Is Frailty Diagnosis Important in Patients with COPD? A Narrative Review of the Literature
Abstract
1. Introduction
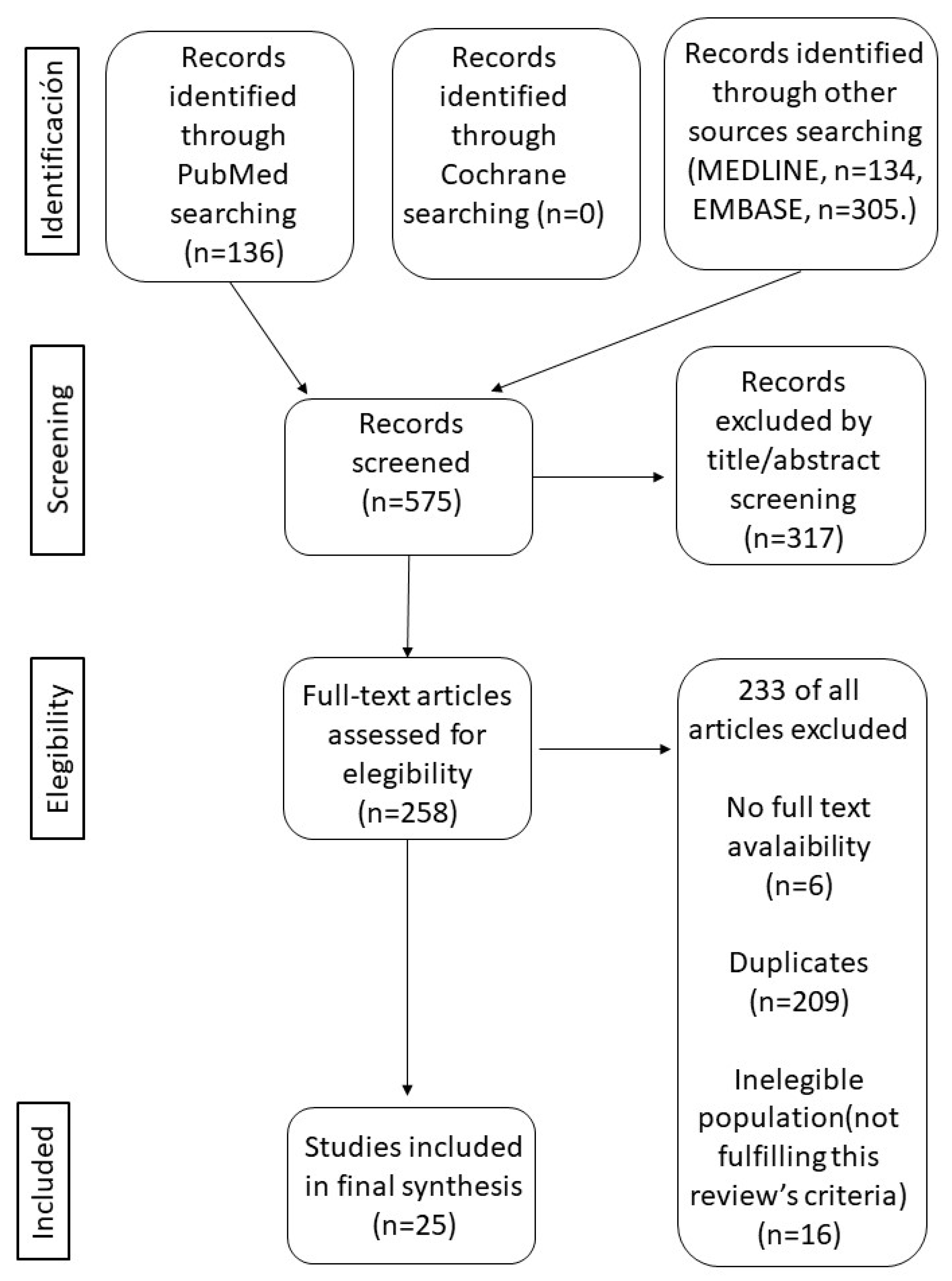
2. Materials and Methods
3. Results
3.1. Frailty Prevalence
The Prevalence of Geriatric Syndromes Linked to Frailty in COPD Patients
3.2. Frailty as a Predictive Factor of Poor Outcomes
3.3. Frailty as a Predictive Factor of Poor Outcomes
3.4. Interventions in Frailty COPD Patients
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Morley, J.E.; Vellas, B.; Abellan van Kan, G.; Anker, S.D.; Bauer, J.M.; Bernabei, R.; Walston, J. Frailty consensus: A call to action. J. Am. Med. Dir. Assoc. 2013, 14, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in older adults: Evidence for a phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, 146–157. [Google Scholar] [CrossRef]
- Kojima, G.; Taniguchi, Y.; Iliffe, S.; Jivraj, S.; Walters, K. Transitions between frailty states among community-dwelling older people: A systematic review and meta-analysis. Ageing Res. Rev. 2019, 50, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Dent, E.; Martin, F.C.; Bergman, H.; Woo, J.; Romero-Ortuno, R.; Walston, J.D. Management of frailty: Opportunities, challenges, and future directions. Lancet 2019, 394, 1376–1386. [Google Scholar] [CrossRef]
- Veronese, N.; Sigeirsdottir, K.; Eiriksdottir, G.; Marques, E.A.; Chalhoub, D.; Philips, C.L.; Launer, L.J.; Maggi, S.; Gudnason, V.; Harris, T.B. Frailty and risk of cardiovascular diseases in older persons: The age, gene/environment susceptibility-Reykjavik study. Rejuvenation Res. 2017, 20, 517–524. [Google Scholar] [CrossRef]
- Bandeen-Roche, K.; Xue, Q.L.; Ferrucci, L.; Walston, J.; Guralnik, J.M.; Chaves, P.; Zeger, S.L.; Fried, L.P. Phenotype of frailty: Characterization in the women’s health and aging studies. J. Gerontol. A Biol. Sci. Med. Sci. 2006, 61, 262–266. [Google Scholar] [CrossRef]
- Vestbo, J.; Hurd, S.S.; Agustí, A.G.; Jones, P.W.; Vogelmeier, C.; Anzueto, A.; Barnes, P.J.; Fabbri, L.M.; Martinez, F.J.; Nishimura, M.; et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am. J. Respir. Crit. Care Med. 2013, 187, 347–365. [Google Scholar] [CrossRef] [PubMed]
- Collard, R.M.; Boter, H.; Schoevers, R.A.; Oude-Voshaar, R.C. Prevalence of frailty in community-dwelling older persons: A systematic review. J. Am. Geriatr. Soc. 2012, 60, 1487–1492. [Google Scholar] [CrossRef] [PubMed]
- Singer, J.P.; Lederer, D.J.; Baldwin, M.R. Frailty in pulmonary and critical care medicine. Ann. Am. Thorac. Soc. 2016, 13, 1394–1404. [Google Scholar] [CrossRef]
- Milne, K.M.; Kwan, J.M.; Guler, S.; Winstone, T.A.; Le, A.; Khalil, N.; Camp, P.G.; Wilcox, P.G.; Ryerson, C.J. Frailty is common and strongly associated with dyspnoea severity in fibrotic interstitial lung disease. Respirology 2017, 22, 728–734. [Google Scholar] [CrossRef]
