Research on the Effects of Environmental Factors on the Emission of Volatile Organic Compounds from Plastic Track
Abstract
:1. Introduction
2. Materials, Instruments and Methods
2.1. Materials
2.2. Experiment Instruments and Reagents
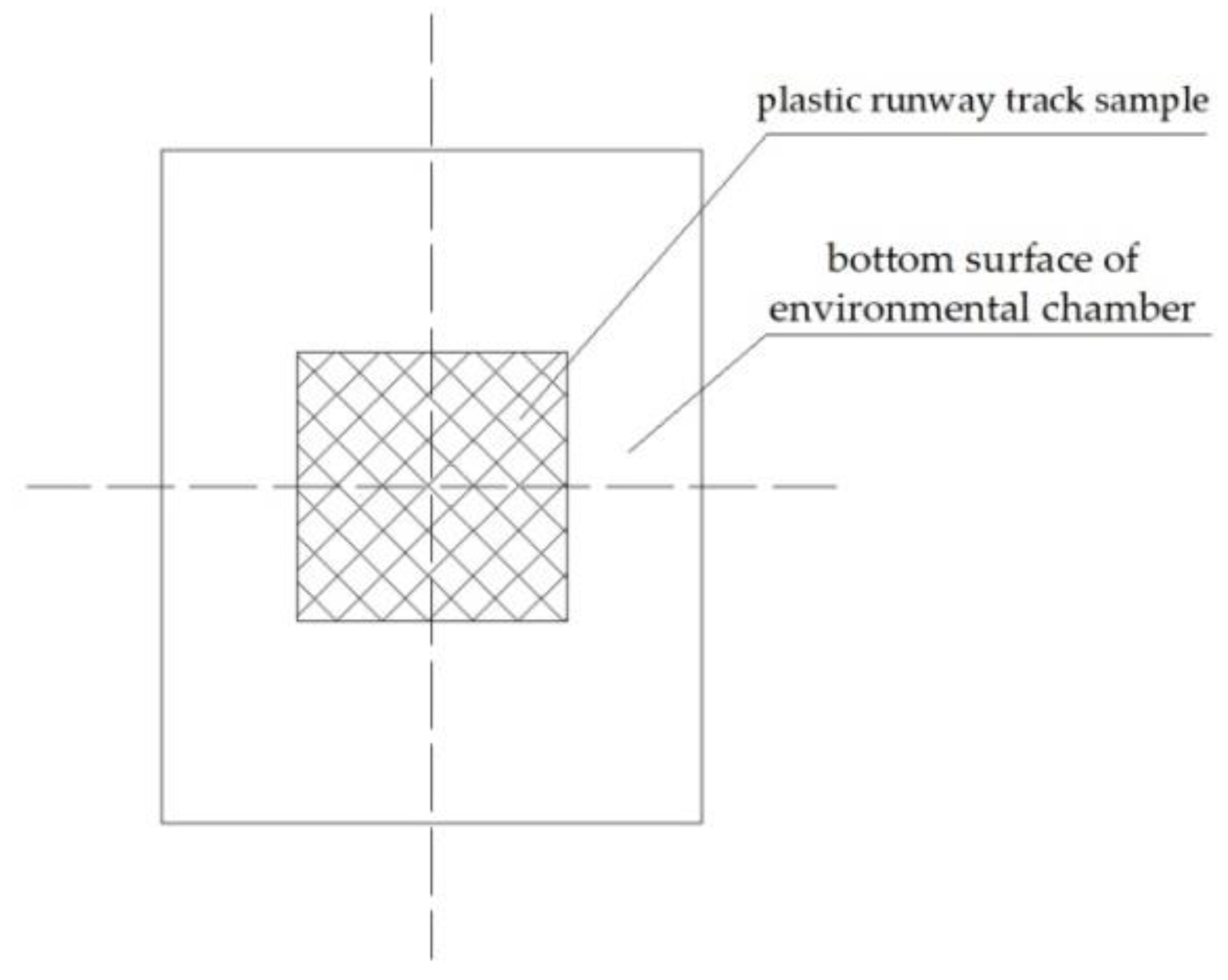
2.3. Methodology
2.4. Experimental Conditions for VOC Release Tests
3. Results
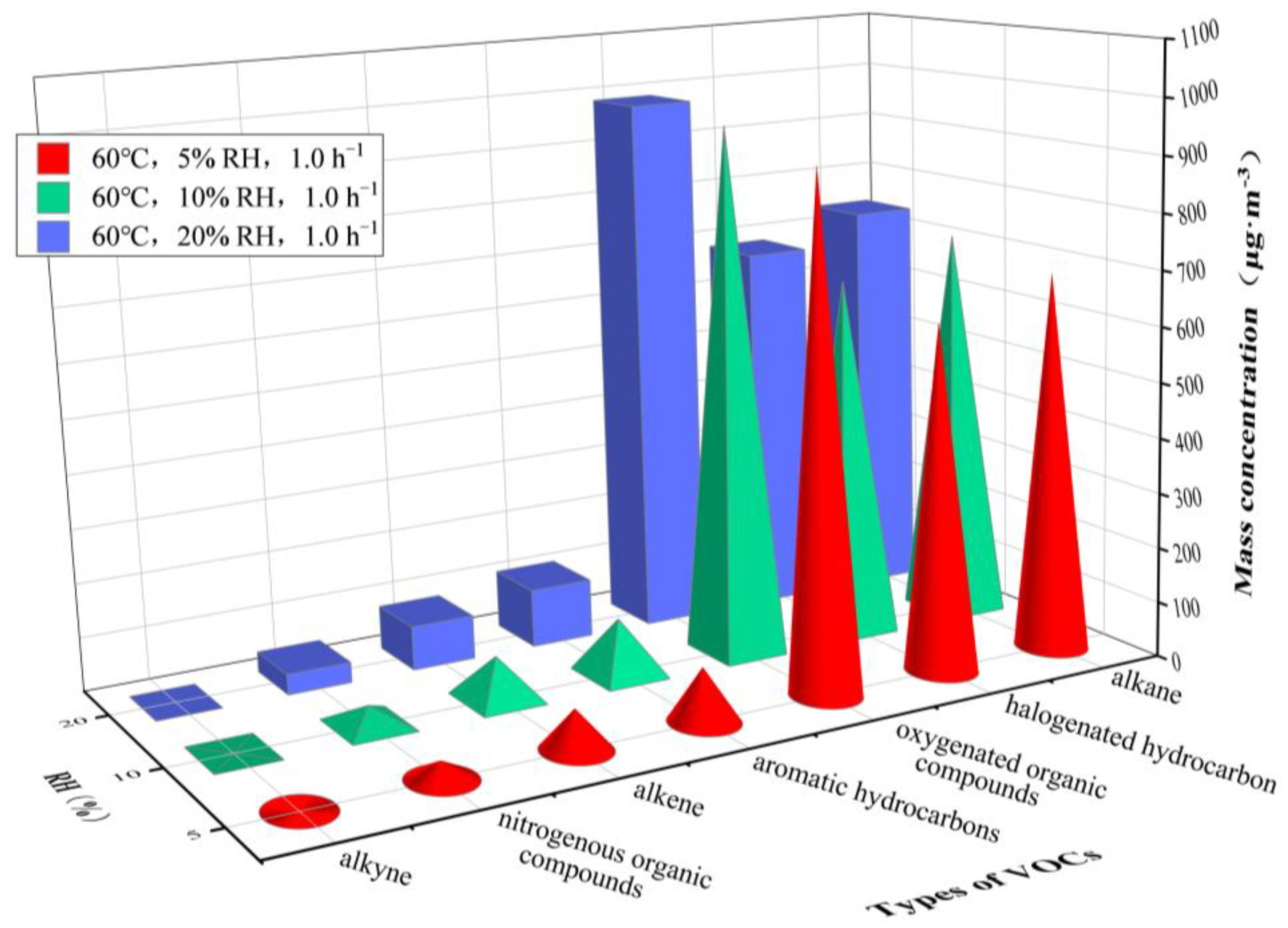
3.1. VOCs Release at Varying Temperature
