The Role of Slurry Reflux in a Corn Stalk Continuous Anaerobic Digestion System: Performance and Microbial Community
Abstract
1. Introduction
2. Materials and Methods
2.1. Substrates and Inoculum
2.2. Experimental Digester and Setup
2.3. Analytical Techniques and Statistical Method
3. Results
3.1. Influence of Slurry Reflux on Methane Production
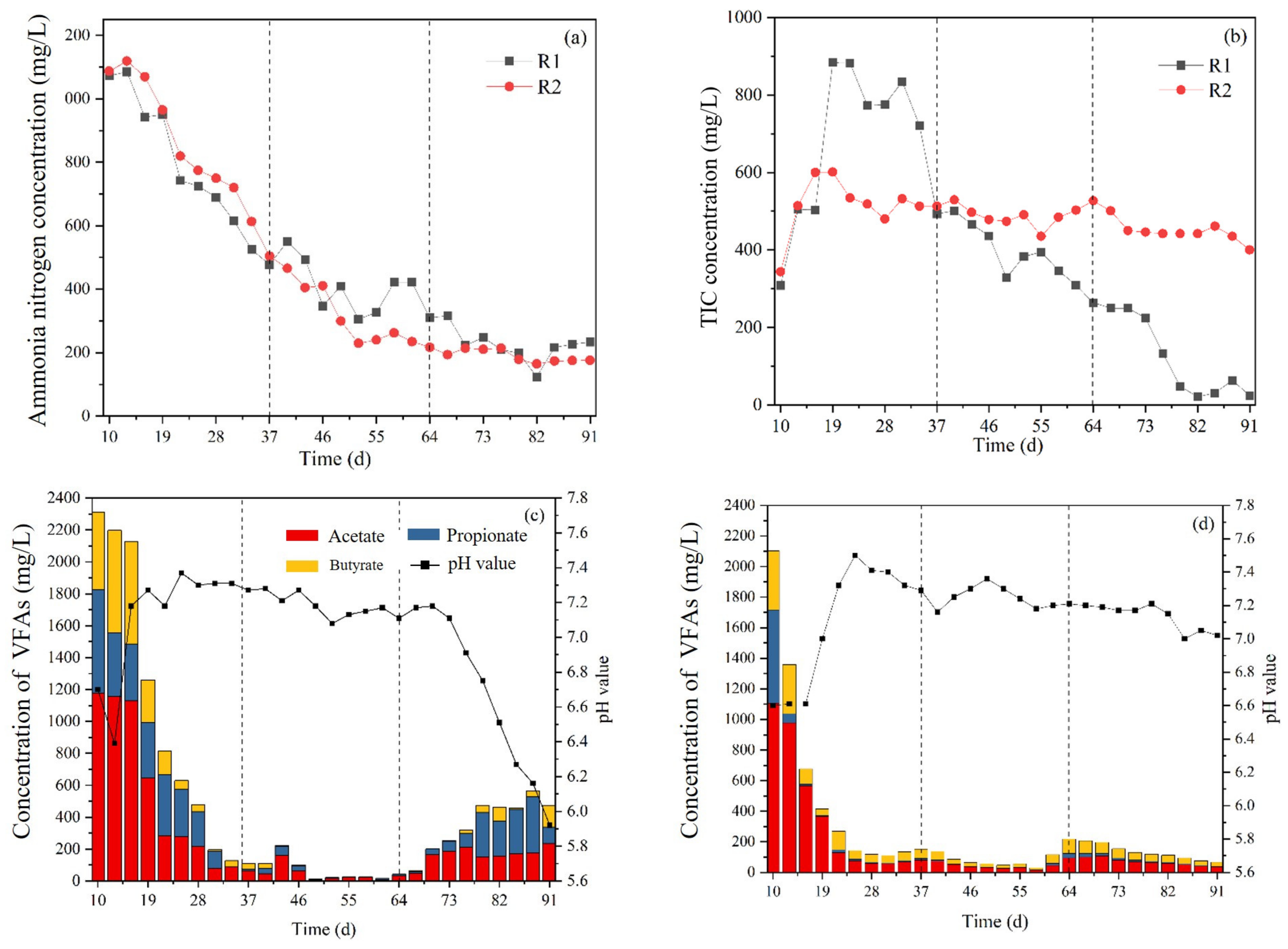
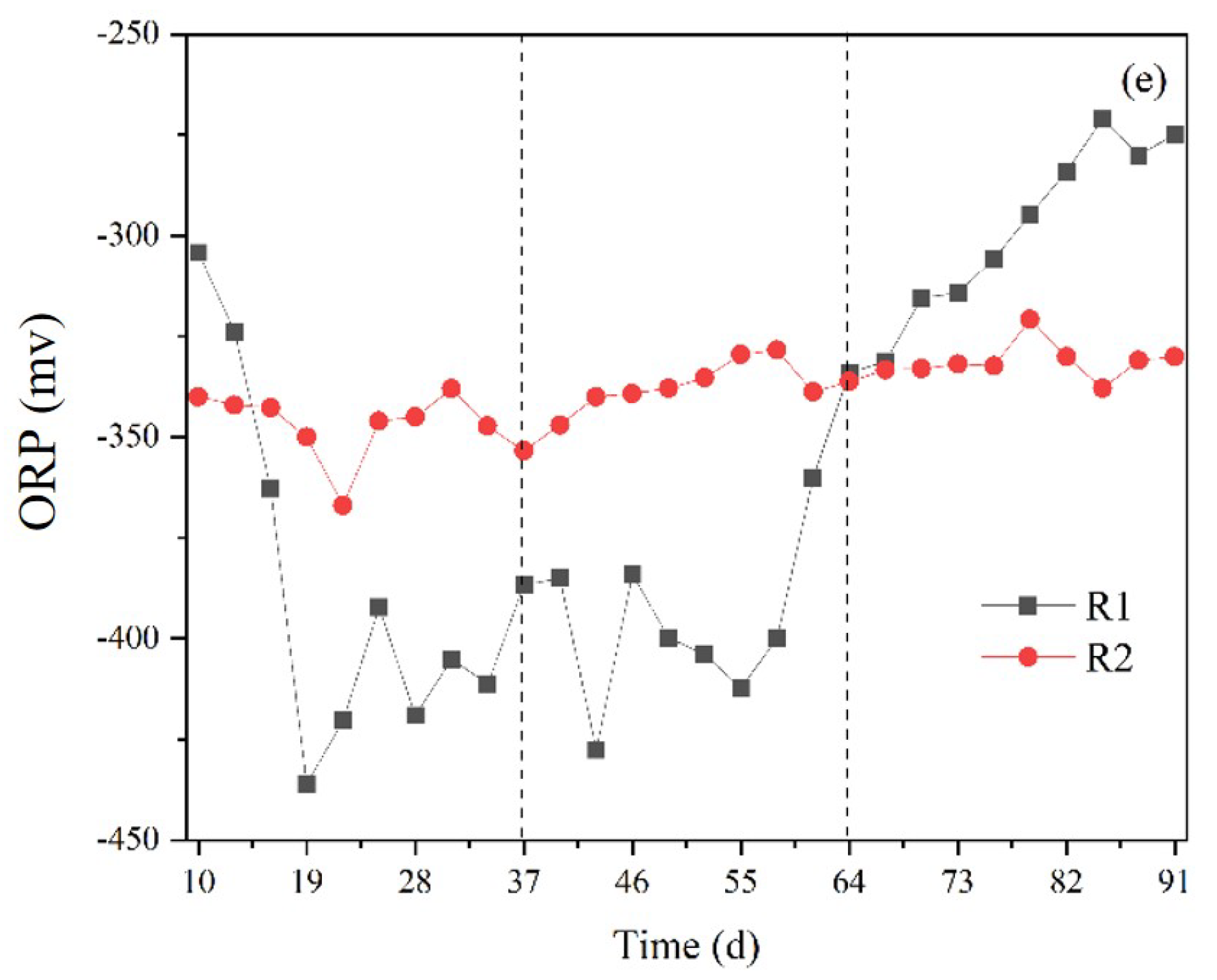
3.2. Influence of Slurry Reflux on System Stability
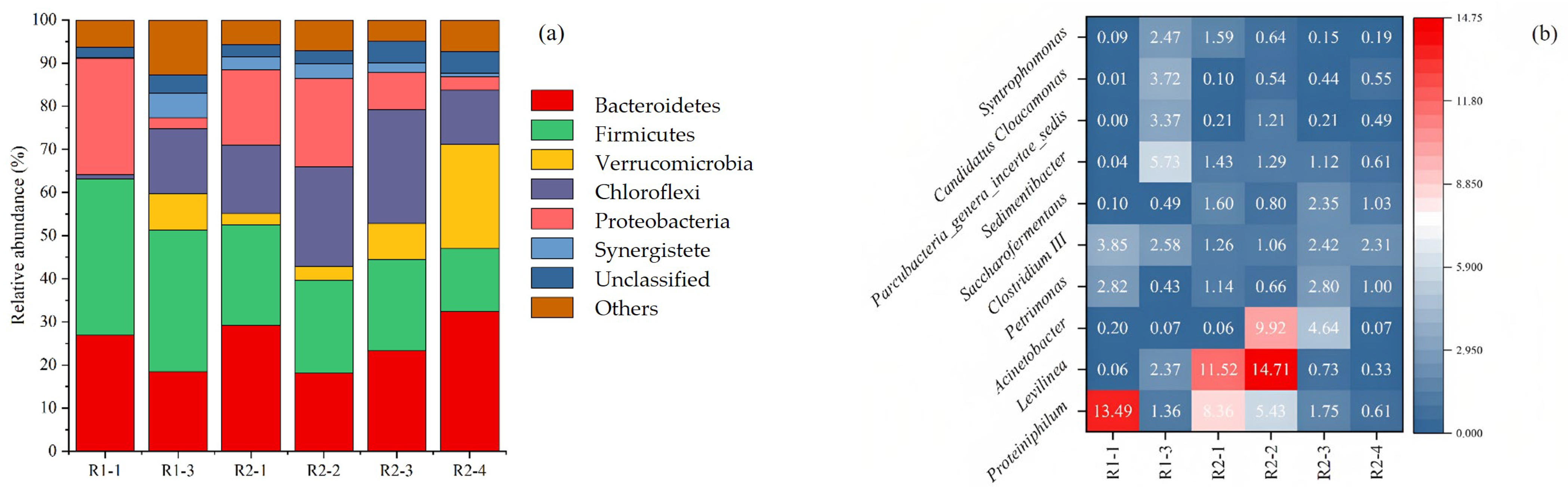
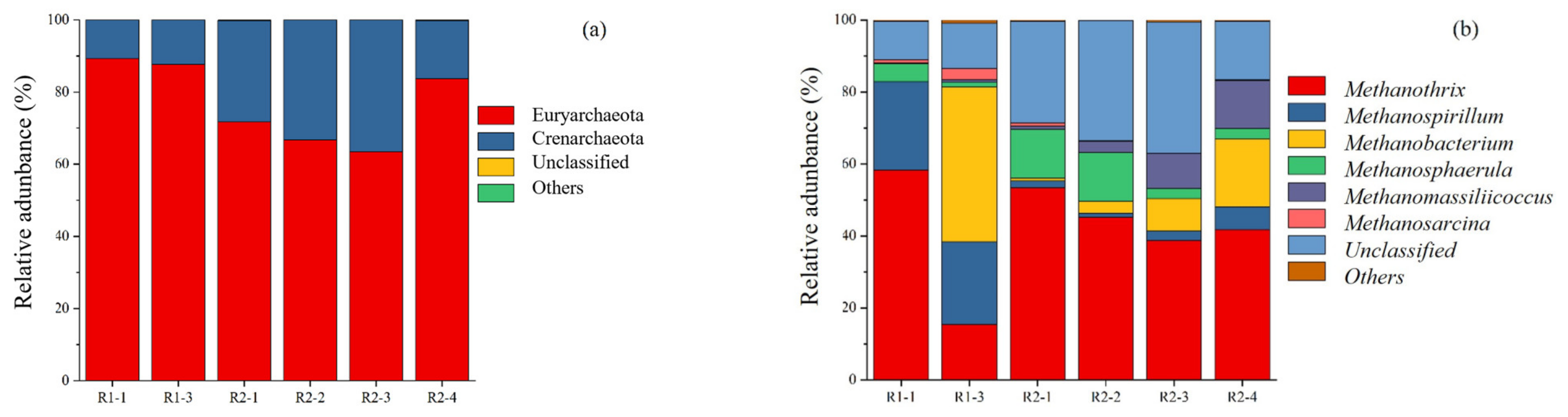
3.3. Microbiological Analysis during Biogas Production Process
3.3.1. Dynamics of Microbial Community Richness and Diversity
Dynamics of Bacterial Populations
Dynamics of Archaeal Populations at the Phylum and Genus Level
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, K.; Liu, R.; Sun, C. A review of methane production from agricultural residues in China. Renew. Sustain. Energy Rev. 2016, 54, 857–865. [Google Scholar] [CrossRef]
- Du, H.; Zhang, Y.X.; Zhang, X.Y.; He, Z.; Mao, W.W. Bibliometric analysis on the research status and development of straw utilization in China. J. Liaoning Univ. Nat. Sci. Ed. 2017, 44, 163–170. [Google Scholar]
- Liu, C.; He, Z.; Lu, X. Optimization analysis of carbon emission reduction from crop straw collection and transportation under the sustainable development goals. Trans. CSAE 2022, 38, 239–248. [Google Scholar]
- Lee, M.; Hidaka, T.; Hagiwara, W.; Tsuno, H. Comparative performance and microbial diversity of hyperthermophilic and thermophilic co-digestion of kitchen garbage and excess sludge. Bioresour. Technol. 2009, 100, 578–585. [Google Scholar] [CrossRef] [PubMed]
- Yanjin, W.; Zhenfeng, W.; Quanguo, Z.; Gaoshen, L.; Chenxi, X. Comparison of bio-hydrogen and bio-methane production performance in continuous two-phase anaerobic fermentation system between co-digestion and digestate recirculation. Bioresour. Technol. 2020, 318, 124269. [Google Scholar]