- Rosenwasser, Y.; Berger, I.; Loewy, Z.G. Therapeutic Approaches for Chronic Obstructive Pulmonary Disease (COPD) Exacerbations. Pathogens 2022, 11, 1513. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J. Cellular and molecular mechanisms of asthma and COPD. Clin. Sci. 2017, 131, 1541–1558. [Google Scholar] [CrossRef]
- Karpman, C.; Benzo, R. Gait speed as a measure of functional status in COPD patients. Int. J. Chron. Obstruct. Pulmon. Dis. 2014, 9, 1315–1320. [Google Scholar]
- Lahousse, L.; Ziere, G.; Verlinden, V.J.; Zillikens, M.C.; Uitterlinden, A.G.; Rivadeneira, F.; Tiemeier, H.; Joos, G.F.; Hofman, A.; Ikram, M.A.; et al. Risk of frailty in elderly with COPD: A population-based study. J. Gerontol. A Biol. Sci. Med. Sci. 2016, 71, 689–695. [Google Scholar] [CrossRef]
- Lippi, L.; Folli, A.; Curci, C.; D’Abrosca, F.; Moalli, S.; Mezian, K.; de Sire, A.; Invernizzi, M. Osteosarcopenia in Patients with Chronic Obstructive Pulmonary Diseases: Which Pathophysiologic Implications for Rehabilitation? Int. J. Environ. Res. Public Health 2022, 19, 14314. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Gandullo, E.; Hidalgo-Molina, A.; Montoro-Ballesteros, F.; Morales-González, M.; Muñoz-Ramírez, I.; Arnedillo-Muñoz, A. Inspiratory Muscle Training in Patients with Chronic Obstructive Pulmonary Disease (COPD) as Part of a Respiratory Rehabilitation Program Implementation of Mechanical Devices: A Systematic Review. Int. J. Environ. Res. Public Health 2022, 19, 5564. [Google Scholar] [CrossRef]
- Marengoni, A.; Vetrano, D.L.; Manes-Gravina, E.; Bernabei, R.; Onder, G.; Palmer, K. The relationship between COPD and frailty: A systematic review and meta-analysis of observational studies. Chest 2018, 154, 21–40. [Google Scholar] [CrossRef] [PubMed]
- Baldi, S.; Jose, P.E.; Bruschi, C.; Pinna, G.D.; Maestri, R.; Rezzani, A.; Bellinzona, E.; Fracchia, C.; Dacosto, E.; Crotti, P.; et al. The mediating role of cytokine IL-6 on the relationship of FEV1 upon 6-minute walk distance in chronic obstructive pulmonary disease. Int. J. Chron. Obstruct. Pulmon. Dis. 2014, 9, 1091–1099. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Ushida, K.; Shimizu, A.; Hori, S.; Yamamoto, Y.; Momosaki, R. Hospital Frailty Risk Score Predicts Outcomes in Chronic Obstructive Pulmonary Disease Exacerbations. Arch. Gerontol. Geriatr. 2022, 100, 104658. [Google Scholar] [CrossRef] [PubMed]
- Neo, H.Y.; Yap, C.W.; Teo, L.M.; Lee, L.T.; Tan, H.W.; Xu, H.Y.; Hum, A.; Abisheganaden, J.A. Palliative Rehabilitation Improves Health Care Utilization and Function in Frail Older Adults with Chronic Lung Diseases. J. Am. Med. Dir. Assoc. 2021, 22, 2478–2485. [Google Scholar] [CrossRef]