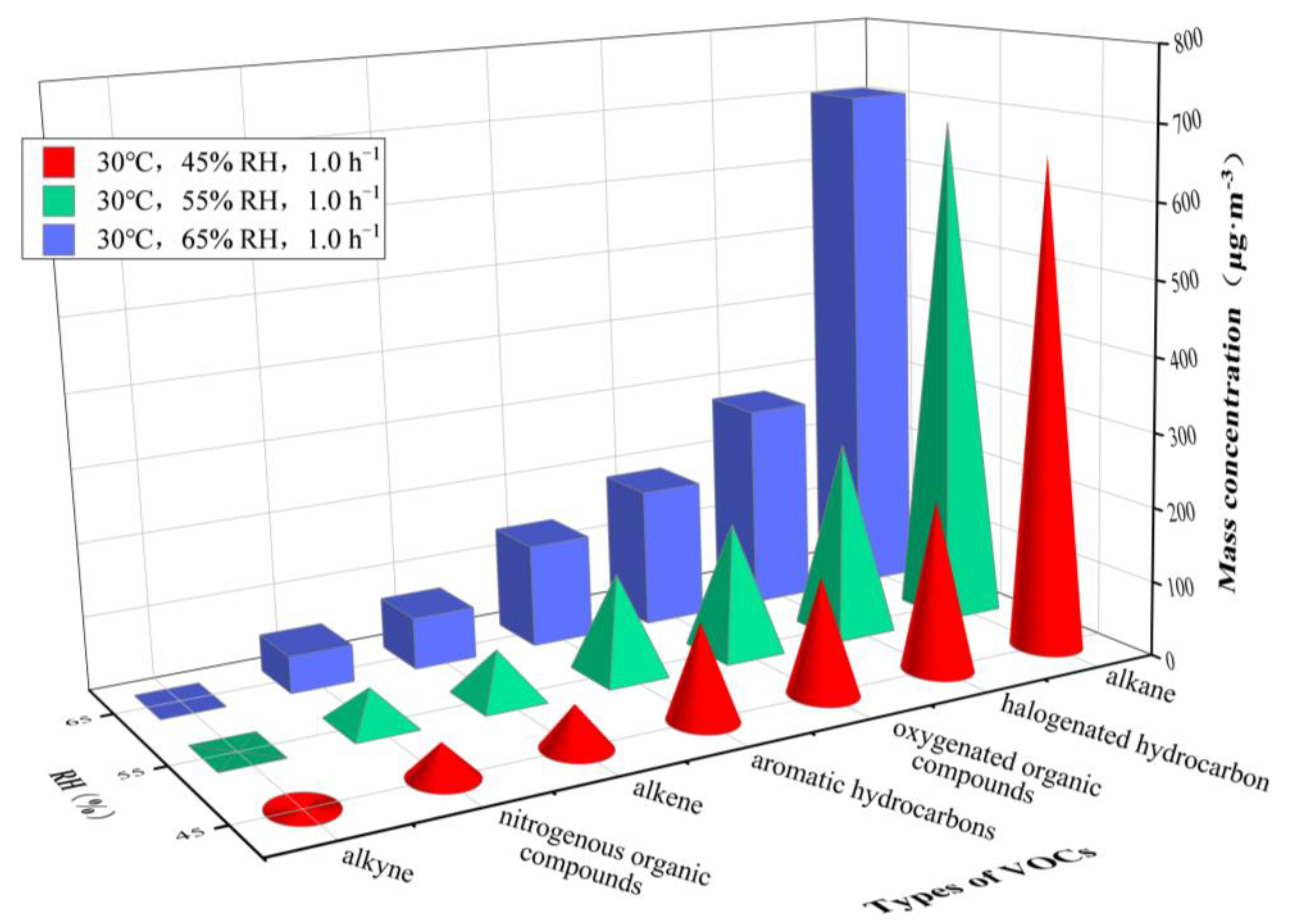
3.2. VOCs Release at Varying Relative Humidity
3.3. VOCs Release at Varying Air Exchange Rate
4. Discussion
4.1. Role of Temperature
4.2. Role of Relative Humidity
4.3. Role of Air Exchange Rate
5. Conclusions
- The increase of environmental temperature and relative humidity can promote the release of VOCs from plastic tracks, while the increase in air exchange rate can significantly reduce this effect.
