Benzo[A]Pyrene Biodegradation by Multiple and Individual Mesophilic Bacteria under Axenic Conditions and in Soil Samples
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microbial Isolation
2.2. Bacterial Enumeration
2.3. Scanning Electron Microscopy (SEM)
2.4. Species Identification
2.5. Assessment of Bacterial Degradation of BaP and Other PAHs and the Effects of pH and Salinity
2.6. BaP Quantification and Substrate Utilization
2.7. Quantification of BaP Utilization in Sandy Soil Samples
2.8. Identification of Metabolites by HPLC-MS/MS
2.9. Statistical Analyses
3. Results and Discussion
3.1. Bacterial Isolation
3.2. Species Identification
3.3. Effects of pH, Temperature, and Salinity on Bacterial Growth
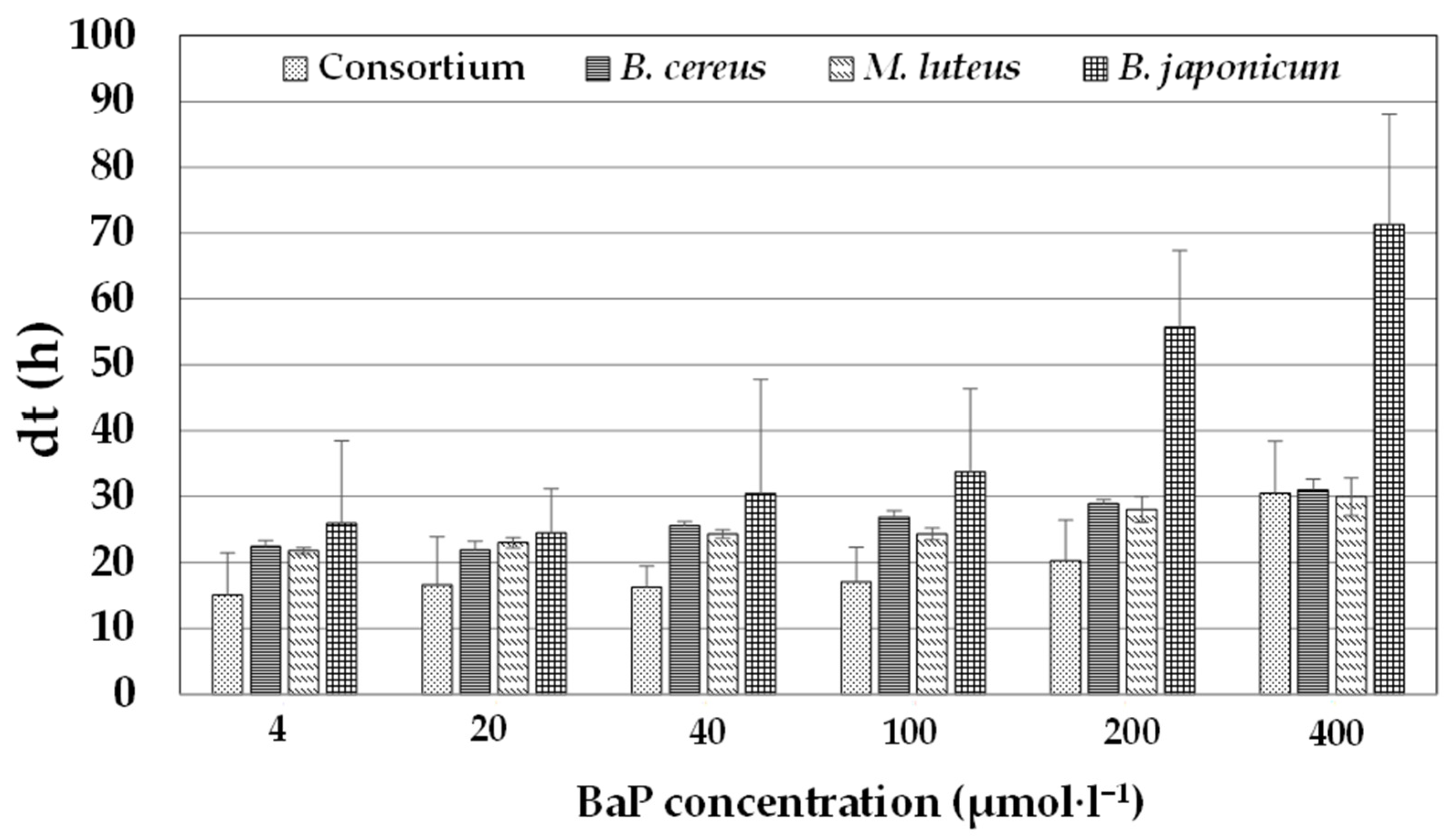
3.4. Growth of the Bacterial Consortium in Comparison with the Individual Strains in the Presence of Increasing BaP Concentrations
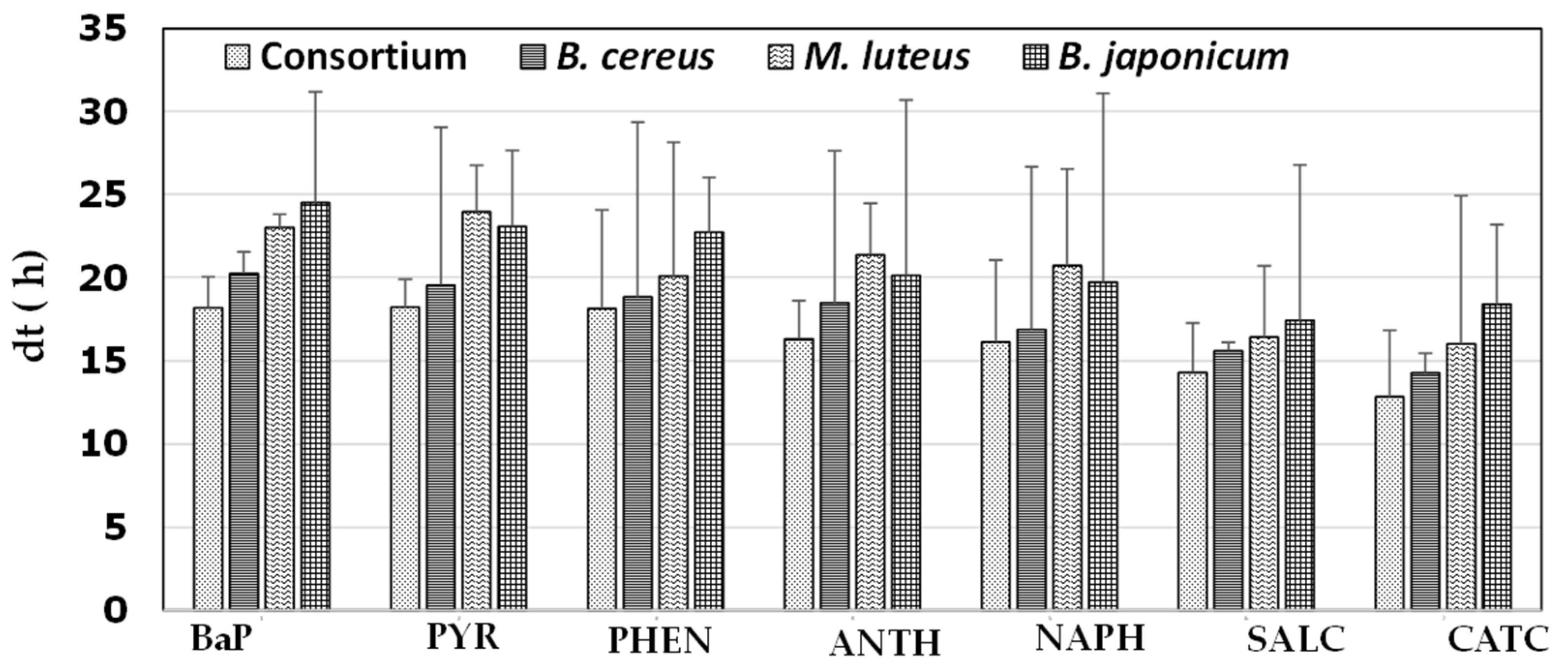
3.5. Growth in the Presence of Various Aromatics
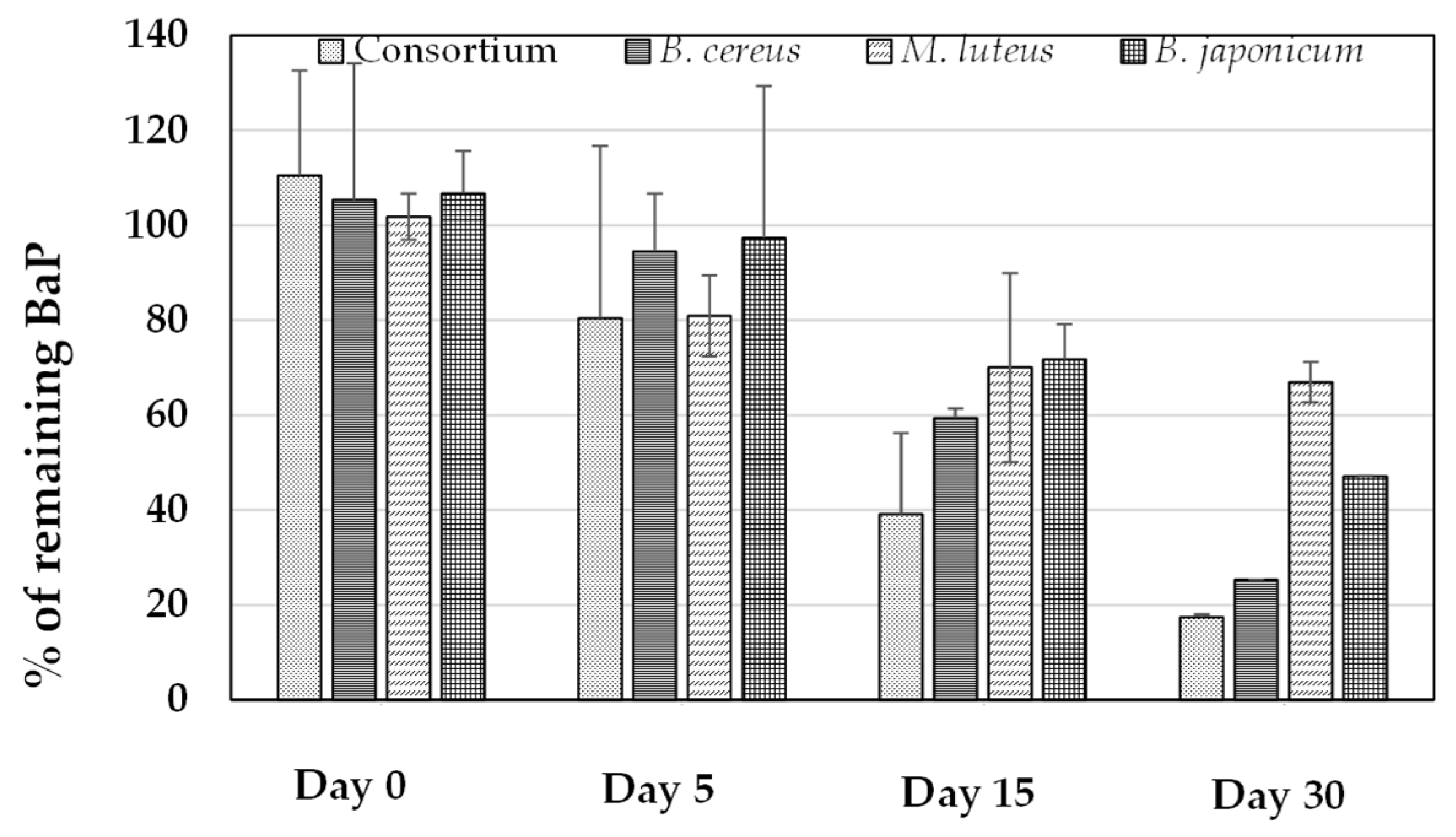
3.6. Quantification of BaP Utilization in BH Medium and Sandy Soil Samples
3.7. Metabolite Identification