- Kennedy, C.C.; Novotny, P.J.; LeBrasseur, N.K.; Wise, R.A.; Sciurba, F.C.; Benzo, R.P. Frailty and Clinical Outcomes in Chronic Obstructive Pulmonary Disease. Ann. Am. Thorac. Soc. 2019, 16, 217–224. [Google Scholar] [CrossRef]
- Witt, L.J.; Spacht, W.A.; Carey, K.A.; Arora, V.M.; White, S.R.; Huisingh-Scheetz, M.; Press, V.G. Weak Handgrip at Index Admission for Acute Exacerbation of COPD Predicts All-Cause 30-Day Readmission. Front. Med. 2021, 8, 611989. [Google Scholar] [CrossRef]
- Luo, J.; Zhang, D.; Tang, W.; Dou, L.Y.; Sun, Y. Impact of Frailty on the Risk of Exacerbations and All-Cause Mortality in Elderly Patients with Stable Chronic Obstructive Pulmonary Disease. Clin. Interv. Aging 2021, 16, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Yee, N.; Locke, E.R.; Pike, K.C.; Chen, Z.; Lee, J.; Huang, J.C.; Nguyen, H.Q.; Fan, V.S. Frailty in Chronic Obstructive Pulmonary Disease and Risk of Exacerbations and Hospitalizations. Int. J. Chron. Obstruct. Pulmon. Dis. 2020, 15, 1967–1976. [Google Scholar] [CrossRef] [PubMed]
- Bernabeu-Mora, R.; Oliveira-Sousa, S.L.; Sánchez-Martínez, M.P.; García-Vidal, J.A.; Gacto-Sánchez, M.; Medina-Mirapeix, F. Frailty transitions and associated clinical outcomes in patients with stable COPD: A longitudinal study. PLoS ONE 2020, 15, e0230116. [Google Scholar] [CrossRef] [PubMed]
- Naval, E.; González, M.C.; Giraldós, S.; Calatayud, J.; Jornet, M.; Lluch, I.; Meseguer, M.; Cubillan, J.J.R.; Viña, J.; Tarazona-Santabalbina, F.J. Frailty Assessment in a Stable COPD Cohort: Is There a COPD-Frail Phenotype? COPD 2021, 18, 525–532. [Google Scholar] [CrossRef]
- Hanlon, P.; Lewsey, J.; Quint, J.K.; Jani, B.D.; Nicholl, B.I.; McAllister, D.A.; Mair, F.S. Frailty in COPD: An analysis of prevalence and clinical impact using UK Biobank. BMJ Open Respir. Res. 2022, 9, e001314. [Google Scholar] [CrossRef]
- Zhang, D.; Tang, W.; Dou, L.Y.; Luo, J.; Sun, Y. Four different frailty models predict health outcomes in older patients with stable chronic obstructive pulmonary disease. BMC Geriatr. 2022, 22, 57. [Google Scholar] [CrossRef]
- Soni, N.; Banerjee, J.; Gunasekaran, V.; Saini, M.; Singh, V.; Kumar, P.; Chakrawarty, A.; Chatterjee, P.; Dey, A.B. Association of geriatric syndromes in older adults with chronic obstructive pulmonary disease. Aging Med. 2022, 5, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Dias, L.S.; Ferreira, A.C.G.; da Silva Junior, J.L.R.; Conte, M.B.; Rabahi, M.F. Prevalence of Frailty and Evaluation of Associated Variables among COPD Patients. Int. J. Chron. Obstruct. Pulmon. Dis. 2020, 15, 1349–1356. [Google Scholar] [CrossRef] [PubMed]