- Environmental temperature, relative humidity and air exchange rate have no definite impact on the main classes and species of VOCs released from plastic tracks—the main classes include alkane, alkene, aromatic hydrocarbons, halogenated hydrocarbon, oxygenated organic compounds and nitrogenous organic compounds, and the main species include n-butane, 3-methylheptane, n-octane, n-decane, trans-2-pentene, toluene, ethylbenzene, styrene, dichloromethane, 1,1,2,2-tetrachloroethane, 1,2-dichloroethene, hexanal, acetone, acetonitrile. However, the increase of the environmental temperature can significantly change the percentage of the mass concentrations of different VOCs in total VOC; when the temperature was less than or equal to 30 ℃, Calkane > Chalogenated hydrocarbon > Coxygenated organic compounds > Caromatic hydrocarbons > Calkene > Cnitrogenous organic compounds > Calkyne, and when the temperature was higher than 35 °C, Coxygenated organic compounds significantly rose to its maximum value because Cacetone rapidly increased.
- Environmental temperature and relative humidity cannot significantly exert an impact on the detection rate of VOC species released from a plastic track, while air exchange rate is the main factor leading to the non-detection of VOCs species; when the air exchange rate was increased from 0.5 h−1 to 1.5 h−1, the number of non-detected VOC species increased from 1 to 7–12.
- Environmental temperature has the most significant effect on the emission of VOCs from plastic tracks, followed by air exchange rate and relative humidity.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chang, F.-H.; Lin, T.-C.; Huang, C.-I.; Chao, H.-R.; Chang, T.-Y.; Lu, C.-S. Emission characteristics of VOCs from athletic tracks. J. Hazard. Mater. 1999, 70, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, G.; Zheng, W.; Xie, Y. Research of National Standards and Experiment’s Verification of Harmful Substances in Polyurethane Plastic Runway. In Proceedings of the 2nd International Conference on Environmental Science and Engineering, Xiamen, China, 14–16 July 2017; pp. 14–16. [Google Scholar]
- Liu, G.; Zheng, W.; Wang, H. Qualitative and Quantitative Analysis of VOCs Released from Plastic Track by GC-MS Using a Small Environmental Chamber. Phys. Test. Chem. Anal. (Part B Chem. Anal.) 2018, 54, 249–254. [Google Scholar]
- Yang, R.; Xu, P.; Wang, W.; Xia, H. Determination of Emission Rate of Total Volatile Organic Compounds in Track Surface Materials by GC with Thermal Desorption Emission Test Chamber. Phys. Test. Chem. Anal. (Part B: Chem. Anal.) 2018, 54, 752–756. [Google Scholar]
- Cui, L.; He, Y.; Mei, L.; Cui, J. Determination of Volatile Organic Compounds in Polyurethane Plastic Track by Bag Sampling-Gas Chromatography/Mass Spectrometry. J. Anal. Sci. 2019, 35, 665–669. [Google Scholar]
- Jiang, B.; Wu, X.; Xiong, G.; Wu, Y. Research of VOC and odor of polyurethane foams. Polyurethane Ind. 2019, 34, 20–23. [Google Scholar]
- Kamarulzaman, N.H.; Le-Minh, N.; Fisher, R.M.; Stuetz, R.M. Quantification of VOCs and the development of odour wheels for rubber processing. Sci. Total Environ. 2019, 657, 154–168. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, W.; Song, X.; Hu, H.; Meng, Y. Determination of Release of 35 Volatile Organic Compounds (VOCs) in Plastic Track by Thermal Desorption-Gas Chromatography-Mass Spectrometry. Chem. Reag. 2020, 42, 542–545. [Google Scholar]
- Zhou, L.; Zhang, X.; Ma, J.; Fang, R.; Hu, W.; Li, S. Determination of 16 organic substances in non-solid raw material of plastic runway surface by GC-MS. China Adhes. 2020, 29, 54–58. [Google Scholar]
- Li, S.; Zhou, L.; Zhang, X.; Ma, J. Determination of Nine VOCs in Adhesives for Synthetic Surface Track by GC. Adhesion 2021, 45, 35–38. [Google Scholar]