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lawal, A.T. Polycyclic Aromatic Hydrocarbons. A Review. Cogent Environ. Sci. 2017, 3, 1339841. [Google Scholar] [CrossRef]
- de Lima, R.F.; Dionello, R.G.; Peralba MD, C.R.; Barrionuevo, S.; Radunz, L.L.; Júnior FW, R. PAHs in Corn Grains Submitted to Drying with Firewood. Food Chem. 2017, 215, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Fatma Aygün, S.; Kabadayi, F. Determination of Benzo[a]Pyrene in Charcoal Grilled Meat Samples by HPLC with Fluorescence Detection. Int. J. Food Sci. Nutr. 2005, 56, 581–585. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, A.; Harris, K.J.; Archibong, A.E. Chapter 40—Reproductive Toxicity of Polycyclic Aromatic Hydrocarbons. In Reproductive and Developmental Toxicology, 2nd ed.; Gupta, R.C., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 745–763. ISBN 978-0-12-804239-7. [Google Scholar]
- Gray, J.P. Benzo(a)Pyrene. In Encyclopedia of Toxicology, 3rd ed.; Wexler, P., Ed.; Academic Press: Cambridge, MA, USA, 2014; pp. 423–428. ISBN 978-0-12-386455-0. [Google Scholar]
- Jesus, F.; Pereira, J.L.; Campos, I.; Santos, M.; Ré, A.; Keizer, J.; Nogueira, A.; Gonçalves, F.J.M.; Abrantes, N.; Serpa, D. A Review on Polycyclic Aromatic Hydrocarbons Distribution in Freshwater Ecosystems and Their Toxicity to Benthic Fauna. Sci. Total Environ. 2022, 820, 153282. [Google Scholar] [CrossRef]
- Ostrem Loss, E.M.; Yu, J.H. Bioremediation and Microbial Metabolism of Benzo(a)Pyrene. Mol. Microbiol. 2018, 109, 433–444. [Google Scholar] [CrossRef] [Green Version]
- Nzila, A. Biodegradation of High-Molecular-Weight Polycyclic Aromatic Hydrocarbons under Anaerobic Conditions: Overview of Studies, Proposed Pathways and Future Perspectives. Environ. Pollut. 2018, 239, 788–802. [Google Scholar] [CrossRef]
- Nzila, A. Current Status of the Degradation of Aliphatic and Aromatic Petroleum Hydrocarbons by Thermophilic Microbes and Future Perspectives. Int. J. Environ. Res. Public Health 2018, 15, 2782. [Google Scholar] [CrossRef] [Green Version]
- Ghosal, D.; Ghosh, S.; Dutta, T.K.; Ahn, Y. Current State of Knowledge in Microbial Degradation of Polycyclic Aromatic Hydrocarbons (PAHs): A Review. Front. Microbiol. 2016, 7, 1369. [Google Scholar] [CrossRef] [Green Version]
- Nzila, A.; Musa, M.M. Current Status of and Future Perspectives in Bacterial Degradation of Benzo[a]Pyrene. Int. J. Environ. Res. Public Health 2020, 18, 262. [Google Scholar] [CrossRef]