- Yu, Q.; Liu, R.; Li, K.; Ma, R. A review of crop straw pretreatment methods for biogas production by anaerobic digestion in China. Renew. Sustain. Energy Rev. 2019, 107, 51–58. [Google Scholar] [CrossRef]
- Rubén, A.; José, M.L.; Manoj, K.; Mohammad, A.S.; Muhammad, U.K.; Abid, S.; Muhammad, S.; Maksim, R.; Muhammad, U. Anaerobic Digestion of Lignocellulose Components: Challenges and Novel Approaches. Energies 2022, 15, 8413. [Google Scholar]
- Lu, J.; Zhu, L.; Hu, G.; Wu, J. Integrating animal manure-based bioenergy production with invasive species control: A case study at Tongren Pig Farm in China. Biomass Bioenergy 2010, 34, 821–827. [Google Scholar] [CrossRef]
- Lamolinara, B.; Pérez-Martínez, A.; Guardado-Yordi, E.; Fiallos, C.G.; Diéguez-Santana, K.; Ruiz-Mercado, G.J. Anaerobic digestate management, environmental impacts, and techno-economic challenges. Waste Manag. 2022, 140, 14–30. [Google Scholar] [CrossRef]
- Chen, C.; Ruan, Z.Y.; Wu, J.; Gao, L.H.; Song, J.L.; Wang, Y.W.; Xu, Y.S.; Wei, X.L.; Xu, F.H. Research Progress on the Comprehensive Disposal and Utilization of Biogas Slurry from Large Scale Biogas Engineering. China Biogas 2013, 31, 25–28+43. [Google Scholar]
- Qiu, S.; Zhao, L.B.; Sun, Y. The influence of aeration rate on intermittent forced-aeration composting of biogas residue. China Environ. Sci. 2016, 36, 2402–2408. [Google Scholar]
- Wu, C.; Huang, Q.; Yu, M.; Ren, Y.; Wang, Q.; Sakai, K. Effects of digestate recirculation on a two-stage anaerobic digestion system, particularly focusing on metabolite correlation analysis. Bioresour. Technol. 2018, 251, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Z.; Wu, S.; Zhang, W.; Dong, R. Effects of organic loading rate and effluent recirculation on the performance of two-stage anaerobic digestion of vegetable waste. Bioresour. Technol. 2013, 146, 556–561. [Google Scholar]