- Kim, K.-H.; Jahan, S.A.; Kabir, E. A review of diseases associated with household air pollution due to the use of biomass fuels. J. Hazard. Mater. 2011, 192, 425–431. [Google Scholar] [CrossRef]
- Annesi-Maesano, I.; Hulin, M.; Lavaud, F.; Raherison, C.; Kopferschmitt, C.; de Blay, F.; Charpin, D.A.; Denis, C. Poor air quality in classrooms related to asthma and rhinitis in primary schoolchildren of the French 6 Cities Study. Thorax 2012, 67, 682–688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, C.; Song, H.; Xu, J.; Wang, M.; Hou, Z.; Wei, C.; Dong, Q.; Wang, S.; Qiao, S.; Zhu, B. Clinical characteristics of the patients with occupational chronic carbon disulfide poisoning in a chemical fiber factory of Nanjing. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi= Zhonghua Laodong Weisheng Zhiyebing Zazhi= Chin. J. Ind. Hyg. Occup. Dis. 2012, 30, 439–442. [Google Scholar]
- Wcisło, E.; Bronder, J.; Bubak, A.; Rodríguez-Valdés, E.; Gallego, J.L.R. Human health risk assessment in restoring safe and productive use of abandoned contaminated sites. Environ. Int. 2016, 94, 436–448. [Google Scholar] [CrossRef] [PubMed]
- van der Wal, J.F.; Moons, A.M.; Cornelissen, H.J. Indoor air quality in renovated Dutch homes. Indoor Air 1991, 1, 621–633. [Google Scholar]
- An, J.Y.; Kim, J.A.; Kim, K.W. The Effect of Temperature for VOCs, Formaldehyde Emission from Particleboards Using VOC Analyzer. In Proceedings of the 2007 Beijing International Bonding Technology Symposium & 2nd Asian Conference on Adhesion, Beijing, China, 31 October 2007. [Google Scholar]
- Deng, Q.; Yang, X.; Zhang, J. Study on a new correlation between diffusion coefficient and temperature in porous building materials. Atmos. Environ. 2009, 43, 2080–2083. [Google Scholar] [CrossRef]
- Wei, W.; Xiong, J.; Zhang, Y. Temperature Impact on the Emissions from VOC and Formaldehyde Reference Sources. In Proceedings of the 8th International Symposium on Heating, Ventilation and Air Conditioning, Xi’an, China, 19–21 October 2013; pp. 389–394. [Google Scholar]
- Wolkoff, P. Impact of air velocity, temperature, humidity, and air on long-term VOC emissions from building products. Atmos. Environ. 1998, 32, 2659–2668. [Google Scholar] [CrossRef]
- Lin, C.-C.; Yu, K.-P.; Zhao, P.; Lee, G.W.-M. Evaluation of impact factors on VOC emissions and concentrations from wooden flooring based on chamber tests. Build. Environ. 2009, 44, 525–533. [Google Scholar] [CrossRef]
- Li, H.; Geng, G.; Li, B.; Xiao, W. Experimental Research on the Emissions of Volatile Organic Compounds(VOCs) from Wet Building Materials. J. Shenyang Jianzhu Univ. (Nat. Sci.) 2012, 28, 697–701. [Google Scholar]
- Cao, L.; Shen, J.; Wang, J.; Shen, X. Effects of relative humidity on VOCs emission characteristics of particle board. J. Beijing For. Univ. 2013, 35, 149–153. [Google Scholar]
- Yu, Y.; Zhang, G.; Yu, D. External factors influencing contamination emissions from porous materials. Heat Vent. Air Cond 2006, 36, 13–19. [Google Scholar]
- Deng, Q.; Yang, X.; Zhang, J.S. Key factor analysis of VOC sorption and its impact on indoor concentrations: The role of ventilation. Build. Environ. 2012, 47, 182–187. [Google Scholar] [CrossRef]
- Rackes, A.; Waring, M. Do time-averaged, whole-building, effective volatile organic compound (VOC) emissions depend on the air exchange rate? A statistical analysis of trends for 46 VOC s in US offices. Indoor Air 2016, 26, 642–659. [Google Scholar] [CrossRef] [PubMed]
- Manoukian, A.; Buiron, D.; Temime-Roussel, B.; Wortham, H.; Quivet, E. Measurements of VOC/SVOC emission factors from burning incenses in an environmental test chamber: Influence of temperature, relative humidity, and air exchange rate. Environ. Sci. Pollut. Res. 2016, 23, 6300–6311. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Education of the People’s Republic of China. Sports Areas with Synthetic Surfaces for Primary and Middle Schools: GB 36246-2018; Standards Press of China: Beijing, China, 2018.