- Nzila, A.; Sankara, S.; Al-Momani, M.; Musa, M.M. Isolation and Characterisation of Bacteria Degrading Polycyclic Aromatic Hydrocarbons: Phenanthrene and Anthracene. Arch. Environ. Prot. 2017, 44, 43–54. [Google Scholar] [CrossRef]
- Weisburg, W.G.; Barns, S.M.; Pelletier, D.A.; Lane, D.J. 16S Ribosomal DNA Amplification for Phylogenetic Study. J. Bacteriol. 1991, 173, 697–703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoon, S.H.; Ha, S.-M.; Kwon, S.; Lim, J.; Kim, Y.; Seo, H.; Chun, J. Introducing EzBioCloud: A Taxonomically United Database of 16S RRNA Gene Sequences and Whole-Genome Assemblies. Int. J. Syst. Evol. Microbiol. 2017, 67, 1613–1617. [Google Scholar] [CrossRef] [PubMed]
- Budiyanto, F.; Thukair, A.; Al-Momani, M.; Musa, M.M.; Nzila, A. Characterization of Halophilic Bacteria Capable of Efficiently Biodegrading the High-Molecular-Weight Polycyclic Aromatic Hydrocarbon Pyrene. Environ. Eng. Sci. 2018, 35, 616–626. [Google Scholar] [CrossRef] [Green Version]
- Anonymous Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: http://www.r-project.org/index.html (accessed on 15 July 2022).