- Zheng, Z.; Cai, Y.; Zhao, Y.; Meng, X.; Zhang, Y.; Lu, C.; Hu, Y.; Cui, Z.; Wang, X. Achieve clean and efficient biomethane production by matching between digestate recirculation and straw-to-manure feeding ratios. J. Clean. Prod. 2020, 263, 121414. [Google Scholar] [CrossRef]
- Hu, Y.; Shen, F.; Yuan, H.; Zou, D.; Pang, Y.; Liu, Y.; Zhu, B.; Chufo, W.A.; Jaffar, M.; Li, X. Influence of recirculation of liquid fraction of the digestate (LFD) on maize stover anaerobic digestion. Biosyst. Eng. 2014, 127, 189–196. [Google Scholar] [CrossRef]
- Yang, Q.; Ju, M.T.; Li, W.Z. Review of methane production from straws anaerobic digestion. Trans. CSAE 2016, 32, 232–242. [Google Scholar]
- Razaviarani, V.; Buchanan, I.D. Anaerobic co-digestion of biodiesel waste glycerin with municipal wastewater sludge: Microbial community structure dynamics and reactor performance. Bioresour. Technol. 2015, 182, 8–17. [Google Scholar] [CrossRef]
- Abbassi-Guendouz, A.; Brockmann, D.; Trably, E.; Dumas, C.; Delgenès, J.-P.; Steyer, J.-P.; Escudié, R. Total solids content drives high solid anaerobic digestion via mass transfer limitation. Bioresour. Technol. 2012, 111, 55–61. [Google Scholar] [CrossRef]
- Rui, J.; Li, J.; Zhang, S.; Yan, X.; Wang, Y.; Li, X. The core populations and co-occurrence patterns of prokaryotic communities in household biogas digesters. Biotechnol. Biofuels 2015, 8, 3–15. [Google Scholar] [CrossRef]
- Li, Y.; Liu, C.; Wachemo, A.C.; Li, X. Effects of liquid fraction of digestate recirculation on system performance and microbial community structure during serial anaerobic digestion of completely stirred tank reactors for corn stover. Energy 2018, 160, 309–317. [Google Scholar] [CrossRef]
- Gottardo, M.; Micolucci, F.; Bolzonella, D.; Uellendahl, H.; Pavan, P. Pilot scale fermentation coupled with anaerobic digestion of food waste—Effect of dynamic digestate recirculation. Renew. Energy 2017, 114, 455–463. [Google Scholar] [CrossRef]
- Wang, X.; Chen, G.; Zhou, S.; Li, J.; Zhao, X. Study on Semi-Continuous Anaerobic Fermentation of Straw Based on Total Reflux of Biogas Slurry. Chin. J. Anhui Norm. Univ. (Nat. Sci.) 2019, 42, 341–345. [Google Scholar]
