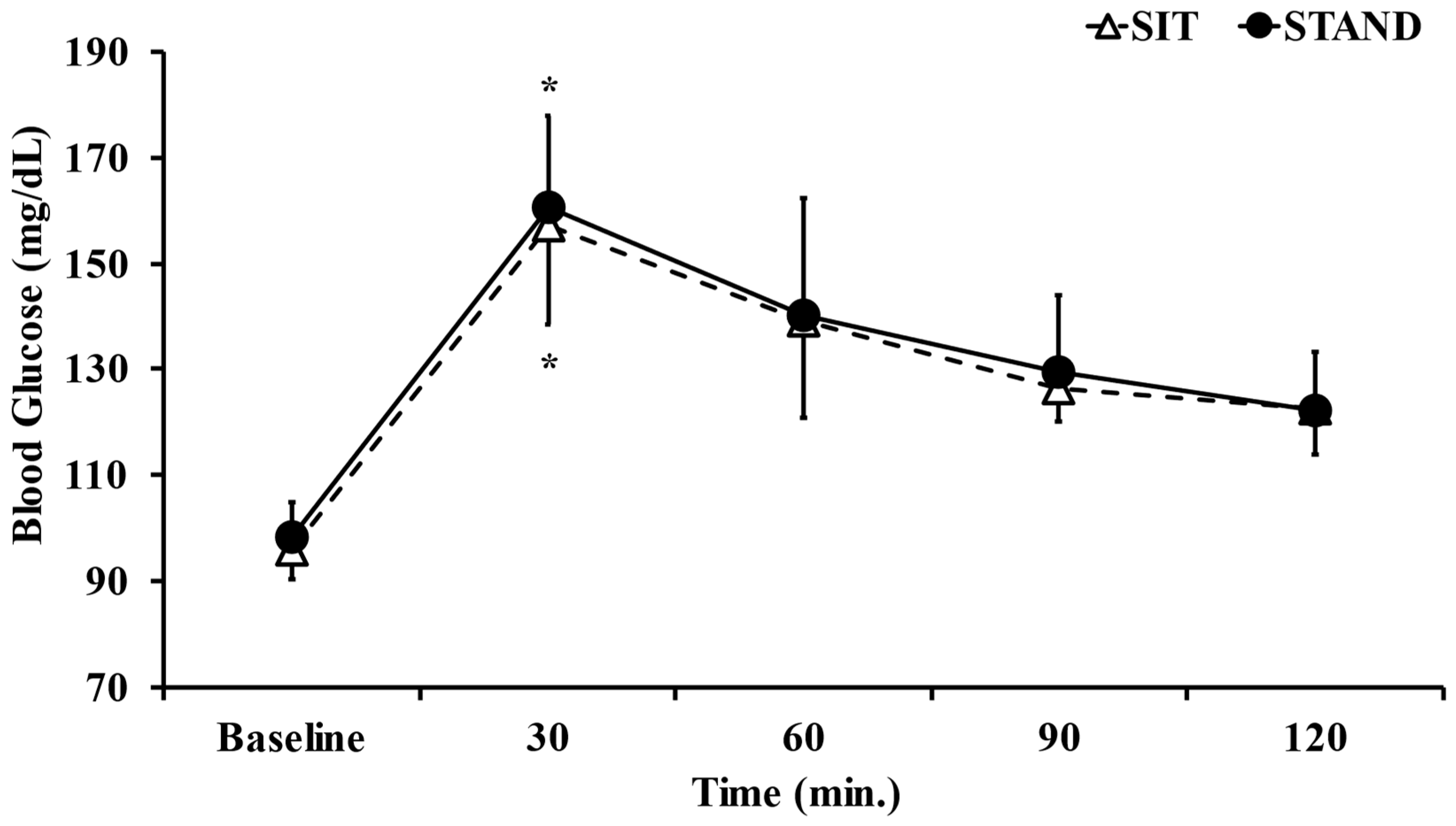
3.2. Blood Glucose
3.3. HR
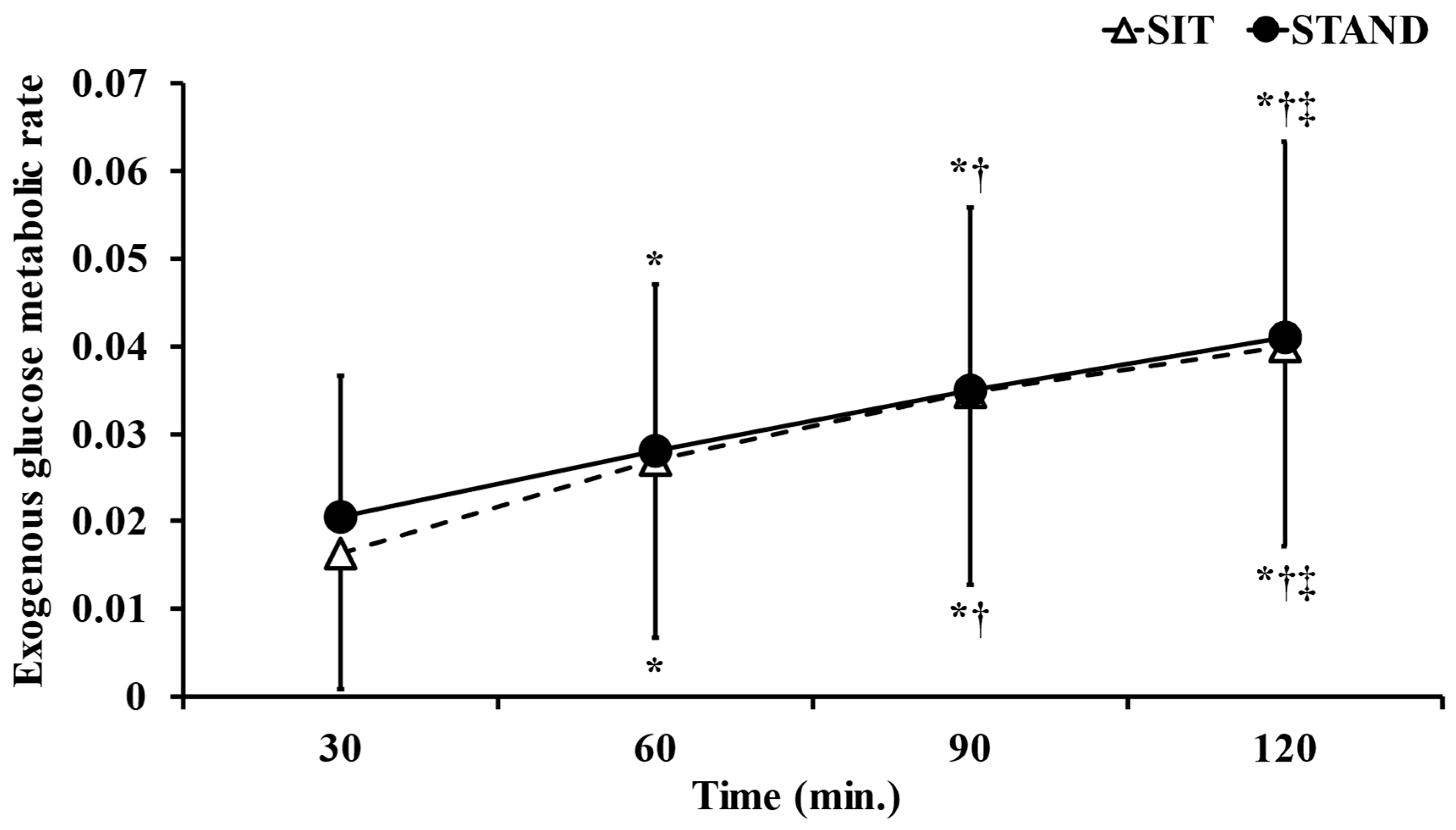
3.4. Exogenous Glucose Metabolic Rate
3.5. RPE
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Obesity: Preventing and managing the global epidemic. Report of a WHO consultation. In World Health Organization Technical Report Series; World Health Organization: Geneva, Switzerland, 2000; Volume 894. [Google Scholar]
- James, A.L.; Lorraine, M.L.; Shelly, K.M.; Shelly, K.M.; Alisa, C.K.; Leslie, R.O.; Paul, H.K.; Mickael, D.J.; Matthew, M.C. Interindividual variation in posture allocation: Possible role in human obesity. Science 2005, 307, 584–586. [Google Scholar] [CrossRef]
- Oscar, C.; Jason, B.; Ineke, V.; Gregoire, B.; Stuart, J.H.B. How Sedentary Are University Students? A Systematic Review and Meta-Analysis. Prev. Sci. 2000, 21, 332–343. [Google Scholar] [CrossRef]
- Francesco, L.; Valentina, C.; Valentina, V.; Gaspare, P. COVID-19 lockdown: Physical activity, sedentary behaviour and sleep in Italian medicine students. Eur. J. Sport Sci. 2021, 21, 1459–1468. [Google Scholar] [CrossRef]
- Fiona, C.B.; Salih, S.A.; Stuart, B.; Katja, B.; Matthew, P.B.; Greet, C.; Catherine, C.; Jean, P.C.; Sebastien, C.; Roger, C.; et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br. J. Sports Med. 2020, 54, 1451–1462. [Google Scholar] [CrossRef]
- Peter, T.K.; Timothy, S.C.; Cora, L.C.; Claude, B. Sitting time and mortality from all causes, cardiovascular disease, and cancer. Med. Sci. Sports Exerc. 2009, 41, 998–1005. [Google Scholar] [CrossRef]
- Shinto, A.; Teruhide, K.; Nagato, K.; Etsuko, O.; Ritei, U. The Association of Daily Physical Activity Behaviors with Visceral Fat. Obes. Res. Clin. Pract. 2020, 14, 531–535. [Google Scholar]
- Antonio, C.; Katherine, E.; Ludovica, P.; Michael, A.I.; Jessica, E.T.; Roberto, T.; Massimo, B.; Dario, G. Oscillating glucose is more deleterious to endothelial function and oxidative stress than mean glucose in normal and type 2 diabetic patients. Diabetes 2008, 57, 1349–1354. [Google Scholar]
