Assessing Ethnic Minority Representation in Fibromyalgia Clinical Trials: A Systematic Review of Recruitment Demographics
Abstract
:1. Introduction
2. Methods
2.1. Literature Searches
2.2. Inclusion Criteria
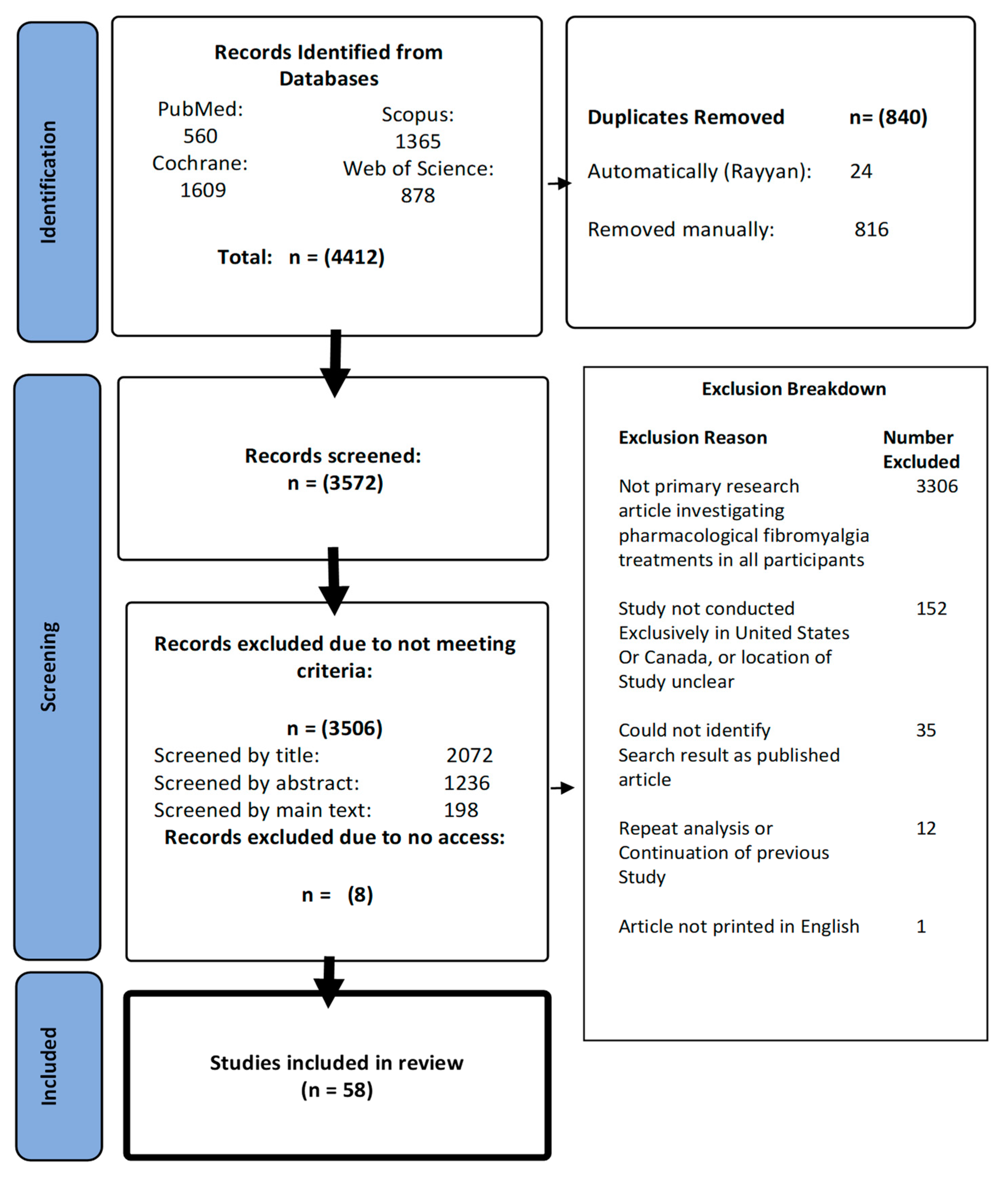
2.3. Screening
2.4. Data Extraction
2.5. Analysis
3. Results
3.1. Identification of Fibromyalgia Research
3.1.1. Study Characteristics
3.1.2. Ethnic Diversity of Fibromyalgia Trials in the United States and Canada (Objective 1)
3.1.3. Ethnic Diversity of Fibromyalgia Trials Conducted Exclusively in the United States (Objective 2)
3.1.4. Longitudinal Assessment of Non-White Participant Representation in Trials (Objective 3)
3.1.5. White and Non-White Participant Retention in Trials (Objective 4)
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Commissioner O of the FDASIA Section 907: Inclusion of Demographic Subgroups in Clinical Trials. FDA. 2019. Available online: https://www.fda.gov/regulatory-information/food-and-drug-administration-safety-and-innovation-act-fdasia/fdasia-section-907-inclusion-demographic-subgroups-clinical-trials (accessed on 29 April 2023).