- Wei, F. Guide to Analytical Methods for Air and Exhaust Monitoring; China Environmental Science Press: Beijing, China, 2006; Volume 1, p. 642. [Google Scholar]
- Twomey, D.; Petrass, L.; Harvey, J.; Otago, L.; LeRossignol, P. Heat experienced on synthetic turf surfaces: An inevitable or preventable risk? J. Sci. Med. Sport 2014, 18, e119–e120. [Google Scholar] [CrossRef]
- Gustin, M.; Fleming, P.R.; Allinson, D.; Watson, S. Modelling surface temperatures on 3G artificial turf. Proceedings 2018, 2, 279. [Google Scholar]
- Verheyen, T.V.; Reynolds, A.J. Yallourn brown coal–The effect of drying conditions on its VOC emissions. Fuel Process Technol. 2017, 155, 88–96. [Google Scholar] [CrossRef]
- Yang, X.; Chen, Q.; Zhang, J.; Magee, R.; Zeng, J.; Shaw, C. Numerical simulation of VOC emissions from dry materials. Build. Environ. 2001, 36, 1099–1107. [Google Scholar] [CrossRef]
- Zhang, Y.; Luo, X.; Wang, X.; Qian, K.; Zhao, R. Influence of temperature on formaldehyde emission parameters of dry building materials. Atmos. Environ. 2007, 41, 3203–3216. [Google Scholar] [CrossRef]
- Yang, X.; Chen, Q.; Bluyssen, P.M. Prediction of short-term and long-term volatile organic compound emissions from SBR bitumen-backed carpet under different temperatures. ASHRAE Trans. 1998, 104, 1297–1308. [Google Scholar]
- Huang, L.; Liang, W.; Qin, M. Effect of Temperature on Formaldehyde and Volatile Organic Compound Emissions. Build. Sci. 2018, 34, 71–76+88. [Google Scholar]
- Jo, W.-J.; Sohn, J.-Y. The effect of environmental and structural factors on indoor air quality of apartments in Korea. Build. Environ. 2009, 44, 1794–1802. [Google Scholar] [CrossRef]
- Yaws, C.L.; Yang, H.C. Vapor pressure data for 700 major organic compounds. Chem. Eng. Des. 1990, 10, 42–59. [Google Scholar]
- Snyder, R.E. Benzene’s toxicity: A consolidated short review of human and animal studies by ha khan. Hum. Exp. Toxicol. 2007, 26, 687–696. [Google Scholar] [CrossRef]
- Axelsson, G.; Barregard, L.; Holmberg, E.; Sallsten, G. Cancer incidence in a petrochemical industry area in Sweden. Sci. Total Environ. 2010, 408, 4482–4487. [Google Scholar] [CrossRef]
- Tong, R.P.; Yang, Y.Y.; Shao, G.H.; Zhang, Y.W.; Dou, S.; Jiang, W. Emission sources and probabilistic health risk of volatile organic compounds emitted from production areas in a petrochemical refinery in Hainan, China. Hum. Ecol. Risk Assess. Int. J. 2020, 26, 1407–1427. [Google Scholar] [CrossRef]
