An Evaluation of the Impact of Air Pollution on the Lung Functions of High School Students Living in a Ceramic Industrial Park Zone
Abstract
1. Introduction
2. Materials and Methods
2.1. Procedures
2.2. Disease Definition
2.3. Air Pollution Data
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thurston, G.D.; Balmes, J.R.; Garcia, E.; Gilliland, F.D.; Rice, M.B.; Schikowski, T.; Van Winkle, L.S.; Annesi-Maesano, I.; Burchard, E.G.; Carlsten, C.; et al. Outdoor Air Pollution and New-Onset Airway Disease. An Official American Thoracic Society Workshop Report. Ann. Am. Thorac. Soc. 2020, 17, 387–398. [Google Scholar] [CrossRef] [PubMed]
- Mehta, S.; Shin, H.; Burnett, R.; North, T.; Cohen, A.J. Ambient particulate air pollution and acute lower respiratory infections: A systematic review and implications for estimating the global burden of disease. Air Qual. Atmos. Health 2013, 6, 69–83. [Google Scholar] [CrossRef] [PubMed]
- Gauderman, W.J.; Avol, E.; Gilliland, F.; Vora, H.; Thomas, D.; Berhane, K.; McConnell, R.; Kuenzli, N.; Lurmann, F.; Rappaport, E.; et al. The Effect of Air Pollution on Lung Development from 10 to 18 Years of Age. N. Engl. J. Med. 2004, 351, 1057–1067. [Google Scholar] [CrossRef] [PubMed]
- Schultz, E.S.; Hallberg, J.; Bellander, T.; Bergström, A.; Bottai, M.; Chiesa, F.; Gustafsson, P.M.; Gruzieva, O.; Thunqvist, P.; Pershagen, G.; et al. Early-Life Exposure to Traffic-Related Air Pollution and Lung Function in Adolescence. Am. J. Respir. Crit. Care Med. 2016, 193, 171–177. [Google Scholar] [CrossRef]
- Air Quality Database. Available online: https://www.who.int/data/gho/data/themes/air-pollution/who-air-quality-database (accessed on 15 June 2022).
- Companhia Ambiental do Estado de São Paulo (CETESB). QUALAR. Available online: https://qualar.cetesb.sp.gov.br/qualar/home.do (accessed on 3 November 2020).
- Oliveira, M. Caracterização Mineralógica e Química Das Fontes de Poeira e Sua Influência na Atmosfera da Região do Polo Cerâmico de Santa Gertrudes (SP). Tese de Doutorado, Universidade Estadual de São Paulo (UNESP), Rio Claro, Brazil, 2014. Available online: https://repositorio.unesp.br/handle/11449/134023 (accessed on 16 June 2022).
- Bhagia, L. Non-Occupational Exposure to Silica Dust. Indian J. Occup. Environ. Med. 2012, 16, 95. [Google Scholar] [CrossRef]
- Norboo, T.; Angchuk, P.T.; Yahya, M.; Kamat, S.R.; Pooley, F.D.; Corrin, B.; Kerr, I.H.; Bruce, N.; Ball, K.P. Silicosis in a Himalayan village population: Role of environmental dust. Thorax 1991, 46, 341–343. [Google Scholar] [CrossRef]
- Porgpermdee, S.; Potchanakaew, F.; Chaiyo, S. A Case of Non-Occupational Silicosis from 3-Year Surveillance at Stone Crushing Factories Neighboring Communities in Surin Province, Thailand. Am. J. Public Health Res. 2018, 6, 57–61. [Google Scholar] [CrossRef]
- Solé, D.; Vanna, A.T.; Yamada, E.; Rizzo, M.C.; Naspitz, C.K. International Study of Asthma and Allergies in Childhood (ISAAC) Written Questionnaire: Validation of the Asthma Component among Brazilian Children. J. Investig. Allergol. Clin. Immunol. 1998, 8, 376–382. [Google Scholar]