- Bøttern, J.; Stage, T.B.; Dunvald, A.C.D. Sex, racial, and ethnic diversity in clinical trials. Clin. Transl. Sci. 2023, 16, 937–945. [Google Scholar] [CrossRef] [PubMed]
- Sirugo, G.; Williams, S.M.; Tishkoff, S.A. The Missing Diversity in Human Genetic Studies. Cell 2019, 177, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Alomar, M.J. Factors affecting the development of adverse drug reactions (Review article). Saudi Pharm. J. 2014, 22, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, S.; Zhang, L.; Huang, S.M. The Role of Ethnicity in Variability in Response to Drugs: Focus on Clinical Pharmacology Studies. Clin. Pharmacol. Ther. 2008, 84, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Burroughs, V.J.; Maxey, R.W.; Levy, R.A. Racial and ethnic differences in response to medicines: Towards individualized pharmaceutical treatment. J. Natl. Med. Assoc. 2002, 94 (Suppl. S10), 1–26. [Google Scholar] [PubMed]
- Delon, C.; Brown, K.F.; Payne, N.W.S.; Kotrotsios, Y.; Vernon, S.; Shelton, J. Differences in cancer incidence by broad ethnic group in England, 2013–2017. Br. J. Cancer 2022, 126, 1765–1773. [Google Scholar] [CrossRef] [PubMed]
- Association, A. 2019 Alzheimer’s disease facts and figures. Alzheimers Dement. 2019, 15, 321–387. [Google Scholar] [CrossRef]
- Lindhorst, J.; Alexander, N.; Blignaut, J.; Rayner, B. Differences in hypertension between blacks and whites: An overview: Review article. Cardiovasc. J. Afr. 2007, 18, 241–247. [Google Scholar]
- Szanto, K.B.; Li, J.; Cordero, P.; Oben, J.A. Ethnic differences and heterogeneity in genetic and metabolic makeup contributing to nonalcoholic fatty liver disease. Diabetes Metab. Syndr. Obes. 2019, 12, 357–367. [Google Scholar] [CrossRef]
- Meeks, K.A.C.; Freitas-Da-Silva, D.; Adeyemo, A.; Beune, E.J.A.J.; Modesti, P.A.; Stronks, K.; Zafarmand, M.H.; Agyemang, C. Disparities in type 2 diabetes prevalence among ethnic minority groups resident in Europe: A systematic review and meta-analysis. Intern. Emerg. Med. 2016, 11, 327–340. [Google Scholar] [CrossRef]
- Number of Clinical Trials by Year, Country, WHO Region and Income Group (1999–2019). 2020. Available online: https://www.who.int/observatories/global-observatory-on-health-research-and-development/monitoring/number-of-clinical-trials-by-year-country-who-region-and-income-group-mar-2020 (accessed on 29 April 2023).
- Venkatakrishnan, K.; Gupta, N.; Smith, P.F.; Lin, T.; Lineberry, N.; Ishida, T.; Wang, L.; Rogge, M. Asia-Inclusive Clinical Research and Development Enabled by Translational Science and Quantitative Clinical Pharmacology: Toward a Culture That Challenges the Status Quo. Clin. Pharmacol. Ther. 2022, 113, 298–309. [Google Scholar] [CrossRef]
- Ali, S.; Egunsola, O.; Babar, Z.U.D.; Hasan, S.S. Clinical trials in Asia: A World Health Organization database study. Perspect. Clin. Res. 2019, 10, 121–124. [Google Scholar] [CrossRef] [PubMed]
- Ramamoorthy, A.; Knepper, T.C.; Merenda, C.; Mendoza, M.; McLeod, H.L.; Bull, J.; Zhang, L.; Pacanowski, M. Demographic Composition of Select Oncologic New Molecular Entities Approved by the FDA between 2008 and 2017. Clin. Pharmacol. Ther. 2018, 104, 940–948. [Google Scholar] [CrossRef] [PubMed]
- Camidge, D.R.; Park, H.; Smoyer, K.E.; Jacobs, I.; Lee, L.J.; Askerova, Z.; McGinnis, J.; Zakharia, Y. Race and ethnicity representation in clinical trials: Findings from a literature review of Phase I oncology trials. Future Oncol. 2021, 17, 3271–3280. [Google Scholar] [CrossRef] [PubMed]
- Crivelli, L. LatAm-FINGERS (Latin America). Alzheimers Dement. 2020, 16, e046953. [Google Scholar] [CrossRef]
- Weiner, M.W.; Veitch, D.P.; Miller, M.J.; Aisen, P.S.; Albala, B.; Beckett, L.A.; Green, R.C.; Harvey, D.; Jack, C.R.; Jagust, W.; et al. Increasing participant diversity in AD research: Plans for digital screening, blood testing, and a community-engaged approach in the Alzheimer’s Disease Neuroimaging Initiative 4. Alzheimers Dement. 2023, 19, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Guerra, C.; Pressman, A.; Hurley, P.; Garrett-Mayer, E.; Bruinooge, S.S.; Howson, A.; Kaltenbaugh, M.; Williams, J.H.; Boehmer, L.; Bernick, L.A.; et al. Increasing Racial and Ethnic Equity, Diversity, and Inclusion in Cancer Treatment Trials: Evaluation of an ASCO-Association of Community Cancer Centers Site Self-Assessment. JCO Oncol. Pract. 2023, 19, e581–e588. [Google Scholar] [CrossRef] [PubMed]
- Center for Biologics Evaluation and Research; Center for Drug Evaluation and Research; US Food and Drug Administration; FDA. Enhancing the Diversity of Clinical Trial Populations—Eligibility Criteria, Enrollment Practices, and Trial Designs Guidance for Industry. 2020. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/enhancing-diversity-clinical-trial-populations-eligibility-criteria-enrollment-practices-and-trial (accessed on 13 May 2023).