- DE-Miranda, B.R.; Castro, S.L.; Rocha, E.M.; Bodle, C.R.; Greenamyre, J.T. The industrial solvent trichloroethylene induces LRRK2 kinase activity and dopaminergic neurodegeneration in a rat model of Parkinson’s disease. Neurobiol. Dis. 2021, 153, 105312. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Xu, K.; Zhao, Y.S.; Liang, T.R.; Song, S.Y. Research on damage consequences of dangerous chemical tanker leakage accidents. Saf. Environ. Eng. 2019, 26, 128–136. [Google Scholar]
- Yang, M.F. Hazard and detection method of acetone in the air of workplace. Guangdong Chem. Ind. 2022, 49, 196–198. [Google Scholar]
- Zhu, H.; Que, Z.; Lu, Z. Emission study of volatile, organic compounds released from bamboo flooring. China Wood Ind 2013, 27, 13–17. [Google Scholar]
- Parthasarathy, S.; Maddalena, R.L.; Russell, M.L.; Apte, M.G. Effect of temperature and humidity on formaldehyde emissions in temporary housing units. J. Air Waste Manag. Assoc. 2011, 61, 689–695. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.; Zhao, Y.; Zhu, M.; Yu, M.; Wang, Q.; Shen, J. Releasing Rule and Characteristics of VOC Emissions from Plywood by the Rapid Detection. J. Northeast For. Univ. 2015, 43, 120–123. [Google Scholar]
- Yang, X.; Chen, Q.; Zeng, J.; Zhang, J.; Shaw, C. Effects of environmental and test conditions on VOC emissions from”wet” coating materials. Indoor Air 2001, 11, 270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, Y.-M.; Hu, W.-H.; Fang, W.-B.; Chen, S.-S.; Chang, C.-T.; Ching, H.-W. A study on dynamic volatile organic compound emission characterization of water-based paints. J. Air Waste Manag. Assoc. 2011, 61, 35–45. [Google Scholar] [CrossRef]
- Xiong, J.; Wang, L.; Bai, Y.; Zhang, Y. Measuring the characteristic parameters of VOC emission from paints. Build. Environ. 2013, 66, 65–71. [Google Scholar] [CrossRef]
- Gan, L.; Weitao, Z.; Hong, W. Influence of Ambient Temperature on TVOC Released from Polyurethane Athletics Track. In Proceedings of the 13th Conference of the International Sports Engineering Association, Online, 22–26 June 2020; p. 25. [Google Scholar]
- Héroux, M.-È.; Gauvin, D.; Gilbert, N.L.; Guay, M.; Dupuis, G.; Legris, M.; Lévesque, B. Housing characteristics and indoor concentrations of selected volatile organic compounds (VOCs) in Quebec City, Canada. Indoor Built Environ. 2008, 17, 128–137. [Google Scholar] [CrossRef]
- Liu, Z. Computational fluid dynamics simulation of formaldehyde emission characteristics and its experimental validation in environment chamber. J. Chongqing Univ. (Engl. Ed.) 2010, 9, 124–132. [Google Scholar]
- Yang, Z.; Shen, J.; Cui, X. Investigation of VOC Released from Three-Layer Parquet by Using A New Rapid Detection Method. Sci. Silvae Sin. 2015, 51, 99–104. [Google Scholar]



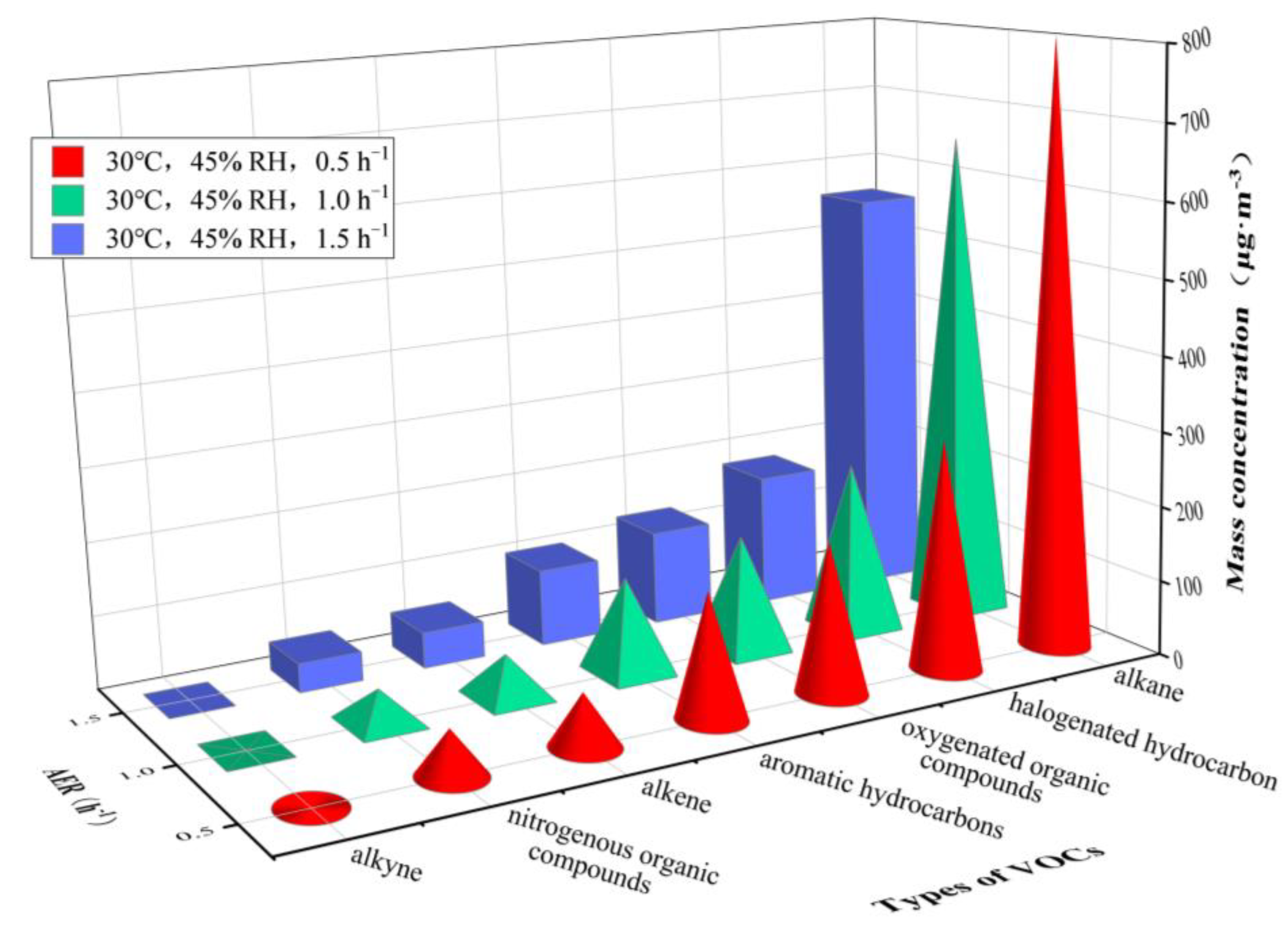
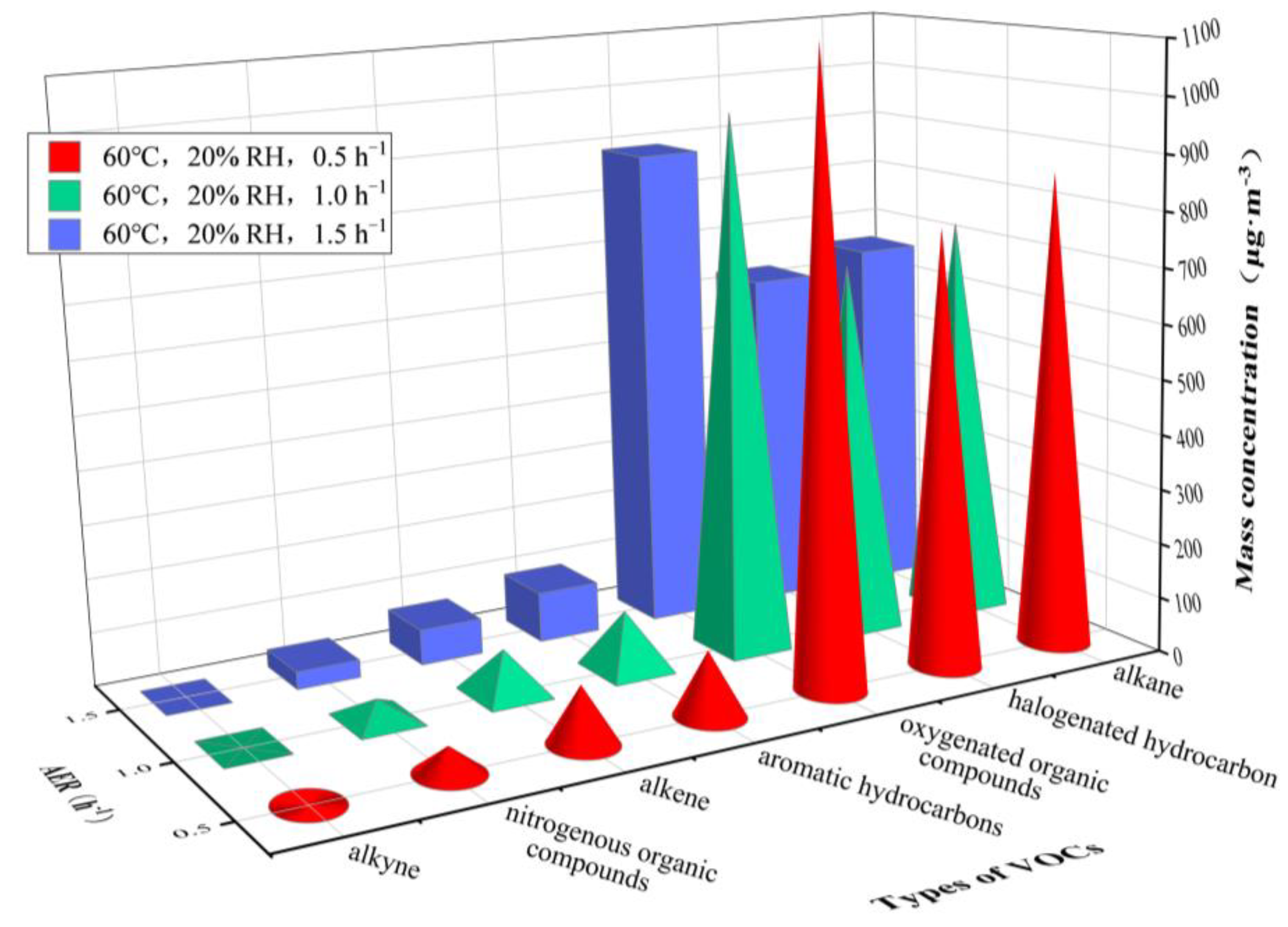