- Gale, N.S.; Albarrati, A.M.; Munnery, M.M.; Hubbard, R.E.; Tal-Singer, R.; Cockcroft, J.R.; Shale, D.J. Frailty: A global measure of the multisystem impact of COPD. Chron. Respir. Dis. 2018, 15, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Albarrati, A.M.; Gale, N.S.; Munnery, M.M.; Reid, N.; Cockcroft, J.R.; Shale, D.J. The Timed up and Go test predicts frailty in patients with COPD. NPJ Prim. Care Respir. Med. 2022, 32, 24. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.J.; Liu, Q.; Zheng, L.J. A Frailty Assessment Tool to Predict In-Hospital Mortality in Patients with Acute Exacerbations of Chronic Obstructive Pulmonary Disease. Int. J. Chron. Obstruct. Pulmon. Dis. 2021, 16, 1093–1100. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, S.; Hirano, T.; Yasuda, K.; Donishi, T.; Suga, K.; Doi, K.; Oishi, K.; Ohata, S.; Murata, Y.; Yamaji, Y.; et al. Impact of Frailty on Hippocampal Volume in Patients with Chronic Obstructive Pulmonary Disease. Biomedicines 2021, 9, 1103. [Google Scholar] [CrossRef] [PubMed]
- Oishi, K.; Matsunaga, K.; Harada, M.; Suizu, J.; Murakawa, K.; Chikumoto, A.; Ohteru, Y.; Matsuda, K.; Uehara, S.; Hamada, K.; et al. A New Dyspnea Evaluation System Focusing on Patients’ Perceptions of Dyspnea and Their Living Disabilities: The Linkage between COPD and Frailty. J. Clin. Med. 2020, 9, 3580. [Google Scholar] [CrossRef] [PubMed]
- Witt, L.J.; Wroblewski, K.E.; Pinto, J.M.; Wang, E.; McClintock, M.K.; Dale, W.; White, S.R.; Press, V.G.; Huisingh-Scheetz, M. Beyond the Lung: Geriatric Conditions Afflict Community-Dwelling Older Adults with Self- Reported Chronic Obstructive Pulmonary Disease. Front. Med. 2022, 9, 814606. [Google Scholar] [CrossRef] [PubMed]
- Bernabeu-Mora, R.; García-Guillamón, G.; Valera-Novella, E.; Giménez-Giménez, L.M.; Escolar-Reina, P.; Medina-Mirapeix, F. Frailty is a predictive factor of readmission within 90 days of hospitalization for acute exacerbations of chronic obstructive pulmonary disease: A longitudinal study. Ther. Adv. Respir. Dis. 2017, 11, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.J.; Yang, K.Y.; Perng, W.C.; Lin, K.C.; Wang, K.Y. Effect of dyspnea on frailty stages and related factors in Taiwanese men with COPD. Int. J. Chron. Obstruct. Pulmon. Dis. 2018, 13, 2463–2469. [Google Scholar] [CrossRef] [PubMed]
- Ter Beek, L.; van der Vaart, H.; Wempe, J.B.; Krijnen, W.P.; Roodenburg, J.L.N.; van der Schans, C.P.; Jager-Wittenaar, H. Coexistence of malnutrition, frailty, physical frailty and disability in patients with COPD starting a pulmonary rehabilitation program. Clin. Nutr. 2020, 39, 2557–2563. [Google Scholar] [CrossRef] [PubMed]