- Bellato, E.; Marini, E.; Castoldi, F.; Barbasetti, N.; Mattei, L.; Bonasia, D.E.; Blonna, D. Fibromyalgia Syndrome: Etiology, Pathogenesis, Diagnosis, and Treatment. Pain. Res. Treat. 2012, 2012, e426130. [Google Scholar] [CrossRef]
- Sluka, K.A.; Clauw, D.J. Neurobiology of fibromyalgia and chronic widespread pain. Neuroscience 2016, 338, 114–129. [Google Scholar] [CrossRef]
- Tzadok, R.; Ablin, J.N. Current and Emerging Pharmacotherapy for Fibromyalgia. Pain. Res. Manag. 2020, 2020, e6541798. [Google Scholar] [CrossRef]
- Heidari, F.; Afshari, M.; Moosazadeh, M. Prevalence of fibromyalgia in general population and patients, a systematic review and meta-analysis. Rheumatol. Int. 2017, 37, 1527–1539. [Google Scholar] [CrossRef]
- Wolfe, F.; Ross, K.; Anderson, J.; Russell, I.J.; Hebert, L. The prevalence and characteristics of fibromyalgia in the general population. Arthritis Rheum. 1995, 38, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Buskila, D.; Neumann, L.; Odes, L.R.; Schleifer, E.; Depsames, R.; Abu-Shakra, M. The prevalence of musculoskeletal pain and fibromyalgia in patients hospitalized on internal medicine wards. Semin. Arthritis Rheum. 2001, 30, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Raphael, K.G.; Janal, M.N.; Nayak, S.; Schwartz, J.E.; Gallagher, R.M. Psychiatric comorbidities in a community sample of women with fibromyalgia. Pain 2006, 124, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Marr, N.C.; Van Liew, C.; Carovich, T.F.; Cecchini, G.A.; McKinley, L.E.; Cronan, T.A. The Effects of Racial/Ethnic Minority Status on Sleep, Mood Disturbance, and Depression in People with Fibromyalgia. Psychol. Res. Behav. Manag. 2020, 13, 343–353. [Google Scholar] [CrossRef]
- Pryma, J. “Even my sister says I’m acting like a crazy to get a check”: Race, gender, and moral boundary-work in women’s claims of disabling chronic pain. Soc. Sci. Med. 2017, 181, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Rayyan—AI Powered Tool for Systematic Literature Reviews. 2021. Available online: https://www.rayyan.ai/ (accessed on 29 May 2023).
- White, H.D.; Brown, L.A.J.; Gyurik, R.J.; Manganiello, P.D.; Robinson, T.D.; Hallock, L.S.; Lewis, L.D.; Yeo, K.J. Treatment of pain in fibromyalgia patients with testosterone gel: Pharmacokinetics and clinical response. Int. Immunopharmacol. 2015, 27, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Wood, P.B.; Kablinger, A.S.; Caldito, G.S. Open trial of pindolol in the treatment of fibromyalgia. Ann. Pharmacother. 2005, 39, 1812–1816. [Google Scholar] [CrossRef]
- Scharf, M.B.; Baumann, M.; Berkowitz, D.V. The effects of sodium oxybate on clinical symptoms and sleep patterns in patients with fibromyalgia. J. Rheumatol. 2003, 30, 1070–1074. [Google Scholar]
- Arnold, L.M.; Hess, E.V.; Hudson, J.I.; Welge, J.A.; Berno, S.E.; Keck, P.E. A randomized, placebo-controlled, double-blind, flexible-dose study of fluoxetine in the treatment of women with fibromyalgia. Am. J. Med. 2002, 112, 191–197. [Google Scholar] [CrossRef]
- Cohen, S.P.; Verdolin, M.H.; Chang, A.S.; Kurihara, C.; Morlando, B.J.; Mao, J. The Intravenous Ketamine Test Predicts Subsequent Response to an Oral Dextromethorphan Treatment Regimen in Fibromyalgia Patients. J. Pain 2006, 7, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Mueller, C.; Ness, T.J.; Younger, J.W. Low-Dose Dextromethorphan for the Treatment of Fibromyalgia Pain: Results from a Longitudinal, Single-Blind, Placebo-Controlled Pilot Trial. J. Pain. Res. 2021, 14, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Roehrs, T.; Withrow, D.; Koshorek, G.; Verkler, J.; Bazan, L.; Roth, T. Sleep and pain in humans with fibromyalgia and comorbid insomnia: Double-blind, crossover study of suvorexant 20 mg versus placebo. J. Clin. Sleep Med. 2020, 16, 415–421. [Google Scholar] [CrossRef]
- Ang, D.C.; Hilligoss, J.; Stump, T. Mast Cell Stabilizer (Ketotifen) in Fibromyalgia: Phase 1 Randomized Controlled Clinical Trial. Clin. J. Pain. 2015, 31, 836–842. [Google Scholar] [CrossRef] [PubMed]