| Test Group | Temperature/°C | RH/% | AER/h−1 |
|---|---|---|---|
| A | 23/30/35/40 | 45 | 1.0 |
| B | 45/50/55/60 | 20 | 1.0 |
| C | 30 | 45/55/65 | 1.0 |
| D | 60 | 5/10/20 | 1.0 |
| E | 30 | 45 | 0.5/1.0/1.5 |
| F | 60 | 20 | 0.5/1.0/1.5 |
| Types of VOCs | Main VOCs Species | Mass Concentrations/μg·m−3 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 45% RH, AER = 1.0 h−1 | 20% RH, AER = 1.0 h−1 | ||||||||
| 23 °C | 30 °C | 35 °C | 40 °C | 45 °C | 50 °C | 55 °C | 60 °C | ||
| alkane | n-butane | 40.71 | 45.94 | 48.84 | 50.15 | 59.37 | 54.70 | 55.21 | 56.57 |
| 3-methylheptane | 106.40 | 139.02 | 141.35 | 146.00 | 155.81 | 168.49 | 180.70 | 198.62 | |
| n-octane | 159.17 | 209.06 | 212.90 | 226.40 | 227.74 | 237.37 | 245.69 | 255.90 | |
| n-decane | 138.80 | 197.84 | 179.36 | 165.96 | 164.72 | 157.37 | 159.49 | 161.48 | |
| alkene | trans-2-pentene | 28.24 | 47.06 | 50.69 | 52.96 | 54.59 | 61.42 | 65.56 | 70.02 |
| aromatic hydrocarbons | toluene | 17.89 | 22.82 | 21.28 | 20.71 | 22.04 | 18.90 | 16.61 | 14.81 |
| ethylbenzene | 9.84 | 12.84 | 10.74 | 9.45 | 10.15 | 10.85 | 11.12 | 12.50 | |
| styrene | 49.19 | 60.65 | 60.56 | 59.78 | 61.84 | 58.44 | 55.59 | 54.34 | |
| halogenated hydrocarbon | dichloromethane | 10.32 | 14.97 | 14.41 | 13.47 | 13.13 | 12.96 | 11.72 | 9.89 |
| 1,1,2,2-tetrachloroethane | 65.16 | 76.65 | 82.42 | 76.87 | 75.85 | 73.72 | 69.15 | 64.35 | |
| 1,2-dichloroethene | 64.76 | 82.69 | 136.17 | 216.19 | 225.29 | 260.75 | 365.46 | 569.33 | |
| oxygenated organic compounds | hexanal | 72.41 | 93.44 | 90.18 | 87.85 | 84.69 | 79.90 | 76.15 | 73.54 |
| acetone | 30.27 | 35.58 | 492.20 | 615.53 | 677.18 | 744.46 | 819.88 | 872.14 | |
| nitrogenous organic compounds | acetonitrile | 34.97 | 45.02 | 40.98 | 31.34 | 31.30 | 29.90 | 28.58 | 37.91 |
| Types of VOCs | Main VOC Species | Mass Concentrations/μg·m−3 | |||||
|---|---|---|---|---|---|---|---|
| 30 °C, AER = 1.0 h−1 | 60 °C, AER = 1.0 h−1 | ||||||
| 45% | 55% | 65% | 5% | 10% | 20% | ||
| alkane | n-butane | 45.94 | 54.92 | 62.45 | 40.37 | 49.83 | 56.57 |
| 3-methylheptane | 139.02 | 153.97 | 169.30 | 185.52 | 192.09 | 198.62 | |
| n-octane | 209.06 | 214.91 | 220.92 | 235.93 | 248.17 | 255.90 | |
| n-decane | 197.84 | 189.79 | 183.92 | 169.26 | 165.18 | 161.48 | |
| alkene | trans-2-pentene | 47.06 | 51.40 | 55.17 | 63.14 | 67.35 | 70.02 |
| aromatic hydrocarbons | toluene | 22.82 | 25.55 | 27.71 | 13.38 | 14.29 | 14.81 |
| ethylbenzene | 12.84 | 12.20 | 11.88 | 11.86 | 12.28 | 12.50 | |
| styrene | 60.65 | 64.47 | 66.17 | 49.79 | 51.48 | 54.34 | |
| halogenated hydrocarbon | dichloromethane | 14.97 | 16.13 | 17.76 | 8.56 | 9.40 | 9.89 |