- Chin, M.; Voduc, N.; Huang, S.; Forster, A.; Mulpuru, S. Practical lessons in implementing frailty assessments for hospitalised patients with COPD. BMJ Open Qual. 2020, 9, e000782. [Google Scholar] [CrossRef] [PubMed]
- Ierodiakonou, D.; Kampouraki, M.; Poulonirakis, I.; Papadokostakis, P.; Lintovoi, E.; Karanassos, D.; Maltezis, K.; Chorti, M.; Petrovitsos, E.; Dimopoulou, S.; et al. Determinants of frailty in primary care patients with COPD: The Greek UNLOCK study. BMC Pulm. Med. 2019, 19, 63. [Google Scholar] [CrossRef] [PubMed]
- Patino-Hernandez, D.; Borda, M.G.; Cano-Gutiérrez, C.A.; Celis-Preciado, C.A.; Pérez-Zepeda, M.U. La fragilidad se asocia con mayor mortalidad en la enfermedad pulmonar obstructiva crónica [Frailty is associated with increased mortality in chronic obstructive pulmonary disease]. Rev. Esp. Geriatr. Gerontol. 2019, 54, 237–238. [Google Scholar] [CrossRef] [PubMed]
- Theou, O.; Walston, J.; Rockwood, K. Operationalizing Frailty Using the Frailty Phenotype and Deficit Accumulation Approaches. Interdiscip. Top Gerontol. Geriatr. 2015, 41, 66–73. [Google Scholar]
- Lim, Y.J.; Ng, Y.S.; Sultana, R.; Tay, E.L.; Mah, S.M.; Chan, C.H.N.; Latib, A.B.; Abu-Bakar, H.M.; Ho, J.C.Y.; Kwek, T.H.H.; et al. Frailty Assessment in Community-Dwelling Older Adults: A Comparison of 3 Diagnostic Instruments. J. Nutr. Health Aging 2020, 24, 582–590. [Google Scholar] [CrossRef]
- Liu, S.; Yan, Q.; Jiang, Y.; Xiao, M.; Zhao, J.; Wang, Y.; Deng, R.; Wang, C.; Yang, Z. The Impact of Frailty on All-Cause Mortality in Patients with HIV Infection: A Systematic Review and Meta-Analysis. AIDS Res. Hum. Retrovir. 2022, 38, 692–699. [Google Scholar] [CrossRef]
- Sepúlveda-Loyola, W.; Osadnik, C.; Phu, S.; Morita, A.A.; Duque, G.; Probst, V.S. Diagnosis, prevalence, and clinical impact of sarcopenia in COPD: A systematic review and meta-analysis. J. Cachexia Sarcopenia Muscle 2020, 11, 1164–1176. [Google Scholar] [CrossRef]
- Boakye, D.; Rillmann, B.; Walter, V.; Jansen, L.; Hoffmeister, M.; Brenner, H. Impact of comorbidity and frailty on prognosis in colorectal cancer patients: A systematic review and meta-analysis. Cancer Treat. Rev. 2018, 64, 30–39. [Google Scholar] [CrossRef]
- Antoniu, S.A.; Boiculese, L.V.; Prunoiu, V. Frailty, a Dimension of Impaired Functional Status in Advanced COPD: Utility and Clinical Applicability. Medicina 2021, 57, 474. [Google Scholar] [CrossRef]
- Kojima, G. Frailty as a predictor of hospitalisation among community-dwelling older people: A systematic review and meta-analysis. J. Epidemiol. Community Health 2016, 70, 722–729. [Google Scholar] [CrossRef]
- Kojima, G.; Iliffe, S.; Walters, K. Frailty index as a predictor of mortality: A systematic review and meta-analysis. Age Ageing 2018, 47, 193–200. [Google Scholar] [CrossRef]