- Graham, B.L.; Steenbruggen, I.; Miller, M.R.; Barjaktarevic, I.Z.; Cooper, B.G.; Hall, G.L.; Hallstrand, T.S.; Kaminsky, D.A.; McCarthy, K.; McCormack, M.C.; et al. Standardization of Spirometry 2019 Update. An Official American Thoracic Society and European Respiratory Society Technical Statement. Am. J. Respir. Crit. Care Med. 2019, 200, e70–e88. [Google Scholar] [CrossRef]
- Quanjer, P.H.; Stanojevic, S.; Cole, T.J.; Baur, X.; Hall, G.L.; Culver, B.H.; Enright, P.L.; Hankinson, J.L.; Ip, M.S.M.; Zheng, J.; et al. Multi-Ethnic Reference Values for Spirometry for the 3–95-Yr Age Range: The Global Lung Function 2012 Equations. Eur. Respir. J. 2012, 40, 1324–1343. [Google Scholar] [CrossRef]
- Stanojevic, S.; Kaminsky, D.A.; Miller, M.R.; Thompson, B.; Aliverti, A.; Barjaktarevic, I.; Cooper, B.G.; Culver, B.; Derom, E.; Hall, G.L.; et al. ERS/ATS Technical Standard on Interpretive Strategies for Routine Lung Function Tests. Eur. Respir. J. 2022, 60, 2101499. [Google Scholar] [CrossRef] [PubMed]
- de Onis, M. Development of a WHO Growth Reference for School-Aged Children and Adolescents. Bull. World Health Organ. 2007, 85, 660–667. [Google Scholar] [CrossRef] [PubMed]
- Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention. 2022. Available online: www.ginasthma.org (accessed on 16 June 2022).
- Companhia Ambiental do Estado de São Paulo (CETESB). Relatório de Qualidade do Ar no Estado de São Paulo, 2018; Relatórios/CETESB; Companhia Ambiental do Estado de São Paulo (CETESB): São Paulo, Brazil, 2019; p. 214. Available online: https://cetesb.sp.gov.br/ar/wp-content/uploads/sites/28/2019/07/Relat%C3%B3rio-de-Qualidade-do-Ar-2018.pdf (accessed on 17 June 2022).
- DustTrakTM II Aerosol Monitor 8530. Available online: https://tsi.com/Products/Aerosol-and-Dust-Monitors/Aerosol-and-Dust-Monitors/DustTrakTM-II-Aerosol-Monitor-8530 (accessed on 4 July 2022).
- Schultz, E.S.; Litonjua, A.A.; Melén, E. Effects of Long-Term Exposure to Traffic-Related Air Pollution on Lung Function in Children. Curr. Allergy Asthma Rep. 2017, 17, 41. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Martinez, R.; Perez-Padilla, R.; Olaiz-Fernandez, G.; Mendoza-Alvarado, L.; Moreno-Macias, H.; Fortoul, T.; McDonnell, W.; Loomis, D.; Romieu, I. Lung Function Growth in Children with Long-Term Exposure to Air Pollutants in Mexico City. Am. J. Respir. Crit. Care Med. 2007, 176, 377–384. [Google Scholar] [CrossRef]
- Mölter, A.; Simpson, A.; Berdel, D.; Brunekreef, B.; Custovic, A.; Cyrys, J.; de Jongste, J.; de Vocht, F.; Fuertes, E.; Gehring, U.; et al. A Multicentre Study of Air Pollution Exposure and Childhood Asthma Prevalence: The ESCAPE Project. Eur. Respir. J. 2015, 45, 610–624. [Google Scholar] [CrossRef]
- Cai, Y.; Zijlema, W.L.; Doiron, D.; Blangiardo, M.; Burton, P.R.; Fortier, I.; Gaye, A.; Gulliver, J.; de Hoogh, K.; Hveem, K.; et al. Ambient Air Pollution, Traffic Noise and Adult Asthma Prevalence: A BioSHaRE Approach. Eur. Respir. J. 2017, 49, 1502127. [Google Scholar] [CrossRef]