- Jaiswal, S.K.; Dakora, F.D. Widespread Distribution of Highly Adapted Bradyrhizobium Species Nodulating Diverse Legumes in Africa. Front. Microbiol. 2019, 10, 310. [Google Scholar] [CrossRef] [Green Version]
- Qu, Y.; Spain, J.C. Biodegradation of 5-Nitroanthranilic Acid by Bradyrhizobium Sp. Strain JS329. Appl. Environ. Microbiol. 2010, 76, 1417–1422. [Google Scholar] [CrossRef] [Green Version]
- Sudtachat, N.; Ito, N.; Itakura, M.; Masuda, S.; Eda, S.; Mitsui, H.; Kawaharada, Y.; Minamisawa, K. Aerobic Vanillate Degradation and C1 Compound Metabolism in Bradyrhizobium Japonicum. Appl. Environ. Microbiol. 2009, 75, 5012–5017. [Google Scholar] [CrossRef] [Green Version]
- Ito, N.; Itakura, M.; Eda, S.; Saeki, K.; Oomori, H.; Yokoyama, T.; Kaneko, T.; Tabata, S.; Ohwada, T.; Tajima, S.; et al. Global Gene Expression in Bradyrhizobium Japonicum Cultured with Vanillin, Vanillate, 4-Hydroxybenzoate and Protocatechuate. Microbes Environ. 2006, 21, 240–250. [Google Scholar] [CrossRef] [Green Version]
- Olowomofe, T.O.; Oluyege, J.O.; Aderiye, B.I.; Oluwole, O.A. Degradation of Poly Aromatic Fractions of Crude Oil and Detection of Catabolic Genes in Hydrocarbon-Degrading Bacteria Isolated from Agbabu Bitumen Sediments in Ondo State. AIMS Microbiol. 2019, 5, 308–323. [Google Scholar] [CrossRef]
- Doddamani, H.P.; Ninnekar, H.Z. Biodegradation of Carbaryl by a Micrococcus Species. Curr. Microbiol. 2001, 43, 69–73. [Google Scholar] [CrossRef]
- Bevinakatti, B.G.; Ninnekar, H.Z. Biodegradation of 4-Chlorobiphenyl by Micrococcus Species. World J. Microbiol. Biotechnol. 1993, 9, 607–608. [Google Scholar] [CrossRef]
- Bevinakatti, B.G.; Ninnekar, H.Z. Degradation of Biphenyl by a Micrococcus Species. Appl. Microbiol. Biotechnol. 1992, 38, 273–275. [Google Scholar] [CrossRef]
- Al-Awadhi, H.; Sulaiman, R.H.D.; Mahmoud, H.M.; Radwan, S.S. Alkaliphilic and Halophilic Hydrocarbon-Utilizing Bacteria from Kuwaiti Coasts of the Arabian Gulf. Appl. Microbiol. Biotechnol. 2007, 77, 183–186. [Google Scholar] [CrossRef] [PubMed]
- Haritash, A.K.; Kaushik, C. Degradation of Low Molecular Weight Polycyclic Aromatic Hydrocarbons by Microorganisms Isolated from Contaminated Soil. Int. J. Environ. Sci. 2016, 6, 472–782. [Google Scholar] [CrossRef]
- Mohandass, R.; Rout, P.; Jiwal, S.; Sasikala, C. Biodegradation of Benzo[a]Pyrene by the Mixed Culture of Bacillus Cereus and Bacillus Vireti Isolated from the Petrochemical Industry. J. Environ. Biol. 2012, 33, 985–989. [Google Scholar] [PubMed]
- Zhao, Z.; Wong, J.W.C. Rapid Biodegradation of Benzo[a]Pyrene by Bacillus Subtilis BUM Under Thermophilic Condition. Environ. Eng. Sci. 2010, 27, 939–945. [Google Scholar] [CrossRef]
- Feitkenhauer, H.; Müller, R.; Märkl, H. Degradation of Polycyclic Aromatic Hydrocarbons and Long Chain Alkanes at 60–70 Degrees by Thermus and Bacillus spp. Biodegradation 2003, 14, 367–372. [Google Scholar] [CrossRef]
- Guevara-Luna, J.; Alvarez-Fitz, P.; Rios-Leal, E.; Acevedo-Quiroz, M.; Encarnacion-Guevara, S.; Moreno-Godinez, M.E.; Castellanos-Escamilla, M.; Toribio-Jimenez, J.; Romero-Ramirez, Y. Biotransformation of Benzo[a]Pyrene by the Thermophilic Bacterium Bacillus Licheniformis M2-7. World J. Microbiol. Biotechnol. 2018, 34, 88. [Google Scholar] [CrossRef]