- Wang, Z.; Hu, X.; Dai, Q. Is it possible to reverse frailty in patients with chronic obstructive pulmonary disease? Clinics 2020, 75, e1778. [Google Scholar] [CrossRef] [PubMed]
- Guan, C.; Niu, H. Frailty assessment in older adults with chronic obstructive respiratory diseases. Clin. Interv. Aging 2018, 13, 1513–1524. [Google Scholar] [CrossRef] [PubMed]
- Brighton, L.J.; Evans, C.J.; Man, W.D.C.; Maddocks, M. Improving Exercise-Based Interventions for People Living with Both COPD and Frailty: A Realist Review. Int. J. Chron. Obstruct. Pulmon. Dis. 2020, 15, 841–855. [Google Scholar] [CrossRef]
- Gephine, S.; Saey, D.; Grosbois, J.M.; Maltais, F.; Mucci, P. Home-based Pulmonary Rehabilitation is Effective in Frail COPD Patients with Chronic Respiratory Failure. Chronic. Obstr. Pulm. Dis. 2022, 9, 15–25. [Google Scholar] [CrossRef]
- Lyer, A.S.; Sullivan, D.R.; Lindell, K.O.; Reinke, L.F. The Role of Palliative Care in COPD. Chest 2022, 161, 1250–1262. [Google Scholar]
- Symvoulakis, E.K.; Kamekis, A.; Drakonaki, E.; Mastrodemou, S.; Ryerson, C.J.; Antoniou, K. Frailty and chronic respiratory disease: The need for a multidisciplinary care model. Sarcoidosis Vasc. Diffuse Lung Dis. 2021, 38, e2021031. [Google Scholar] [PubMed]

| Article | Type | Sample | Age (Years) | Tool Used | Frailty Prevalence | Spirometric COPD Confirmation |
|---|---|---|---|---|---|---|
| Ushida, K. et al. [20] | Retrospective cohort | 3396 COPD patients | 75.9 (SD 11.2) | HFRS | 14% | No (ICD-10 codes: J41–J44) |
| Neo, H.Y. et al. [21] | Prospective, propensity score-match study | 100 matched pairs | 73.9 (SD 8.2) | HFRS | 57% | No |
| Kennedy, C. C. et al. [22] | Retrospective cohort | 902 COPD patients | 67 [IQR 63–70] | FFP | 6% | Yes |
| Witt, L. J. et al. [23] | Observational study | 70 patients admitted due to COPD exacerbation | 63.5 (SD 58.1, 71.3) | FFP | 67% | Yes |
| Luo, J. et al. [24] | Cross-sectional study | 309 COPD patients | 86 [IQR 80–90] | FFP | 49.8% | Yes |
| Yee, N. et al. [25] | Prospective cohort | 280 COPD patients | 68.6 (SD 9.2) | FFP | 23% | Yes |
| Bernabeu-Mora, R. et al. [26] | Prospective study | 119 COPD patients | 66.9 (SD 7.9) | FFP | 7.6% | Yes |
| Naval, E. et al. [27] | Cross-sectional study | 127 COPD patients | 66.5 (SD 7.9) | FFP | 24.4% | Yes |
| Hanlon, P. et al. [28] | Observational study | 3132 COPD patients | 61.9 (SD 5.9) | FFP FI | 17% FFP 32% FI | Yes |
| Zhang, D. et al. [29] | Prospective study | 302 COPD patients | 86 [IQR 80–90] | FFP CFS FI-CD SPPB | FFP 51% CFS 64% FI 58.6% SPPB 59.6% | Yes |
| Soni, N. et al. [30] | Case-control study | 150 COPD 150 Controls | 65.98 (SD 5.43) 65.72 (SD 5.65) | FRAIL | 25.3% | Yes |
| Dias, L. S. et al. [31] | Cross-sectional study | 150 COPD patients | 67.0 (SD 61.0–71.5) | FRAIL | 50.3% | Yes |