- Anderson, H.R.; Favarato, G.; Atkinson, R.W. Long-Term Exposure to Outdoor Air Pollution and the Prevalence of Asthma: Meta-Analysis of Multi-Community Prevalence Studies. Air Qual. Atmos. Health 2013, 6, 57–68. [Google Scholar] [CrossRef]
- Voraphani, N.; Stern, D.A.; Zhai, J.; Wright, A.L.; Halonen, M.; Sherrill, D.L.; Hallberg, J.; Kull, I.; Bergström, A.; Murray, C.S.; et al. The Role of Growth and Nutrition in the Early Origins of Spirometric Restriction in Adult Life: A Longitudinal, Multicohort, Population-Based Study. Lancet Respir. Med. 2022, 10, 59–71. [Google Scholar] [CrossRef]
- Kotecha, S.J.; Watkins, W.J.; Henderson, A.J.; Kotecha, S. The Effect of Birth Weight on Lung Spirometry in White, School-Aged Children and Adolescents Born at Term: A Longitudinal Population Based Observational Cohort Study. J. Pediatr. 2015, 166, 1163–1167. [Google Scholar] [CrossRef]
- Saad, N.J.; Patel, J.; Burney, P.; Minelli, C. Birth Weight and Lung Function in Adulthood: A Systematic Review and Meta-Analysis. Ann. Am. Thorac. Soc. 2017, 14, 994–1004. [Google Scholar] [CrossRef]
- Ferreira, M.S.; Marson, F.A.L.; Wolf, V.L.W.; Ribeiro, J.D.; Mendes, R.T. Lung Function in Obese Children and Adolescents without Respiratory Disease: A Systematic Review. BMC Pulm. Med. 2020, 20, 281. [Google Scholar] [CrossRef] [PubMed]
- Freedman, D.S.; Wang, J.; Maynard, L.M.; Thornton, J.C.; Mei, Z.; Pierson, R.N.; Dietz, W.H.; Horlick, M. Relation of BMI to Fat and Fat-Free Mass among Children and Adolescents. Int. J. Obes. 2005, 29, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Peralta, G.P.; Fuertes, E.; Granell, R.; Mahmoud, O.; Roda, C.; Serra, I.; Jarvis, D.; Henderson, J.; Garcia-Aymerich, J. Childhood Body Composition Trajectories and Adolescent Lung Function. Findings from the ALSPAC Study. Am. J. Respir. Crit. Care Med. 2019, 200, 75–83. [Google Scholar] [CrossRef] [PubMed]
- de Castro, H.A.; da Cunha, M.F.; Mendonça, G.A.S.; Junger, W.L.; Cunha-Cruz, J.; de Leon, A.P. Efeitos Da Poluição Do Ar Na Função Respiratória de Escolares, Rio de Janeiro, RJ. Rev. Saúde Pública 2009, 43, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Riguera, D.; André, P.A.; Zanetta, D.M.T. Poluição Da Queima de Cana e Sintomas Respiratórios Em Escolares de Monte Aprazível, SP. Rev. Saúde Pública 2011, 45, 878–886. [Google Scholar] [CrossRef] [PubMed][Green Version]
- da Jacobson, L.S.V.; de Hacon, S.S.; de Castro, H.A.; Ignotti, E.; Artaxo, P.; Saldiva, P.H.N.; de Leon, A.C.M.P. Acute Effects of Particulate Matter and Black Carbon from Seasonal Fires on Peak Expiratory Flow of Schoolchildren in the Brazilian Amazon. PLoS ONE 2014, 9, e104177. [Google Scholar] [CrossRef] [PubMed]
- Toledo, M.F.; Saraiva-Romanholo, B.M.; Oliveira, R.C.; da Silva, L.F.; Solé, D. Air Pollution and Its Relationship to Lung Function among Adolescents from Taubate, São Paulo, Brazil. Allergol. Immunopathol. 2018, 46, 160–166. [Google Scholar] [CrossRef]
- de Oliveira, B.B.B. Avaliação da Função Respiratória de Crianças em Santos e Sua Correlação Com a Qualidade do ar. Tese de Doutorado; Universidade Católica de Santos: Santos, Brazil, 2019; Available online: https://tede.unisantos.br/handle/tede/5201 (accessed on 22 June 2022).