- Antonio, C.; Markolf, H.; Lawrence, L.; Louis, M.; Alan, M.; David, O.; Naoko, T.; Jaakko, T. Postprandial glucose regulation and diabetic complications. Arch. Intern. Med. 2004, 164, 2090–2095. [Google Scholar]
- Chinmay, M.; James, A.L.; Debashis, K.N.; Ahmed, S.; Chiara, D.M.; Shelly, K.M.S.; Rita, B.; Claudio, C.; Rickey, E.C.; Ananda, B.; et al. The Effect of Walking on Postprandial Glycemic Excursion in Patients with Type 1 Diabetes and Healthy People. Diabetes Care 2012, 35, 2493–2499. [Google Scholar] [CrossRef]
- Kidokoro, T.; Shimizu, Y.; Edamoto, K.; Michael, A. Classroom standing desks and time-series variation in sedentary behavior and physical activity among primary school children. Int. J. Environ. Res. Public Health 2019, 16, 1892. [Google Scholar] [CrossRef]
- Miyachi, M.; Kurita, S.; Tripette, J.; Ryo, T.; Yoshiko, Y.; Haruka, M. Installation of a stationary high desk in the workplace: Effect of a 6-week intervention on physical activity. BMC Public Health 2015, 15, 368. [Google Scholar] [CrossRef] [PubMed]
- Christopher, R.; Kara, M.; Donald, R.D. Difference in caloric expenditure in sitting versus standing desks. J. Phys. Act. Health 2012, 9, 1009–1011. [Google Scholar] [CrossRef]
- Alicia, A.T.; Bronwyn, A.K.; Parneet, S.; Louise, H.; Neville, O.; David, W.D. Alternating bouts of sitting and standing attenuate postprandial glucose responses. Med. Sci. Sports Exerc. 2014, 46, 2053–2061. [Google Scholar]
- Dobashi, S.; Kawaguchi, S.; Ando, D.; Koyama, K. Alternating work posture improves postprandial glucose response without reducing computer task performance in the early afternoon. Physiol. Behav. 2021, 237, 113431. [Google Scholar] [CrossRef]
- Lopez, J.F. Standing for healthier lives—Literally. Eur. Heart J. 2015, 36, 2650–2652. [Google Scholar] [CrossRef]
- Trinity, J.D. Something is definitely better than nothing: Simple strategies to prevent vascular dysfunction. Clin. Sci. 2017, 131, 1055–1058. [Google Scholar] [CrossRef]