| Gale, N. S. et al. [32] | Case-control study | 520 COPD 150 controls | (66.1 (SD 7.6)) (65 (SD 7.4)) | FI | 28% | Yes |
| Albarrati, A. M. et al. [33] | Case-control study | 520 COPD 120 controls | 66.1 (SD 7.6) (65 (SD 7.4)) | FI | 76% | Yes |
| Gu, J. J. et al. [34] | Observational retrospective study | 154 COPD patients | 79.73 (SD 8.38) | FI-lab | 75.3% | Yes |
| Takahashi, S. et al. [35] | Cross-sectional study | 40 COPD patients | 70.6 (SD 8.21) | KCL | 50% | Yes |
| Oishi, K. et al. [36] | Observational study | 128 COPD patients | 73 [IQR 69–78] | KCL | 37.5% | Yes |
| Witt, L. J. et al. [37] | Cross-sectional study | 322 COPD patients | 69.6 (SD 7.4) | modified frailty | 16% | No |
| Bernabeu-Mora, R. et al. [38] | Prospective cohort | 103 hospitalized COPD patients | 71 (SD 9.1) | REFS | 35.9% moderate or severe frailty | Yes |
| Chen, P. J. et al. [39] | Cross-sectional study | 125 COPD patients | 77.36 (SD 10.26) | Chinese Canadian study of health and aging clinical frailty scale | 85.9% dyspnea group 26.7% non-dyspnea group | Yes |
| Ter Beek, L. et al. [40] | Cross-sectional study | 57 COPD patients | 61.2 (SD 8.7) | EFIP | 83% | Yes |
| Chin, M. et al. [41] | Prospective study | 46 patients admitted due to COPD exacerbation Mild frailty Moderate frailty Severe frailty | 72 (SD 9) 72 (SD 10) 76 (SD 12) | CFS | 54% | No described |
| Ierodiakonou, D. et al. [42] | Cross-sectional study | 257 COPD patients | (65 (SD 12.3)) | FiND (frail non-disabled) | 82% | Yes |
| Article | Type | Sample | Poor Outcomes Associated with Frailty |
|---|---|---|---|
| Ushida, K. et al. [20] | Retrospective cohort | 3396 COPD patients | Hospital admissions (32.9% vs. 17.5%) In-hospital mortality (16.4% vs. 12.5%) Greater difficulty in returning home (34.6% vs. 22.9%) |
| Kennedy, C. C. et al. [22] | Retrospective cohort | 902 | Increased rate of hospitalization: Adjusted HR, 1.6 (95% CI: 1.1–2.5) Increase in hospital use of 8.0 days: (95% CI: 4.4–11.6) Higher mortality rate: Adjusted HR 1.4 (95% CI: 0.97–2.0); p = 0.07 |
| Witt, L. J. et al. [23] | Observational study | 70 patients admitted due to COPD exacerbation | 30-day readmissions: OR 11.2 (95% CI: 1.3–93.2) |
| Luo, J. et al. [24] | Cross-sectional study | 309 | AECOPD: IRR = 1.75 (95% CI: 1.09–2.82) All-cause hospitalizations: IRR = 1.4 (95% CI: 1.0–1.9) All-cause mortality risk: HR = 2.5 (95% CI: 1.0–6.4) |
| Yee, N. et al. [25] | Cohort study | 280 | Handgrip strength increased AECOPD risk: IRR 1.46 (95% CI: 1.09–1.97) |
| Naval, E. et al. [26] | Cross-sectional study | 127 | AECOPD: Frail COPD patients 2.2 (SD 1.7) vs. fit COPD patients 1.0 (SD 1.0) |