- Sen, L. Study on the Effect of the Reflux of Biogas Slurry on the Anaerobic Fermentation Characteristics of Corn Straw. Master’s Thesis, Shenyang Agricultural University, Shenyang, China, 30 June 2018. [Google Scholar]
- APHA. Standard Methods for the Examination of Water and Wastewater, 22nd ed.; American Public Health Association: Washington, DC, USA, 2012. [Google Scholar]
- Zhong, M.; Duan, N.; Lin, C.; Zhang, D.; Liang, S.; Sun, H. Effects of Organic Loading Rate and Additive on Corn Stalk Anaerobic Digestion. J. Biobased Mater. Bioenergy 2016, 10, 219–224. [Google Scholar] [CrossRef]
- Yang, J.; Wang, D.; Luo, Z.; Zeng, W. Influence of reflux ratio on the anaerobic digestion of pig manure in leach beds coupled with continuous stirred tank reactors. Waste Manag. 2019, 97, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Duan, N.; Lin, C.; Su, S. Analysis of pH Control Measures of Straw Anaerobic Digestion system. J. Yunnan Norm. Univ. 2011, 9, 54–56. [Google Scholar]
- Gulhane, M.; Pandit, P.; Khardenavis, A.; Singh, D.; Purohit, H. Study of microbial community plasticity for anaerobic digestion of vegetable waste in Anaerobic Bafflfled Reactor. Renew. Energy 2017, 101, 59–66. [Google Scholar] [CrossRef]
- Zhu, G.F.; Li, J.Z.; Wu, P.; Jin, H.Z.; Wang, Z. The performance and phase separated characteristics of an anaerobic bafflfled reactor treating soybean protein processing wastewater. Bioresour. Technol. 2008, 99, 8027–8033. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Wen, H.; Huang, X.; Li, X.; Liu, X.; Li, D. Biogas production usingcornstalks and prokaryotic community composition. Chin. J. Chem. Eng. 2014, 65, 1784–1791. [Google Scholar]
- Jing, N.; Mingdian, Z.; Xiaofang, P.; Chunxing, L.; Nan, L.; Tao, W.; Guanjing, C.; Ruming, W.; Junjie, L.; Gefu, Z. Simultaneous biogas and biogas slurry production from co-digestion of pig manure and corn straw: Performance optimization and microbial community shift. Bioresour. Technol. 2019, 282, 37–47. [Google Scholar]