- Seo, J.; Keum, Y.; Li, Q.X. Bacterial Degradation of Aromatic Compounds. Int. J. Environ. Res. Public Health 2009, 6, 278–309. [Google Scholar] [CrossRef] [Green Version]
- Haritash, A.K.; Kaushik, C.P. Biodegradation Aspects of Polycyclic Aromatic Hydrocarbons (PAHs): A Review. J. Hazard. Mater. 2009, 169, 1–15. [Google Scholar] [CrossRef]
- Tarafdar, A.; Sinha, A.; Masto, R.E. Biodegradation of Anthracene by a Newly Isolated Bacterial Strain, Bacillus Thuringiensis AT.ISM.1, Isolated from a Fly Ash Deposition Site. Lett. Appl. Microbiol. 2017, 65, 327–334. [Google Scholar] [CrossRef]
- Doddamani, H.P.; Ninnekar, H.Z. Biodegradation of Phenanthrene by a Bacillus Species. Curr. Microbiol. 2000, 41, 11–14. [Google Scholar] [CrossRef] [PubMed]
- Bibi, N.; Hamayun, M.; Khan, S.A.; Iqbal, A.; Islam, B.; Shah, F.; Khan, M.A.; Lee, I.J. Anthracene Biodegradation Capacity of Newly Isolated Rhizospheric Bacteria Bacillus Cereus S13. PLoS ONE 2018, 13, e0201620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arulazhagan, P.; Vasudevan, N. Biodegradation of Polycyclic Aromatic Hydrocarbons by a Halotolerant Bacterial Strain Ochrobactrum Sp. VA1. Mar. Pollut. Bull. 2011, 62, 388–394. [Google Scholar] [CrossRef] [PubMed]
- Arulazhagan, P.; Vasudevan, N. Role of a Moderately Halophilic Bacterial Consortium in the Biodegradation of Polyaromatic Hydrocarbons. Mar. Pollut. Bull. 2009, 58, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Swaathy, S.; Kavitha, V.; Pravin, A.S.; Mandal, A.B.; Gnanamani, A. Microbial Surfactant Mediated Degradation of Anthracene in Aqueous Phase by Marine Bacillus Licheniformis MTCC 5514. Biotechnol. Rep. 2014, 4, 161–170. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; He, T.; Zhong, M.; Zhang, Y.; Li, E.; Huang, T.; Hu, Z. Isolation of Marine Benzo[a]Pyrene-Degrading Ochrobactrum Sp. BAP5 and Proteins Characterization. J. Environ. Sci. 2009, 21, 1446–1451. [Google Scholar] [CrossRef]
- Yan, Z.; Zhang, Y.; Wu, H.; Yang, M.; Zhang, H.; Hao, Z.; Jiang, H. Isolation and Characterization of a Bacterial Strain Hydrogenophaga Sp. PYR1 for Anaerobic Pyrene and Benzo[a]Pyrene Biodegradation. RSC Adv. 2017, 7, 46690–46698. [Google Scholar] [CrossRef] [Green Version]
- Qin, W.; Fan, F.; Zhu, Y.; Huang, X.; Ding, A.; Liu, X.; Dou, J. Anaerobic Biodegradation of Benzo(a)Pyrene by a Novel Cellulosimicrobium Cellulans CWS2 Isolated from Polycyclic Aromatic Hydrocarbon-Contaminated Soil. Braz. J. Microbiol. 2018, 49, 258–268. [Google Scholar] [CrossRef]
- Yessica, G.P.; Alejandro, A.; Ronald, F.C.; José, A.J.; Esperanza, M.R.; Samuel, C.S.J.; Remedios, M.L.M.; Ormeño-Orrillo, E. Tolerance, Growth and Degradation of Phenanthrene and Benzo[a]Pyrene by Rhizobium Tropici CIAT 899 in Liquid Culture Medium. Appl. Soil Ecol. 2013, 63, 105–111. [Google Scholar] [CrossRef]
- Ping, L.; Zhang, C.; Zhang, C.; Zhu, Y.; He, H.; Wu, M.; Tang, T.; Li, Z.; Zhao, H. Isolation and Characterization of Pyrene and Benzo[a]Pyrene-Degrading Klebsiella Pneumonia PL1 and Its Potential Use in Bioremediation. Appl. Microbiol. Biotechnol. 2014, 98, 3819–3828. [Google Scholar] [CrossRef]
- Liang, L.; Song, X.; Kong, J.; Shen, C.; Huang, T.; Hu, Z. Anaerobic Biodegradation of High-Molecular-Weight Polycyclic Aromatic Hydrocarbons by a Facultative Anaerobe Pseudomonas Sp. JP1. Biodegradation 2014, 25, 825–833. [Google Scholar] [CrossRef] [PubMed]