| Hanlon, P. et al. [27] | Observational study | 3132 | FFP Mortality risk: HR 2.3 (95% CI: 1.8–3.0) MACE: HR 2.7; 95% CI: 1.7–4.5 Hospital admissions HR 3.4 (95% CI: 2.8–4.1) AECOPD hospital admissions: HR 5.2; 95% CI: 3.8–7.1 Community exacerbations: HR 2.1 (95% CI: 1.8–2.5) FI Mortality HR 2.6 (95% CI: 1.7–4.0) MACE HR 6.8 (95% CI: 2.7–17.0) Hospital admission HR 3.7 (95% CI: 2.5–5.4) AECOP hospital admissions HR 4.3; 95% CI: 2.4–7.7 Community exacerbations HR 2.4 (95% CI: 1.7–3.3) |
| Zhang, D. et al. [28] | Prospective study | 302 | 1-year mortality risk FFP: HR = 3.11 (95% CI: 1.30–7.44) CFS: HR = 3.68 (95% CI: 1.03–13.16) SPPB: HR = 3.74 (95% CI: 1.39–10.06) |
| Gu, J. J. et al. [34] | Observational retrospective study | 154 | FI-lab increased AECOPD and mortality: OR 8.705 (95% CI: 3.646–20.782) |
| Bernabeu-Mora, R. et al. [38] | Prospective cohort | 103 hospitalized COPD patients | Hospital readmission (45% vs. 18%) 90-day readmission (OR = 5.19; 95% CI: 1.26–21.50) |
| Chin, M. et al. [41] | Prospective study | 46 | Severe frailty vs. managing well and vulnerable: Total length of stay: 11 days [IQR 10–12] vs. 4 [IQR 2–7] Total cost CAD 14,109 [IQR 13,182–15,037] vs. 4366 [IQR 2490–7094] Previous hospitalization in the last 2 years, 6 [IQR 6–6] vs. 1 [IQR 0–2] |
| Patino-Hernandez, D. et al. [42] | Longitudinal study | 2706 patients (76.4 years) | 3-year mortality risk HR 1.95 (95% CI: 1.18–3.2) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tarazona-Santabalbina, F.J.; Naval, E.; De la Cámara-de las Heras, J.M.; Cunha-Pérez, C.; Viña, J. Is Frailty Diagnosis Important in Patients with COPD? A Narrative Review of the Literature. Int. J. Environ. Res. Public Health 2023, 20, 1678. https://doi.org/10.3390/ijerph20031678
Tarazona-Santabalbina FJ, Naval E, De la Cámara-de las Heras JM, Cunha-Pérez C, Viña J. Is Frailty Diagnosis Important in Patients with COPD? A Narrative Review of the Literature. International Journal of Environmental Research and Public Health. 2023; 20(3):1678. https://doi.org/10.3390/ijerph20031678
Chicago/Turabian StyleTarazona-Santabalbina, Francisco José, Elsa Naval, Juan María De la Cámara-de las Heras, Cristina Cunha-Pérez, and José Viña. 2023. "Is Frailty Diagnosis Important in Patients with COPD? A Narrative Review of the Literature" International Journal of Environmental Research and Public Health 20, no. 3: 1678. https://doi.org/10.3390/ijerph20031678
APA StyleTarazona-Santabalbina, F. J., Naval, E., De la Cámara-de las Heras, J. M., Cunha-Pérez, C., & Viña, J. (2023). Is Frailty Diagnosis Important in Patients with COPD? A Narrative Review of the Literature. International Journal of Environmental Research and Public Health, 20(3), 1678. https://doi.org/10.3390/ijerph20031678