- Bassani, I.; Kougias, P.G.; Treu, L.; Porté, H.; Campanaro, S.; Angelidaki, I. Optimization of hydrogen dispersion in thermophilic up-flow reactors for ex situ biogas upgrading. Bioresour. Technol. 2017, 234, 310–319. [Google Scholar] [CrossRef]
- Girma, M.D.; Freya, M.; James, W.A.; Daniela, P.; Markus, G.; Frank, K.; Lund, N.J.; Anders, F. Exogenous addition of H2 for an in situ biogas upgrading through biological reduction of carbon dioxide into methane. Waste Manag. 2017, 68, 146–156. [Google Scholar]
- Bassani, I.; Kougias, P.G.; Treu, L.; Angelidaki, I. Biogas Upgrading via Hydrogenotrophic Methanogenesis in Two-Stage Continuous Stirred Tank Reactors at Mesophilic and Thermophilic Conditions. Environ. Sci. Ecotechnol. 2015, 49, 12585–12593. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Park, J.-G.; Shin, W.-B.; Tian, D.-J.; Jun, H.-B. Microbial communities change in an anaerobic digestion after application of microbial electrolysis cells. Bioresour. Technol. 2017, 234, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Han, W.; Yifeng, Z.; Irini, A. Ammonia inhibition on hydrogen enriched anaerobic digestion of manure under mesophilic and thermophilic conditions. Water Res. 2016, 105, 90–121. [Google Scholar]
- Zhang, Y.P.; Chen, G.Y.; Hei, K.L.; Yang, Y.F.; Xu, C.Y.; Chang, Z.Z. Effects of Full Continuous Reflux of Biogas Slurry on Characteristics of Rice Straw Anaerobic Digestion. J. Ecol. Rural Environ. 2017, 33, 845–851. [Google Scholar]
- Liu, T.; Sung, S. Ammonia inhibition on thermophilic aceticlastic methanogens. Water Sci. Technol. 2002, 45, 113–120. [Google Scholar] [CrossRef]
- Guo, X.; Wang, C.; Sun, F.; Zhu, W.; Wu, W. A comparison of microbial characteristics between the thermophilic and mesophilic anaerobic digesters exposed to elevated food waste loadings. Bioresour. Technol. 2014, 152, 420–428. [Google Scholar] [CrossRef]