- Olmedo, A.P.B.; Silva, M.G.; Muccilo-Baisch, A.L.; Soares, M.C.F. Avaliação Da Função Pulmonar Em Escolares Expostos à Poluição Atmosférica Em Rio Grande. VITTALLE Rev. Ciênc. Saúde 2015, 24, 11–18. [Google Scholar]
- Wang, Y.; Xiao, S.; Zhang, Y.; Chang, H.; Martin, R.V.; Van Donkelaar, A.; Gaskins, A.; Liu, Y.; Liu, P.; Shi, L. Long-Term Exposure to PM2.5 Major Components and Mortality in the Southeastern United States. Environ. Int. 2022, 158, 106969. [Google Scholar] [CrossRef]
- Oberdörster, G.; Oberdörster, E.; Oberdörster, J. Nanotoxicology: An Emerging Discipline Evolving from Studies of Ultrafine Particles. Environ. Health Perspect 2005, 113, 823–839. [Google Scholar] [CrossRef]
- Castellano, J.F. LEI No 2.163, DE 09 DE NOVEMBRO DE 1979. 1979. Available online: https://www.al.sp.gov.br/repositorio/legislacao/lei/1979/original-lei-2163-09.11.1979.html (accessed on 24 August 2022).
- Doria, J. LEI No 17.469, DE 13 DE DEZEMBRO DE 2021. 2021. Available online: https://www.al.sp.gov.br/repositorio/legislacao/lei/2021/lei-17469-13.12.2021.html (accessed on 24 August 2022).
- Spindel, E.R.; McEvoy, C.T. The Role of Nicotine in the Effects of Maternal Smoking during Pregnancy on Lung Development and Childhood Respiratory Disease. Implications for Dangers of E-Cigarettes. Am. J. Respir. Crit. Care Med. 2016, 193, 486–494. [Google Scholar] [CrossRef] [PubMed]
- Martinez, F.D. Early-Life Origins of Chronic Obstructive Pulmonary Disease. N. Engl. J. Med. 2016, 375, 871–878. [Google Scholar] [CrossRef] [PubMed]