- Boomershine, C.S.; Koch, T.A.; Morris, D. A Blinded, Randomized, Placebo-Controlled Study to Investigate the Efficacy and Safety of Ferric Carboxymaltose in Iron-Deficient Patients with Fibromyalgia. Rheumatol. Ther. 2018, 5, 271–281. [Google Scholar] [CrossRef]
- North, J.M.; Hong, K.J.; Rauck, R.L. The Effect of a Novel form of Extended-Release Gabapentin on Pain and Sleep in Fibromyalgia Subjects: An Open-Label Pilot Study. Pain. Pract. 2016, 16, 720–729. [Google Scholar] [CrossRef]
- Natelson, B.H.; Vu, D.; Mao, X.; Weiduschat, N.; Togo, F.; Lange, G.; Blate, M.; Kang, G.; Coplan, J.D.; Shungu, D.C. Effect of Milnacipran Treatment on Ventricular Lactate in Fibromyalgia: A Randomized, Double-Blind, Placebo-Controlled Trial. J. Pain 2015, 16, 1211–1219. [Google Scholar] [CrossRef]
- Schmidt-Wilcke, T.; Ichesco, E.; Hampson, J.P.; Kairys, A.; Peltier, S.; Harte, S.; Clauw, D.J.; Harris, R.E. Resting state connectivity correlates with drug and placebo response in fibromyalgia patients. NeuroImage Clin. 2014, 6, 252–261. [Google Scholar] [CrossRef]
- Staud, R.; Weyl, E.; Bartley, E.; Price, D.; Robinson, M. Analgesic and anti-hyperalgesic effects of muscle injections with lidocaine or saline in patients with fibromyalgia syndrome. Eur. J. Pain 2014, 18, 803–812. [Google Scholar] [CrossRef]
- Harris, R.E.; Napadow, V.; Huggins, J.P.; Pauer, L.; Kim, J.; Hampson, J.; Sundgren, P.C.; Foerster, B.; Petrou, M.; Schmidt-Wilcke, T.; et al. Pregabalin rectifies aberrant brain chemistry, connectivity, and functional response in chronic pain patients. Anesthesiology 2013, 119, 1453–1464. [Google Scholar] [CrossRef]
- Rutledge, D.N.; Jones, C.J. Effects of Topical Essential Oil on Exercise Volume After a 12-Week Exercise Program for Women with Fibromyalgia: A Pilot Study. J. Altern. Complement. Med. 2007, 13, 1099–1106. [Google Scholar] [CrossRef] [PubMed]
- Holman, A.J.; Myers, R.R. A randomized, double-blind, placebo-controlled trial of pramipexole, a dopamine agonist, in patients with fibromyalgia receiving concomitant medications. Arthritis Rheum. 2005, 52, 2495–2505. [Google Scholar] [CrossRef] [PubMed]
- Younger, J.; Mackey, S. Fibromyalgia Symptoms Are Reduced by Low-Dose Naltrexone: A Pilot Study. Pain. Med. Malden Mass. 2009, 10, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Engen, D.J.; McAllister, S.J.; Whipple, M.O.; Cha, S.S.; Dion, L.J.; Vincent, A.; Bauer, B.A.; Wahner-Roedler, D.L. Effects of transdermal magnesium chloride on quality of life for patients with fibromyalgia: A feasibility study. J. Integr. Med. 2015, 13, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Younger, J.; Noor, N.; McCue, R.; Mackey, S. Low-dose naltrexone for the treatment of fibromyalgia: Findings of a small, randomized, double-blind, placebo-controlled, counterbalanced, crossover trial assessing daily pain levels. Arthritis Rheum. 2013, 65, 529–538. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.; Aamir, R.; Jishi, Z.; Scharf, M.B. The Effects of Milnacipran on Sleep Disturbance in Fibromyalgia: A Randomized, Double-Blind, Placebo-Controlled, Two-Way Crossover Study. J. Clin. Sleep Med. 2016, 12, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Price, D.D.; Staud, R.; Robinson, M.E.; Mauderli, A.P.; Cannon, R.; Vierck, C.J. Enhanced temporal summation of second pain and its central modulation in fibromyalgia patients. Pain 2002, 99, 49–59. [Google Scholar] [CrossRef]
- Mease, P.J.; Russell, I.J.; Arnold, L.M.; Florian, H.; Young, J.P.; Martin, S.A.; Sharma, U. A randomized, double-blind, placebo-controlled, phase III trial of pregabalin in the treatment of patients with fibromyalgia. J. Rheumatol. 2008, 35, 502–514. [Google Scholar]
- Pridgen, W.L.; Duffy, C.; Gendreau, J.F.; Gendreau, R.M. A famciclovir + celecoxib combination treatment is safe and efficacious in the treatment of fibromyalgia. J. Pain Res. 2017, 10, 451–460. [Google Scholar] [CrossRef]