- Aaron, R.C.; Kaitlin, M.G.; Benjamin, T.H.; Megan, E.R.C.; Marcus, P.; Bryce, D.; Matthew, S.G. Prolonged standing increases lower limb arterial stiffness. Eur. J. Appl. Physiol. 2018, 118, 2249–2258. [Google Scholar] [CrossRef]
- Dechristian, F.B.; Luiz, A.B.; Svend, E.M.; Ana, B.O. Effects of Time in Sitting and Standing on Pleasantness, Acceptability, Fatigue, and Pain When Using a Sit-Stand Desk: An Experiment on Overweight and Normal-Weight Subjects. J. Phys. Act. Health 2020, 17, 1222–1230. [Google Scholar]
- Weir, J.B. New methods for calculating metabolic rate with special reference to protein metabolism. J. Physiol. 1949, 109, 1–9. [Google Scholar] [CrossRef]
- Sumi, D.; Hayashi, N.; Yatsutani, H.; Goto, K. Exogenous glucose oxidation during endurance exercise in hypoxia. Physiol. Rep. 2020, 8, 14457. [Google Scholar] [CrossRef]
- Borg, G.A. Psychophysical bases of perceived exertion. Med. Sci. Sports Exerc. 1982, 14, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Barbara, E.A.; William, L.H.; Stephen, D.H.; Nathanael, M.; David, R.B., Jr.; Catrine, T.L.; Jennifer, L.G.; Jesse, V.; Melicia, C.W.G.; Arthur, S.L. 2011 Compendium of Physical Activities: A second update of codes and MET values. Med. Sci. Sports Exerc. 2011, 43, 1575–1581. [Google Scholar] [CrossRef]
- Kapria, J.J.; Élise, L.L.; Sylvain, S.; Pierre, M.L.; Marie, E.M. Acute Impact of the Use of a Standing Desk on Appetite Sensations and Energy Intake. J. Phys. Act. Health 2020, 17, 1240–1246. [Google Scholar] [CrossRef]
- Manuel, C.; Hana, K.; Jihad, A.; Nora, N.B.; Rosendo, A.F.; Melissa, L.B.; Neal, D.B. The Thermic Effect of Food: A Review. J. Am. Coll. Nutr. 2019, 38, 547–551. [Google Scholar] [CrossRef]
- James, A.L. Non-exercise activity thermogenesis (NEAT). Best Pract. Res. Clin. Endocrinol. Metab. 2002, 16, 679–702. [Google Scholar]
- Fox, S. Human Physiology; McGraw-Hill: New York, NY, USA, 2016. [Google Scholar]