- Atlas Brasil. Available online: http://www.atlasbrasil.org.br/ (accessed on 20 June 2022).
- Petruccelli, J.L. A Cor Denominada: Estudos Sobre a Classificação Étnico-Racial; Coleção Políticas da cor; LPP/Uerj; DP&A Editores: Rio de Janeiro, Brazil, 2007. [Google Scholar]
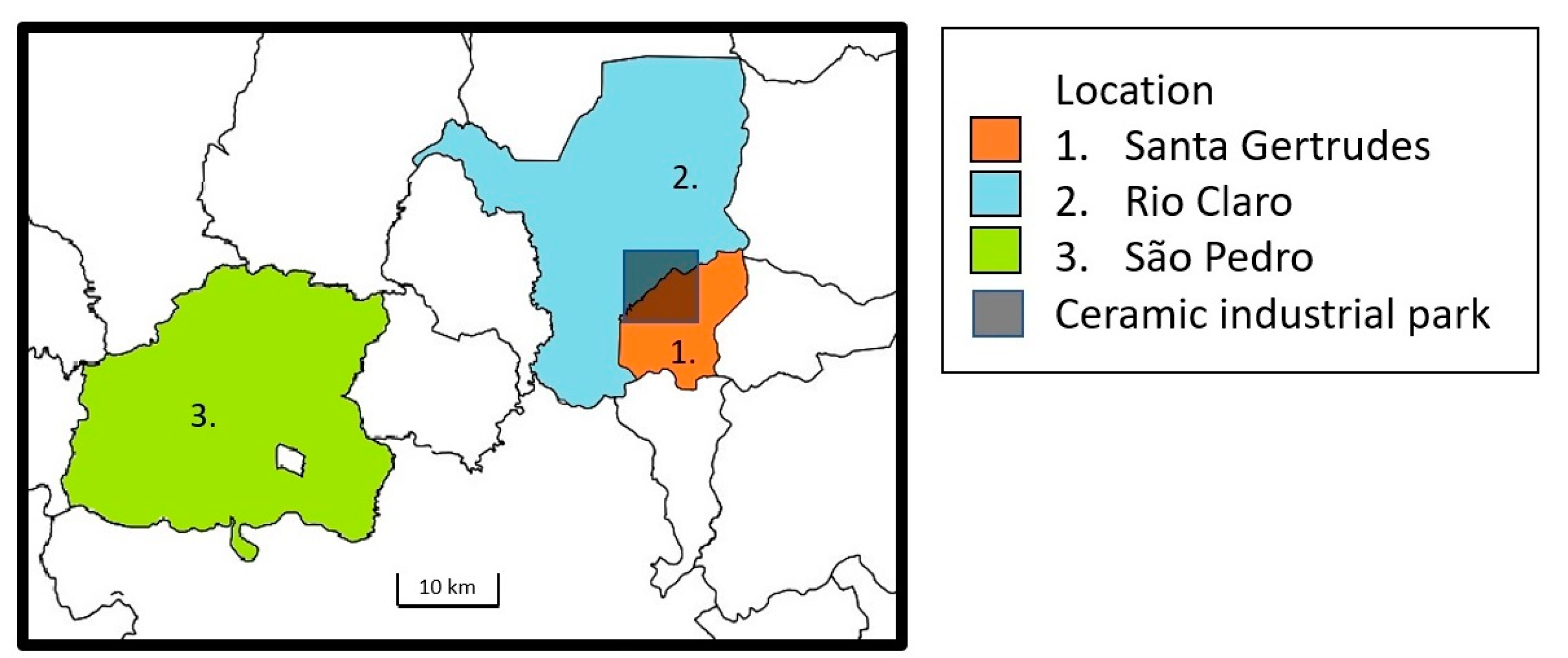
| Santa Gertrudes (SG) | Rio Claro (RC) | São Pedro (SP) | |
|---|---|---|---|
| Sample (n) | 158 | 153 | 139 |
| Female sex | 88 (55.70%) | 80 (52.29%) | 63 (45.32%) |
| Age (years) * | 17.05 ± 0.82 | 16.90 ± 0.64 | 17.30 ± 0.69 † |
| Time living in the same location (years) | 15.46 ± 2.20 | 15.63 ± 2.11 | 15.84 ± 2.00 |
| Height (m) | 1.69 ± 0.09 | 1.68 ± 0.09 | 1.68 ± 0.09 |
| Weight (kg) | 65.35 ± 14.02 | 66.07 ± 15.78 | 69.37 ± 14.03 |
| BMI (kg/m2) * | 22.60 ± 4.02 | 23.23 ± 4.70 | 24.47 ± 5.00 †† |
| Malnutrition a | 2 (1.27%) | 3 (1.96%) | 1 (0.72%) |
| Obesity b,* | 9 (5.70%) | 16 (10.45%) | 26 (18.70%) †† |
| Current/ex-smokers | 16 (13.29%) | 9 (7.19%) | 15 (17.27%) |
| AS ≥ 6 | 30 (18.98%) | 25 (16.34%) | 23 (16.55%) |
| Asthma diagnosis c | 6 (3.80%) | 4 (2.61%) | 1 (0.72%) |
| Santa Gertrudes (SG) | Rio Claro (RC) | São Pedro (SP) | |
|---|---|---|---|
| Sample (n) | 158 | 153 | 139 |
| Mean FVC% * | 95.0 ± 11.8 | 98.8 ± 12.9 † | 102.4 ± 13.8 †† |
| Mean FEV1% * | 95.7 ± 10.4 | 99.7 ± 12.0 † | 103.2 ± 12.0 †† |
| Obstructive pattern | 16 (10.13%) | 13 (8.50%) | 10 (7.19%) |
| Non-specific pattern | 3 (1.90%) | 2 (1.31%) | 1 (0.72%) |
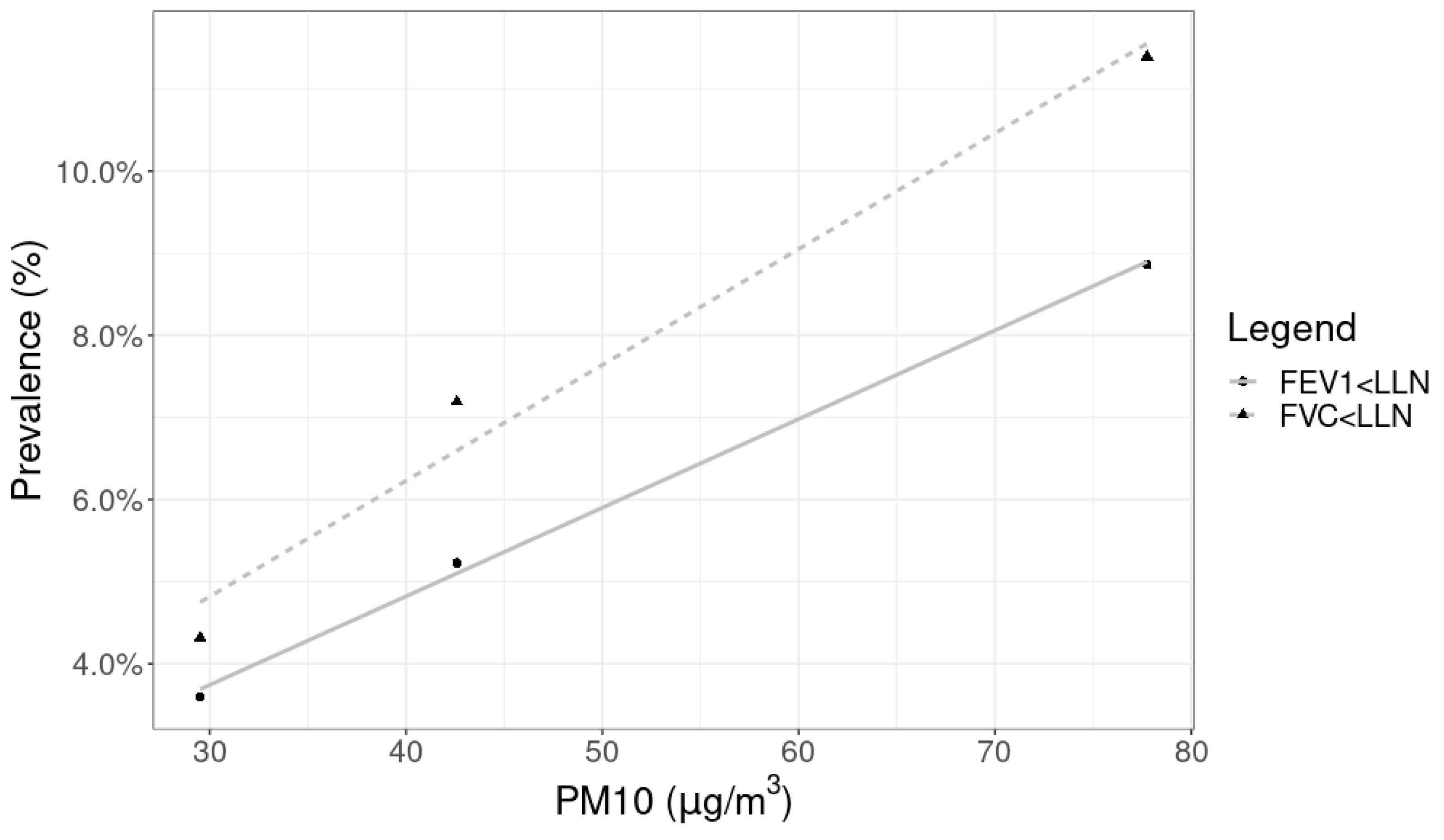