- Nzila, A.; Musa, M.M.; Sankara, S.; Al-Momani, M.; Xiang, L.; Li, Q.X. Degradation of Benzo[a]Pyrene by Halophilic Bacterial Strain Staphylococcus Haemoliticus Strain 10SBZ1A. PLoS ONE 2021, 16, e0247723. [Google Scholar] [CrossRef] [PubMed]
- Wanapaisan, P.; Laothamteep, N.; Vejarano, F.; Chakraborty, J.; Shintani, M.; Muangchinda, C.; Morita, T.; Suzuki-Minakuchi, C.; Inoue, K.; Nojiri, H.; et al. Synergistic Degradation of Pyrene by Five Culturable Bacteria in a Mangrove Sediment-Derived Bacterial Consortium. J. Hazard. Mater. 2018, 342, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Colombo, M.; Cavalca, L.; Bernasconi, S.; Andreoni, V. Bioremediation of Polyaromatic Hydrocarbon Contaminated Soils by Native Microflora and Bioaugmentation with Sphingobium Chlorophenolicum Strain C3R: A Feasibility Study in Solid- and Slurry-Phase Microcosms. Int. Biodeterior. Biodegrad. 2011, 65, 191–197. [Google Scholar] [CrossRef]
- Teng, Y.; Luo, Y.; Sun, M.; Liu, Z.; Li, Z.; Christie, P. Effect of Bioaugmentation by Paracoccus Sp. Strain HPD-2 on the Soil Microbial Community and Removal of Polycyclic Aromatic Hydrocarbons from an Aged Contaminated Soil. Bioresour. Technol. 2010, 101, 3437–3443. [Google Scholar] [CrossRef] [Green Version]
- Szulc, A.; Ambrożewicz, D.; Sydow, M.; Ławniczak, Ł.; Piotrowska-Cyplik, A.; Marecik, R.; Chrzanowski, Ł. The Influence of Bioaugmentation and Biosurfactant Addition on Bioremediation Efficiency of Diesel-Oil Contaminated Soil: Feasibility during Field Studies. J. Environ. Manag. 2014, 132, 121–128. [Google Scholar] [CrossRef]
- Silva, I.S.; Santos Eda, C.; Menezes, C.R.; Faria, A.F.; Franciscon, E.; Grossman, M.; Durrant, L.R. Bioremediation of a Polyaromatic Hydrocarbon Contaminated Soil by Native Soil Microbiota and Bioaugmentation with Isolated Microbial Consortia. Bioresour. Technol. 2009, 100, 4669–4675. [Google Scholar] [CrossRef]
- Saponaro, S.; Bonomo, L.; Petruzzelli, G.; Romele, L.; Barbafieri, M. Polycyclic Aromatic Hydrocarbons (PAHs) Slurry Phase Bioremediation of a Manufacturing Gas Plant (MGP) Site Aged Soil. Water Air Soil Pollut. 2002, 135, 219–236. [Google Scholar] [CrossRef]
- Launen, L.A.; Buggs, V.H.; Eastep, M.E.; Enriquez, R.C.; Leonard, J.W.; Blaylock, M.J.; Huang, J.W.; Häggblom, M.M. Bioremediation of Polyaromatic Hydrocarbon-Contaminated Sediments in Aerated Bioslurry Reactors. Bioremediat. J. 2002, 6, 125–141. [Google Scholar] [CrossRef]
- Bidja Abena, M.T.; Li, T.; Shah, M.N.; Zhong, W. Biodegradation of Total Petroleum Hydrocarbons (TPH) in Highly Contaminated Soils by Natural Attenuation and Bioaugmentation. Chemosphere 2019, 234, 864–874. [Google Scholar] [CrossRef]
- Ferraro, A.; Massini, G.; Miritana, V.M.; Panico, A.; Pontoni, L.; Race, M.; Rosa, S.; Signorini, A.; Fabbricino, M.; Pirozzi, F. Bioaugmentation Strategy to Enhance Polycyclic Aromatic Hydrocarbons Anaerobic Biodegradation in Contaminated Soils. Chemosphere 2021, 275, 130091. [Google Scholar] [CrossRef] [PubMed]
- Gibson, D.T.; Mahadevan, V.; Jerina, D.M.; Yogi, H.; Yeh, H.J. Oxidation of the Carcinogens Benzo [a] Pyrene and Benzo [a] Anthracene to Dihydrodiols by a Bacterium. Science 1975, 189, 295–297. [Google Scholar] [CrossRef] [PubMed]