- Hayashi, T.; Wojtaszewski, J.F.; Goodyear, L.J. Exercise regulation of glucose transport in skeletal muscle. Am. J. Physiol. 1997, 273, 1039–1051. [Google Scholar] [CrossRef]
- Harris, R.C.; Hultman, E.; Sahlin, K. Glycolytic intermediates in human muscle after isometric contraction. Pflügers Arch. Eur. J. Physiol. 1981, 389, 277–282. [Google Scholar] [CrossRef]
- Okada, M. An electromyographic estimation of the relative muscular load in different human postures. J. Hum. Ergol. 1972, 1, 75–93. [Google Scholar] [CrossRef]
- Karpovich, P.V.; Sinning, W.E. Physiology of Muscular Activity; Saunders: Philadelphia, PA, USA, 1971. [Google Scholar]
- Polonsky, K.S.; Given, B.D.; Cauter, E.V. Twenty-four-hour profiles and pulsatile patterns of insulin secretion in normal and obese subjects. J. Clin. Investig. 1988, 81, 442–448. [Google Scholar] [CrossRef]


| Sit | Stand | p Value | |
|---|---|---|---|
| Energy expenditure (kcal/day) | 1918.1 ± 169.3 | 2154.2 ± 235.7 | p < 0.05 |
| RER a | 0.9 ± 0.03 | 0.89 ± 0.03 | p = 0.79 |
| AUC b of blood glucose (min mg/dL) | 15,947 ± 889 | 16,221 ± 1416.4 | p = 0.49 |
| HR c (bpm.) | 63.6 ± 9.6 | 71.6 ± 11 | p < 0.05 |
| AUC b of exogenous glucose metabolic rate | 2.7 ± 1.8 | 2.8 ± 1.7 | p = 0.75 |
| RPE d | 8.1 ± 1.8 | 11.3 ± 2.4 | p < 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kono, H.; Furuta, K.; Sakamoto, T.; Ueda, S.-y. Effects of Standing after a Meal on Glucose Metabolism and Energy Expenditure. Int. J. Environ. Res. Public Health 2023, 20, 6934. https://doi.org/10.3390/ijerph20206934
Kono H, Furuta K, Sakamoto T, Ueda S-y. Effects of Standing after a Meal on Glucose Metabolism and Energy Expenditure. International Journal of Environmental Research and Public Health. 2023; 20(20):6934. https://doi.org/10.3390/ijerph20206934
Chicago/Turabian StyleKono, Hiroya, Kento Furuta, Takumi Sakamoto, and Shin-ya Ueda. 2023. "Effects of Standing after a Meal on Glucose Metabolism and Energy Expenditure" International Journal of Environmental Research and Public Health 20, no. 20: 6934. https://doi.org/10.3390/ijerph20206934