- Trugman, J.M.; Palmer, R.H.; Ma, Y. Milnacipran effects on 24-hour ambulatory blood pressure and heart rate in fibromyalgia patients: A randomized, placebo-controlled, dose-escalation study. Curr. Med. Res. Opin. 2014, 30, 589–597. [Google Scholar] [CrossRef]
- Nasser, K.; Kivitz, A.J.; Maricic, M.J.; Silver, D.S.; Silverman, S.L. Twice daily versus once nightly dosing of pregabalin for fibromyalgia: A double-blind randomized clinical trial of efficacy and safety. Arthritis Care Res. 2014, 66, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Mease, P.J.; Farmer, M.V.; Palmer, R.H.; Gendreau, R.M.; Trugman, J.M.; Wang, Y. Milnacipran combined with pregabalin in fibromyalgia: A randomized, open-label study evaluating the safety and efficacy of adding milnacipran in patients with incomplete response to pregabalin. Ther. Adv. Musculoskelet. Dis. 2013, 5, 113–126. [Google Scholar] [CrossRef] [PubMed]
- Bateman, L.; Palmer, R.H.; Trugman, J.M.; Lin, Y. Results of switching to milnacipran in fibromyalgia patients with an inadequate response to duloxetine: A phase IV pilot study. J. Pain. Res. 2013, 6, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Russell, I.J.; Holman, A.J.; Swick, T.J.; Alvarez-Horine, S.; Wang, Y.G.; Guinta, D. Sodium oxybate reduces pain, fatigue, and sleep disturbance and improves functionality in fibromyalgia: Results from a 14-week, randomized, double-blind, placebo-controlled study. Pain 2011, 152, 1007–1017. [Google Scholar] [CrossRef] [PubMed]
- Clauw, D.J.; Mease, P.; Palmer, R.H.; Gendreau, R.M.; Wang, Y. Milnacipran for the treatment of fibromyalgia in adults: A 15-week, multicenter, randomized, double-blind, placebo-controlled, multiple-dose clinical trial. Clin. Ther. 2008, 30, 1988–2004. [Google Scholar] [CrossRef]
- Gendreau, R.M.; Thorn, M.D.; Gendreau, J.F.; Kranzler, J.D.; Ribeiro, S.; Gracely, R.H.; Williams, D.A.; Mease, P.J.; McLean, S.A.; Clauw, D.J. Efficacy of milnacipran in patients with fibromyalgia. J. Rheumatol. 2005, 32, 1975–1985. [Google Scholar]
- Russell, I.J.; Perkins, A.T.; Michalek, J.E.; Bennett, R.M.; Price, M.; Barron, A.; Evans, T.; Diab, I.; Lacombe, J.; Habros, J.S.; et al. Sodium oxybate relieves pain and improves function in fibromyalgia syndrome: A randomized, double-blind, placebo-controlled, multicenter clinical trial. Arthritis Rheum. 2009, 60, 299–309. [Google Scholar] [CrossRef]
- Arnold, L.M.; Blauwet, M.B.; Tracy, K.; Cai, N.; Walzer, M.; Blahunka, P.; Marek, G.J. Efficacy and Safety of ASP0819 in Patients with Fibromyalgia: Results of a Proof-of-Concept, Randomized, Double-Blind, Placebo-Controlled Trial. J. Pain Res. 2020, 13, 3355–3369. [Google Scholar] [CrossRef]
- Arnold, L.M.; Rosen, A.; Pritchett, Y.L.; D’Souza, D.N.; Goldstein, D.J.; Iyengar, S.; Wernicke, J.F. A randomized, double-blind, placebo-controlled trial of duloxetine in the treatment of women with fibromyalgia with or without major depressive disorder. Pain 2005, 119, 5–15. [Google Scholar] [CrossRef]
- Arnold, L.M.; Lu, Y.; Crofford, L.J.; Wohlreich, M.; Detke, M.J.; Iyengar, S.; Goldstein, D.J. A double-blind, multicenter trial comparing duloxetine with placebo in the treatment of fibromyalgia patients with or without major depressive disorder. Arthritis Rheum. 2004, 50, 2974–2984. [Google Scholar] [CrossRef]
- Mease, P.J.; Clauw, D.J.; Gendreau, R.M.; Rao, S.G.; Kranzler, J.; Chen, W.; Palmer, R.H. The efficacy and safety of milnacipran for treatment of fibromyalgia. a randomized, double-blind, placebo-controlled trial. J. Rheumatol. 2009, 36, 398–409. [Google Scholar] [CrossRef] [PubMed]
- Arnold, L.M.; Russell, I.J.; Diri, E.W.; Duan, W.R.; Young, J.P.; Sharma, U.; Martin, S.A.; Barrett, J.A.; Haig, G. A 14-week, Randomized, Double-Blinded, Placebo-Controlled Monotherapy Trial of Pregabalin in Patients With Fibromyalgia. J. Pain 2008, 9, 792–805. [Google Scholar] [CrossRef] [PubMed]
- Allen, R.; Sharma, U.; Barlas, S. Clinical Experience With Desvenlafaxine in Treatment of Patients With Fibromyalgia Syndrome. Clin. Pharmacol. Drug Dev. 2017, 6, 224–233. [Google Scholar] [CrossRef]