- Schneider, J.; Grosser, R.; Jayasimhulu, K.; Xue, W.; Warshawsky, D. Degradation of Pyrene, Benz[a]Anthracene, and Benzo[a]Pyrene by Mycobacterium Sp. Strain RJGII-135, Isolated from a Former Coal Gasification Site. Appl. Environ. Microbiol. 1996, 62, 13–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moody, J.D.; Freeman, J.P.; Fu, P.P.; Cerniglia, C.E. Degradation of Benzo[a]Pyrene by Mycobacterium Vanbaalenii PYR-1. Appl. Environ. Microbiol. 2004, 70, 340–345. [Google Scholar] [CrossRef] [Green Version]
- Kanaly, R.; Bartha, R.; Fogel, S.; Findlay, M. Biodegradation of [(Sup14)C]Benzo[a]Pyrene Added in Crude Oil to Uncontaminated Soil. Appl. Environ. Microbiol. 1997, 63, 4511–4515. [Google Scholar] [CrossRef]


| dt (h) | |||||
|---|---|---|---|---|---|
| Condition | Consortium | B. cereus | B. japonicum | M. luteus | |
| pH | 5 | 30.3 ± 4.2 a* | 30.3 ± 3.0 a | ND | 29.8 ± 2.3 |
| 6 | 24.6 ± 7.2 b | 23.3 ± 1.9 | 23.3 ± 1.9 | 26.3 ± 2.0 | |
| 7 | 18.1 ± 1.9 c | 20.2 ± 1.3 a | 33.8 ± 3.4 | 23.0 ± 1.6 | |
| 8 | 35.1 ± 7.7 d | 27.1 ± 0.6 | 24.5 ± 5.9 | 31.2 ± 2.9 | |
| 9 | 63.3 ± 8.4 a,b,c,d | ND | 62.9 ± 41.8 | ND | |
| Temperature (°C) | 30 | 22.2 ± 1.4 | 25.2 ± 3.8 a | 29.1 ± 5.0 a | 24.7 ± 1.3 |
| 35 | 20.4 ± 1.5 | 21.2 ± 3.7 b | 22.2 ± 1.3 b | 22.9 ± 4.4 | |
| 37 | 18.1 ± 1.9 | 20.2 ± 1.7 c | 24.5 ± 6.0 c | 23.0 ± 1.6 | |
| 40 | 23.0 ± 6.7 | 23.4 ± 1.9 d | 34.2 ± 4.0 | 26.3 ± 2.0 | |
| 45 | 53.2 ± 19.4 | 52.1 ± 9.0 a,b,c,d | 51.8 ± 7.9 a,b,c | ND | |
| Salinity (% NaCl) | 0.5 | 18.1 ± 1.9 | 20.2 ± 1.5 a | 24.5 ± 6.0 | 23.0 ± 1.6 |
| 2 | 24.2 ± 0.5 | 22.5 ± 1.5 b | 30.3 ± 4.2 | 33.4 ± 7.4 | |
| 4 | 25.9 ± 3.5 | 30.4 ± 1.5 a,b | 37.6 ± 8.8 | ND |
| Metabolite | Observed Molecular Ion Mass | Molecular Formula | Calculated Exact Molar Mass | Strain(s) in Which This Metabolite Is Observed |
|---|---|---|---|---|
| Dihydroxy-BaP | 285.091 [M + 1] | C20H12O2 | 285.091 [M + 1] | JBZ5E |
| Dihydroxy-BaP | 281.247 [M − 1] | C20H10O2 | 281.060 [M − 1] | JBZ2B, CON |
| Methylated-dihydrodiol-BaP | 299.258 [M − 1] | C21H16O2 | 299.107 [M − 1] | JBZ1A, JBZ2B, JBZ5E, CON |
| 4-Formylchrysene-5-carboxylic acid | 299.259 [M − 1] | C20H12O3 | 299.071 [M − 1] | CON |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nzila, A.; Musa, M.M.; Afuecheta, E.; Al-Thukair, A.; Sankaran, S.; Xiang, L.; Li, Q.X. Benzo[A]Pyrene Biodegradation by Multiple and Individual Mesophilic Bacteria under Axenic Conditions and in Soil Samples. Int. J. Environ. Res. Public Health 2023, 20, 1855. https://doi.org/10.3390/ijerph20031855
Nzila A, Musa MM, Afuecheta E, Al-Thukair A, Sankaran S, Xiang L, Li QX. Benzo[A]Pyrene Biodegradation by Multiple and Individual Mesophilic Bacteria under Axenic Conditions and in Soil Samples. International Journal of Environmental Research and Public Health. 2023; 20(3):1855. https://doi.org/10.3390/ijerph20031855
Chicago/Turabian StyleNzila, Alexis, Musa M. Musa, Emmanuel Afuecheta, Assad Al-Thukair, Saravanan Sankaran, Lei Xiang, and Qing X. Li. 2023. "Benzo[A]Pyrene Biodegradation by Multiple and Individual Mesophilic Bacteria under Axenic Conditions and in Soil Samples" International Journal of Environmental Research and Public Health 20, no. 3: 1855. https://doi.org/10.3390/ijerph20031855