| FVC < LLN | 18 (11.39%) | 11 (7.19%) | 6 (4.32%) |
| FVC < 80% ** | 18 (11.39%) | 9 (5.88%) | 5 (3.60%) |
| FVC < 90% ** | 54 (34.18%) | 40 (26.14%) | 20 (14.39%) † |
| FEV1 < LLN | 14 (8.86%) | 8 (5.23%) | 5 (3.60%) |
| FEV1 < 80% | 11 (6.96%) | 8 (5.23%) | 4 (2.88%) |
| FEV1 < 90% * | 51 (32.28%) | 27 (17.65%) † | 18 (12.95%) † |
| Location | Mean PM10 (µg/m3) | Monitoring Period | Type of Monitor |
|---|---|---|---|
| Santa Gertrudes (SG) | 77.75 ± 38.08 | 1 January 2018–31 December 2018 | Fixed |
| Rio Claro (RC) | 42.59 ± 23.46 | 1 January 2018–31 December 2018 | Fixed |
| São Pedro (SP) | 29.52 ± 9.87 | 6 August 2018–8 August 2018 | Portable |
| FVC % | FEV1 % | |
|---|---|---|
| BMI (per 1 kg/m2) | 1.05 | 0.43 |
| (0.81; 1.29) | (0.20; 0.66) | |
| p < 0.01 | p < 0.01 | |
| Malnutrition a | −19.2 | −14.41 |
| (−29.64; −8.76) | (−23.91; −4.91) | |
| p < 0.01 | p < 0.01 | |
| Obesity b | 9.23 | 1.93 |
| (5.49; 12.97) | (−1.54; 5.40) | |
| p < 0.01 | p = 0.28 | |
| Ever smoker | −1.18 | −0.09 |
| (−4.95; 2.59) | (−3.50; 3.32) | |
| p = 0.54 | p = 0.96 | |
| PM10 (per 10 µg/m3) | −1.43 | −1.45 |
| (−2.01; −0.85) | (−1.97; −0.93) | |
| p < 0.01 | p < 0.01 | |
| AS ≥ 6 | −1.59 | −3.57 |
| (−4.8; 1.62) | (−6.46; −0.68) | |
| p = 0.33 | p = 0.02 | |
| Asthma diagnosis c | −2.90 | −11.10 |
| (−10.39; 4.59) | (−17.81; −4.39) | |
| p = 0.45 | p < 0.01 |
| FVC% | FEV1% | |
|---|---|---|
| BMI (per 1 kg/m2) | 0.98 | 0.35 |
| (0.74; 1.22) | (0.12; 0.58) | |
| p < 0.01 | p < 0.01 | |
| PM10 (per 10 µg/m3) | −1.10 | −1.27 |
| (−1.65; −0.55) | (−1.79; −0.75) | |