- Arnold, L.M.; Bateman, L.; Palmer, R.H.; Lin, Y. Preliminary experience using milnacipran in patients with juvenile fibromyalgia: Lessons from a clinical trial program. Pediatr. Rheumatol. 2015, 13, 27. [Google Scholar] [CrossRef] [PubMed]
- Crofford, L.J.; Mease, P.J.; Simpson, S.L.; Young, J.P.; Martin, S.A.; Haig, G.M.; Sharma, U. Fibromyalgia relapse evaluation and efficacy for durability of meaningful relief (FREEDOM): A 6-month, double-blind, placebo-controlled trial with pregabalin. Pain 2008, 136, 419–431. [Google Scholar] [CrossRef] [PubMed]
- Bennett, R.M.; Kamin, M.; Karim, R.; Rosenthal, N. Tramadol and acetaminophen combination tablets in the treatment of fibromyalgia pain: A double-blind, randomized, placebo-controlled study. Am. J. Med. 2003, 114, 537–545. [Google Scholar] [CrossRef]
- Arnold, L.M.; Chatamra, K.; Hirsch, I.; Stoker, M. Safety and efficacy of esreboxetine in patients with fibromyalgia: An 8-week, multicenter, randomized, double-blind, placebo-controlled study. Clin. Ther. 2010, 32, 1618–1632. [Google Scholar] [CrossRef]
- Crofford, L.J.; Rowbotham, M.C.; Mease, P.J.; Russell, I.J.; Dworkin, R.H.; Corbin, A.E.; Young, J.P.; LaMoreaux, L.K.; Martin, S.A.; Sharma, U. Pregabalin for the treatment of fibromyalgia syndrome: Results of a randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2005, 52, 1264–1273. [Google Scholar] [CrossRef]
- Patkar, A.A.; Masand, P.S.; Krulewicz, S.; Mannelli, P.; Peindl, K.; Beebe, K.L.; Jiang, W. A Randomized, Controlled, Trial of Controlled Release Paroxetine in Fibromyalgia. Am. J. Med. 2007, 120, 448–454. [Google Scholar] [CrossRef]
- Kim, J.L.; Rele, S.; Marks, D.M.; Masand, P.S.; Yerramsetty, P.; Millet, R.A.; Keefe, R.S.; Patkar, A.A. Effects of Milnacipran on Neurocognition, Pain, and Fatigue in Fibromyalgia: A 13-Week, Randomized, Placebo-Controlled, Crossover Trial. Prim. Care Companion CNS Disord. 2013, 15, 26298. [Google Scholar] [CrossRef]
- Vitton, O.; Gendreau, M.; Gendreau, J.; Kranzler, J.; Rao, S.G. A double-blind placebo-controlled trial of milnacipran in the treatment of fibromyalgia. Hum. Psychopharmacol. Clin. Exp. 2004, 19, S27–S35. [Google Scholar] [CrossRef]
- Russell, J.I.; Kamin, M.; Bennett, R.M.; Schnitzer, T.J.; Green, J.A.; Katz, W.A. Efficacy of Tramadol in Treatment of Pain in Fibromyalgia. JCR J. Clin. Rheumatol. 2000, 6, 250. [Google Scholar] [CrossRef] [PubMed]
- Russell, I.J.; Mease, P.J.; Smith, T.R.; Kajdasz, D.K.; Wohlreich, M.M.; Detke, M.J.; Walker, D.J.; Chappell, A.S.; Arnold, L.M. Efficacy and safety of duloxetine for treatment of fibromyalgia in patients with or without major depressive disorder: Results from a 6-month, randomized, double-blind, placebo-controlled, fixed-dose trial. Pain 2008, 136, 432–444. [Google Scholar] [CrossRef] [PubMed]
- Arnold, L.M.; Gendreau, R.M.; Palmer, R.H.; Gendreau, J.F.; Wang, Y. Efficacy and safety of milnacipran 100 mg/day in patients with fibromyalgia: Results of a randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2010, 62, 2745–2756. [Google Scholar] [CrossRef] [PubMed]
- Arnold, L.M.; Clauw, D.; Wang, F.; Ahl, J.; Gaynor, P.J.; Wohlreich, M.M. Flexible Dosed Duloxetine in the Treatment of Fibromyalgia: A Randomized, Double-blind, Placebo-controlled Trial. J. Rheumatol. 2010, 37, 2578–2586. [Google Scholar] [CrossRef] [PubMed]
- Arnold, L.M.; Hirsch, I.; Sanders, P.; Ellis, A.; Hughes, B. Safety and efficacy of esreboxetine in patients with fibromyalgia: A fourteen-week, randomized, double-blind, placebo-controlled, multicenter clinical trial. Arthritis Rheum. 2012, 64, 2387–2397. [Google Scholar] [CrossRef] [PubMed]
- Arnold, L.M.; Palmer, R.H.; Ma, Y. A 3-Year, Open-Label, Flexible-Dosing Study of Milnacipran for the Treatment of Fibromyalgia. Clin. J. Pain 2013, 29, 1021. [Google Scholar] [CrossRef] [PubMed]
- Curtis, K.; Katz, J.; Djaiani, C.; O’Leary, G.; Uehling, J.; Carroll, J.; Santa Mina, D.; Clarke, H.; Gofeld, M.; Katznelson, R. Evaluation of a Hyperbaric Oxygen Therapy Intervention in Individuals with Fibromyalgia. Pain Med. 2021, 22, 1324–1332. [Google Scholar] [CrossRef] [PubMed]