| 1,1,2,2-tetrachloroethane | 76.65 | 81.91 | 83.60 | 59.73 | 62.90 | 64.35 | |
| 1,2-dichloroethene | 82.69 | 102.02 | 114.52 | 528.26 | 551.09 | 569.33 | |
| oxygenated organic compounds | hexanal | 93.44 | 98.06 | 103.32 | 70.53 | 72.18 | 73.54 |
| acetone | 35.58 | 37.21 | 40.59 | 823.40 | 855.35 | 872.14 | |
| nitrogenous organic compounds | acetonitrile | 45.02 | 47.94 | 50.13 | 33.28 | 35.96 | 37.91 |
| Types of VOCs | Main VOC Species | Mass Concentrations/μg·m−3 | |||||
|---|---|---|---|---|---|---|---|
| 30 °C, 45% RH | 60 °C, 20% RH | ||||||
| 0.5 h−1 | 1.0 h−1 | 1.5 h−1 | 0.5 h−1 | 1.0 h−1 | 1.5 h−1 | ||
| alkane | n-butane | 58.85 | 45.94 | 35.22 | 65.10 | 56.57 | 51.85 |
| 3-methylheptane | 173.96 | 139.02 | 107.49 | 239.14 | 198.62 | 176.66 | |
| n-octane | 251.20 | 209.06 | 187.14 | 293.16 | 255.90 | 235.70 | |
| n-decane | 226.15 | 197.84 | 181.31 | 181.01 | 161.48 | 147.42 | |
| alkene | trans-2-pentene | 56.17 | 47.06 | 42.22 | 89.13 | 70.02 | 55.46 |
| aromatic hydrocarbons | toluene | 29.02 | 22.82 | 18.09 | 16.13 | 14.81 | 12.23 |
| ethylbenzene | 16.31 | 12.84 | 10.05 | 14.01 | 12.50 | 11.40 | |
| styrene | 69.27 | 60.65 | 55.96 | 59.28 | 54.34 | 47.56 | |
| halogenated hydrocarbon | dichloromethane | 19.78 | 14.97 | 10.82 | 12.27 | 9.89 | 9.45 |
| 1,1,2,2-tetrachloroethane | 101.77 | 76.65 | 64.19 | 72.44 | 64.35 | 61.78 | |
| 1,2-dichloroethene | 90.98 | 82.69 | 73.89 | 656.14 | 569.33 | 526.86 | |
| oxygenated organic compounds | hexanal | 113.86 | 93.44 | 82.75 | 79.73 | 73.54 | 70.14 |
| acetone | 46.76 | 35.58 | 28.31 | 995.69 | 872.14 | 789.03 | |
| nitrogenous organic compounds | acetonitrile | 60.83 | 45.02 | 39.21 | 48.48 | 37.91 | 32.68 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, G.; Zheng, W.; Wang, H.; Liu, L.; Meng, Y.; Huang, Y.; Ma, Y. Research on the Effects of Environmental Factors on the Emission of Volatile Organic Compounds from Plastic Track. Int. J. Environ. Res. Public Health 2023, 20, 1828. https://doi.org/10.3390/ijerph20031828
Liu G, Zheng W, Wang H, Liu L, Meng Y, Huang Y, Ma Y. Research on the Effects of Environmental Factors on the Emission of Volatile Organic Compounds from Plastic Track. International Journal of Environmental Research and Public Health. 2023; 20(3):1828. https://doi.org/10.3390/ijerph20031828
Chicago/Turabian StyleLiu, Gan, Weitao Zheng, Hong Wang, Lin Liu, Yanrong Meng, Yu Huang, and Yong Ma. 2023. "Research on the Effects of Environmental Factors on the Emission of Volatile Organic Compounds from Plastic Track" International Journal of Environmental Research and Public Health 20, no. 3: 1828. https://doi.org/10.3390/ijerph20031828