| p < 0.01 | p < 0.01 | |
| Asthma diagnosis | - | −10.2 |
| (−16.68; −3.72) | ||
| p < 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mizutani, R.F.; de Paula Santos, U.; Arbex, R.F.; Arbex, M.A.; Terra-Filho, M. An Evaluation of the Impact of Air Pollution on the Lung Functions of High School Students Living in a Ceramic Industrial Park Zone. Int. J. Environ. Res. Public Health 2023, 20, 6964. https://doi.org/10.3390/ijerph20216964
Mizutani RF, de Paula Santos U, Arbex RF, Arbex MA, Terra-Filho M. An Evaluation of the Impact of Air Pollution on the Lung Functions of High School Students Living in a Ceramic Industrial Park Zone. International Journal of Environmental Research and Public Health. 2023; 20(21):6964. https://doi.org/10.3390/ijerph20216964
Chicago/Turabian StyleMizutani, Rafael Futoshi, Ubiratan de Paula Santos, Renata Ferlin Arbex, Marcos Abdo Arbex, and Mario Terra-Filho. 2023. "An Evaluation of the Impact of Air Pollution on the Lung Functions of High School Students Living in a Ceramic Industrial Park Zone" International Journal of Environmental Research and Public Health 20, no. 21: 6964. https://doi.org/10.3390/ijerph20216964
APA StyleMizutani, R. F., de Paula Santos, U., Arbex, R. F., Arbex, M. A., & Terra-Filho, M. (2023). An Evaluation of the Impact of Air Pollution on the Lung Functions of High School Students Living in a Ceramic Industrial Park Zone. International Journal of Environmental Research and Public Health, 20(21), 6964. https://doi.org/10.3390/ijerph20216964