- Gilron, I.; Robb, S.; Tu, D.; Holden, R.; Towheed, T.; Ziegler, D.; Wang, L.; Milev, R.; Gray, C. Double-blind, randomized, placebo-controlled crossover trial of alpha-lipoic acid for the treatment of fibromyalgia pain: The IMPALA trial. Pain 2021, 162, 561. [Google Scholar] [CrossRef]
- Ware, M.A.; Fitzcharles, M.A.; Joseph, L.; Shir, Y. The Effects of Nabilone on Sleep in Fibromyalgia: Results of a Randomized Controlled Trial. Anesth. Analg. 2010, 110, 604. [Google Scholar] [CrossRef]
- Gilron, I.; Chaparro, L.E.; Tu, D.; Holden, R.R.; Milev, R.; Towheed, T.; DuMerton-Shore, D.; Walker, S. Combination of pregabalin with duloxetine for fibromyalgia: A randomized controlled trial. Pain 2016, 157, 1532. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, A.; Paisley, D.; Kouassi, E.; Gendron, A. Quetiapine Fumarate Extended-Release for the Treatment of Major Depression With Comorbid Fibromyalgia Syndrome: A Double-Blind, Randomized, Placebo-Controlled Study. Arthritis Rheumatol. 2014, 66, 451–461. [Google Scholar] [CrossRef] [PubMed]
- Saxe, P.A.; Arnold, L.M.; Palmer, R.H.; Gendreau, R.M.; Chen, W. Short-term (2-week) effects of discontinuing milnacipran in patients with fibromyalgia. Curr. Med. Res. Opin. 2012, 28, 815–821. [Google Scholar] [CrossRef] [PubMed]
- Mease, P.J.; Clauw, D.J.; Trugman, J.M.; Palmer, R.H.; Wang, Y. Efficacy of long-term milnacipran treatment in patients meeting different thresholds of clinically relevant pain relief: Subgroup analysis of a randomized, double-blind, placebo-controlled withdrawal study. J. Pain. Res. 2014, 7, 679–687. [Google Scholar] [CrossRef] [PubMed]
- Ford, J.G.; Howerton, M.W.; Lai, G.Y.; Gary, T.L.; Bolen, S.; Gibbons, M.C.; Tilburt, J.; Baffi, C.; Tanpitukpongse, T.P.; Wilson, R.F.; et al. Barriers to recruiting underrepresented populations to cancer clinical trials: A systematic review. Cancer 2008, 112, 228–242. [Google Scholar] [CrossRef] [PubMed]
- Clark, L.T.; Watkins, L.; Piña, I.L.; Elmer, M.; Akinboboye, O.; Gorham, M.; Jamerson, B.; McCullough, C.; Pierre, C.; Polis, A.B.; et al. Increasing Diversity in Clinical Trials: Overcoming Critical Barriers. Curr. Probl. Cardiol. 2019, 44, 148–172. [Google Scholar] [CrossRef] [PubMed]
- Bonevski, B.; Randell, M.; Paul, C.; Chapman, K.; Twyman, L.; Bryant, J.; Brozek, I.; Hughes, C. Reaching the hard-to-reach: A systematic review of strategies for improving health and medical research with socially disadvantaged groups. BMC Med. Res. Methodol. 2014, 14, 42. [Google Scholar] [CrossRef]
- George, S.; Duran, N.; Norris, K. A Systematic Review of Barriers and Facilitators to Minority Research Participation Among African Americans, Latinos, Asian Americans, and Pacific Islanders. Am. J. Public. Health 2014, 104, e16–e31. [Google Scholar] [CrossRef]
- Warren, R.C.; Shedlin, M.G.; Alema-Mensah, E.; Obasaju, C.; Hodge, D.A. Clinical Trials Participation Among African Americans and the Ethics of Trust: Leadership Perspectives. J. Healthc. Sci. Humanit. 2020, 10, 104–123. [Google Scholar] [CrossRef]
- Coakley, M.; Fadiran, E.O.; Parrish, L.J.; Griffith, R.A.; Weiss, E.; Carter, C. Dialogues on Diversifying Clinical Trials: Successful Strategies for Engaging Women and Minorities in Clinical Trials. J. Womens Health 2012, 21, 713–716. [Google Scholar] [CrossRef]
- Cardenas-Rojas, A.; Castelo-Branco, L.; Pacheco-Barrios, K.; Shaikh, E.S.; Uygur-Kucukseymen, E.; Giannoni-Luza, S.; Felippe, L.V.; Gonzalez-Mego, P.; Luna-Cuadros, M.A.; Gianlorenco, A.C.L.; et al. Recruitment characteristics and non-adherence associated factors of fibromyalgia patients in a randomized clinical trial: A retrospective survival analysis. Contemp. Clin. Trials Commun. 2021, 24, 100860. [Google Scholar] [CrossRef] [PubMed]
- Institute for Fiscal Studies. Are Some Ethnic Groups More Vulnerable to COVID-19 Than Others? Available online: https://ifs.org.uk/publications/are-some-ethnic-groups-more-vulnerable-COVID-19-others (accessed on 28 November 2023).