- Ma, X.; Yu, M.; Song, N.; Xu, B.; Gao, M.; Wu, C.; Wang, Q. Effect of ethanol pre-fermentation on organic load rate and stability of semi-continuous anaerobic digestion of food waste. Bioresour. Technol. 2020, 299, 122587. [Google Scholar] [CrossRef]
- Hui, Z.; Ming, G.; Miao, Y.; Wenyu, Z.; Shuang, Z.; Chuanfu, W.; Yukihiro, T.; Qunhui, W. Methane production from food waste via mesophilic anaerobic digestion with ethanol pre-fermentation: Methanogenic pathway and microbial community analyses. Bioresour. Technol. 2020, 297, 122450. [Google Scholar]
- Tian, G.; Zhang, W.; Dong, M.; Yang, B.; Zhu, R.; Yin, F.; Zhao, X.; Wang, Y.; Xiao, W.; Wang, Q.; et al. Metabolic pathway analysis based on high-throughput sequencing in a batch biogas production process. Energy 2017, 139, 571–579. [Google Scholar] [CrossRef]
- Ros, M.; Filho, J.d.S.O.; Murcia, M.D.P.; Bustamante, M.A.; Moral, R.; Coll, M.D.; Santisima-Trinidad, A.B.L.; Pascual, J.A. Mesophilic anaerobic digestion of pig slurry and fruit and vegetable waste: Dissection of the microbial community structure. J. Clean. Prod. 2017, 156, 757–765. [Google Scholar] [CrossRef]

| Parameters | Corn Stalk | Inoculum |
|---|---|---|
| TS (%w/w) | 90.23 ± 0.07 | 17.46 ± 0.12 |
| VS (%w/w) | 84.62 ± 0.04 | 6.12 ± 0.08 |
| C (%w/w) | 42.75 ± 0.21 | 11.32 ± 0.14 |
| N (%w/w) | 0.75 ± 0.07 | 0.77 ± 0.09 |
| pH | - | 7.60 ± 0.1 |
| Parameters | Phase Ⅰ | Phase Ⅱ | Phase Ⅲ | |||
|---|---|---|---|---|---|---|
| R1 | R2 | R1 | R2 | R1 | R2 | |
| Time (d) | 11–37 | 38–64 | 65–91 | |||
| Organic load rate (gTS/(Lreactor·d)) | 2.0 | 2.0 | 3.0 | 3.0 | 4.0 | 4.0 |
| Average methane production rate (mL/gVS) | 202.35 | 260.25 | 154.80 | 209.95 | 118.70 | 202.36 |
| Average volumetric methane production rate (L/(Lreactor.d)) | 1.03 | 1.32 | 1.18 | 1.60 | 1.20 | 2.05 |
| Cumulative methane production (L) | 74.00 | 95.10 | 84.88 | 115.12 | 86.76 | 147.92 |
| Groups | R1-1 | R1-3 | R2-1 | R2-2 | R2-3 | R2-4 | |
|---|---|---|---|---|---|---|---|
| Bacterial | Seq num | 50,509 | 58,538 | 55,398 | 43,665 | 22,254 | 40,155 |