- Fawzy, A.; Wu, T.D.; Wang, K.; Robinson, M.L.; Farha, J.; Bradke, A.; Golden, S.H.; Xu, Y.; Garibaldi, B.T. Racial and Ethnic Discrepancy in Pulse Oximetry and Delayed Identification of Treatment Eligibility Among Patients with COVID-19. JAMA Intern. Med. 2022, 182, 730–738. [Google Scholar] [CrossRef] [PubMed]
- Corneli, A.; Hanlen-Rosado, E.; McKenna, K.; Araojo, R.; Corbett, D.; Vasisht, K.; Siddiqi, B.; Johnson, T.; Clark, L.T.; Calvert, S.B. Enhancing Diversity and Inclusion in Clinical Trials. Clin. Pharmacol. Ther. 2023, 113, 489–499. [Google Scholar] [CrossRef] [PubMed]
- US Census Bureau QuickFacts: United States. Available online: https://www.census.gov/quickfacts/fact/table/US/PST045222 (accessed on 28 May 2023).
- Bureau, U.C. Census.gov. The Chance That Two People Chosen at Random Are of Different Race or Ethnicity Groups Has Increased Since 2010. Available online: https://www.census.gov/library/stories/2021/08/2020-united-states-population-more-racially-ethnically-diverse-than-2010.html (accessed on 28 July 2023).
- Haviland, M.G.; Morton, K.R.; Oda, K.; Fraser, G.E. Traumatic experiences, major life stressors, and self-reporting a physician-given fibromyalgia diagnosis. Psychiatry Res. 2010, 177, 335–341. [Google Scholar] [CrossRef]
- Murthy, V.H.; Krumholz, H.M.; Gross, C.P. Participation in Cancer Clinical TrialsRace-, Sex-, and Age-Based Disparities. JAMA 2004, 291, 2720–2726. [Google Scholar] [CrossRef]
- Duma, N.; Vera Aguilera, J.; Paludo, J.; Haddox, C.L.; Gonzalez Velez, M.; Wang, Y.; Leventakos, K.; Hubbard, J.M.; Mansfield, A.S.; Go, R.S.; et al. Representation of Minorities and Women in Oncology Clinical Trials: Review of the Past 14 Years. J. Oncol. Pract. 2018, 14, e1–e10. [Google Scholar] [CrossRef]
- Murphy, E.J.; Kassem, L.; Chemerinski, A.; Rush, A.J.; Laje, G.; McMahon, F.J. Retention and Attrition Among African Americans in the STAR*D Study: What Causes Research Volunteers to Stay or Stray? Depress. Anxiety 2013, 30, 1137–1144. [Google Scholar] [CrossRef]



| Number of Included Articles | |
| Total articles in review | 58 |
| Total trials recruiting new participants (Objectives 1–3) | 56 |
| Participant numbers across all trials recruiting new participants | |
| Total participants | 14,977 |
| Total female participants | 13,591 (~90%) Data unavailable 125 |
| Study sample size range | 10–1227 |
| Number/proportion of participants in multi-centre trials | 13,996 (93.4%) |
| Characteristics of trials recruiting new participants | |
| Multi-centre trials (non-phase III) | 26 |
| Phase III multi-centre trials | 3 |
| Single centre trials | 27 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Henley, P.; Martins, T.; Zamani, R. Assessing Ethnic Minority Representation in Fibromyalgia Clinical Trials: A Systematic Review of Recruitment Demographics. Int. J. Environ. Res. Public Health 2023, 20, 7185. https://doi.org/10.3390/ijerph20247185
Henley P, Martins T, Zamani R. Assessing Ethnic Minority Representation in Fibromyalgia Clinical Trials: A Systematic Review of Recruitment Demographics. International Journal of Environmental Research and Public Health. 2023; 20(24):7185. https://doi.org/10.3390/ijerph20247185
Chicago/Turabian StyleHenley, Peter, Tanimola Martins, and Reza Zamani. 2023. "Assessing Ethnic Minority Representation in Fibromyalgia Clinical Trials: A Systematic Review of Recruitment Demographics" International Journal of Environmental Research and Public Health 20, no. 24: 7185. https://doi.org/10.3390/ijerph20247185
APA StyleHenley, P., Martins, T., & Zamani, R. (2023). Assessing Ethnic Minority Representation in Fibromyalgia Clinical Trials: A Systematic Review of Recruitment Demographics. International Journal of Environmental Research and Public Health, 20(24), 7185. https://doi.org/10.3390/ijerph20247185