| OTUs | 1227 | 1133 | 1284 | 1105 | 1116 | 1354 | |
| Ace | 1494.53 | 1386.81 | 1468.03 | 1434.84 | 1992.30 | 1705.42 | |
| Shannon | 4.59 | 4.54 | 4.55 | 4.55 | 4.26 | 3.98 | |
| Chao1 | 1406.35 | 1332.23 | 1414.02 | 1357.67 | 1514.35 | 1543.50 | |
| Archaea | Seq num | 35,502 | 72,156 | 66,784 | 79,634 | 59,710 | 66,585 |
| OTUs | 141 | 219 | 150 | 159 | 108 | 212 | |
| Ace | 271.08 | 420.89 | 294.22 | 234.75 | 273.60 | 356.38 | |
| Shannon | 1.59 | 2.21 | 1.63 | 1.92 | 1.95 | 2.02 | |
| Chao1 | 220.50 | 304.00 | 211.22 | 209.32 | 174.00 | 277.54 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, L.; Gao, Y.; Sun, J.; Wang, Y.; Wang, C.; Yu, S.; Wang, Z.; Li, J.; Liu, R.; Kou, W. The Role of Slurry Reflux in a Corn Stalk Continuous Anaerobic Digestion System: Performance and Microbial Community. Int. J. Environ. Res. Public Health 2023, 20, 1687. https://doi.org/10.3390/ijerph20031687
Zhao L, Gao Y, Sun J, Wang Y, Wang C, Yu S, Wang Z, Li J, Liu R, Kou W. The Role of Slurry Reflux in a Corn Stalk Continuous Anaerobic Digestion System: Performance and Microbial Community. International Journal of Environmental Research and Public Health. 2023; 20(3):1687. https://doi.org/10.3390/ijerph20031687
Chicago/Turabian StyleZhao, Ling, Yang Gao, Jiaxing Sun, Yanan Wang, Congxin Wang, Shuai Yu, Zhen Wang, Jingyang Li, Ronghou Liu, and Wei Kou. 2023. "The Role of Slurry Reflux in a Corn Stalk Continuous Anaerobic Digestion System: Performance and Microbial Community" International Journal of Environmental Research and Public Health 20, no. 3: 1687. https://doi.org/10.3390/ijerph20031687
APA StyleZhao, L., Gao, Y., Sun, J., Wang, Y., Wang, C., Yu, S., Wang, Z., Li, J., Liu, R., & Kou, W. (2023). The Role of Slurry Reflux in a Corn Stalk Continuous Anaerobic Digestion System: Performance and Microbial Community. International Journal of Environmental Research and Public Health, 20(3), 1687. https://doi.org/10.3390/ijerph20031687





