The Association between Perceived Annoyances in the Indoor Home Environment and Respiratory Infections: A Danish Cohort Study with up to 19 Years of Follow-Up
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
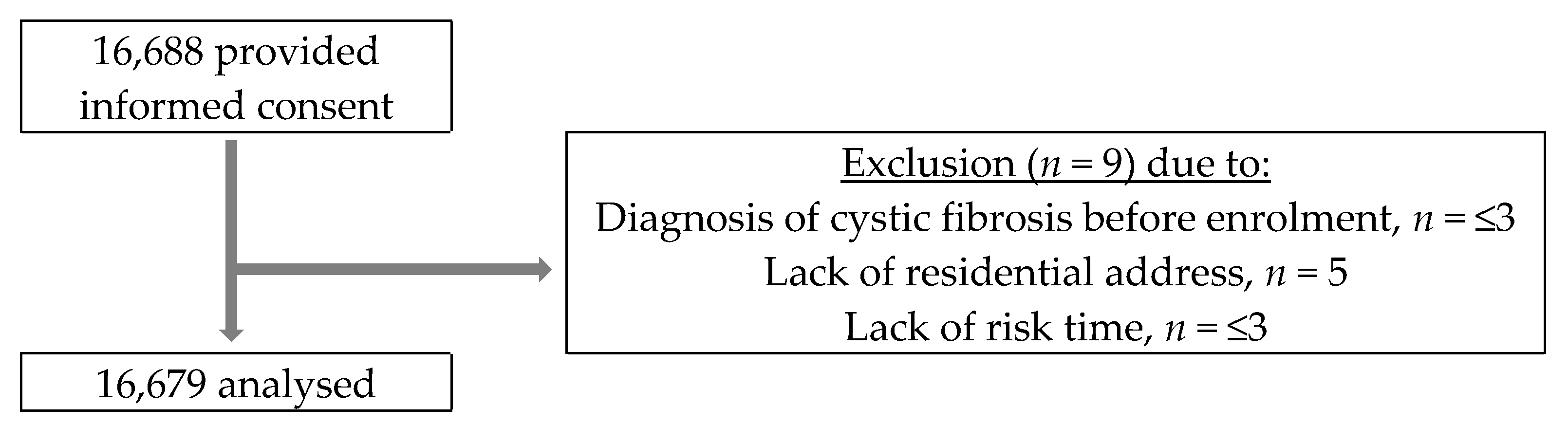
2.2. Study Population
2.3. Assessment of Perceived Annoyances of Indoor Environment
2.4. Respiratory Infection Outcomes
2.5. Confounders and Other Variables
2.6. Risk Time
2.7. Statistical Analysis
3. Results
3.1. Study Participants
3.2. Follow-Up Time and Number of Respiratory Infections
3.3. Associations between Perceived Annoyances and Respiratory Infections
3.4. Sensitivity Analyses
4. Discussion
4.1. Summary of Findings
4.2. Comparison with Other Studies
4.3. Interpretations
4.4. Strengths and Limitations
4.5. Implications and Impact
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ekholm, O.; Kjøller, M.; Davidsen, M.; Hesse, U.; Eriksen, L.; Christensen, A.I.; Grønbæk, M. Sundhed og Sygelighed i Danmark 2005 & Udviklingen Siden 1987; Syddansk Universitet, Statens Institut for Folkesundhed: Copenhagen, Denmark, 2007. [Google Scholar]
- Ekholm, O.; Christensen, A.I.; Davidsen, M.; Juel, K. Boligmiljø: Resultater fra Sundheds-og Sygelighedsundersøgelsen 2013; Statens Institut for Folkesundhed: Copenhagen, Denmark, 2014. [Google Scholar]
- Udesen, C.H.; Jensen, H.A.R.; Davidsen, M.; Christensen, A.I.; Ekholm, O. Boligmiljø: Sundheds-og Sygelighedsundersøgelsen 2017; Syddansk Universitet. Statens Institut for Folkesundhed: Copenhagen, Denmark, 2019. [Google Scholar]
- Kjøller, M.; Rasmussen, N.K. Sundhed og Sygelighed i Danmark 2000: Og Udviklingen Siden 1987; Syddansk Universitet, Statens Institut for Folkesundhed: Copenhagen, Denmark, 2002. [Google Scholar]
- Christensen, A.I.; Ekholm, O.; Davidsen, M.; Juel, K. Sundhed og Sygelighed i Danmark 2010 & Udviklingen Siden 1987; Syddansk Universitet, Statens Institut for Folkesundhed: Copenhagen, Denmark, 2012. [Google Scholar]
- Rasmussen, B.; Ekholm, O. Neighbour and traffic noise annoyance at home-prevalence and trends among Danish adults. Proc. EuroNoise 2015, 2015, 1895–1900. [Google Scholar]
- Pinter-Wollman, N.; Jelić, A.; Wells, N.M. The impact of the built environment on health behaviours and disease transmission in social systems. Philos. Trans. R. Soc. B Biol. Sci. 2018, 373, 20170245. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Fenech, B.; Blackmore, C.; Chen, Y.; Rodgers, G.; Gulliver, J.; Hansell, A.L. Association between Noise Annoyance and Mental Health Outcomes: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2022, 19, 2696. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization Burden of Disease from Environmental Noise: Quantification of Healthy Life Years Lost in Europe; World Health Organization, Regional Office for Europe: Copenhagen, Denmark, 2011; ISBN 92-890-0229-8.
- Hahad, O.; Beutel, M.; Gori, T.; Schulz, A.; Blettner, M.; Pfeiffer, N.; Rostock, T.; Lackner, K.; Sørensen, M.; Prochaska, J.H.; et al. Annoyance to different noise sources is associated with atrial fibrillation in the Gutenberg Health Study. Int. J. Cardiol. 2018, 264, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Lercher, P.; Hörtnagl, J.; Kofler, W.W. Work noise annoyance and blood pressure: Combined effects with stressful working conditions. Int. Arch. Occup. Environ. Health 1993, 65, 23–28. [Google Scholar] [CrossRef]
- Fendrick, A.M.; Monto, A.S.; Nightengale, B.; Sarnes, M. The Economic Burden of Non–Influenza-Related Viral Respiratory Tract Infection in the United States. Arch. Intern. Med. 2003, 163, 487–494. [Google Scholar] [CrossRef]
- Moriyama, M.; Hugentobler, W.J.; Iwasaki, A. Seasonality of respiratory viral infections. Annu. Rev. Virol. 2020, 7, 83–101. [Google Scholar] [CrossRef]
- Lee, K.K.; Bing, R.; Kiang, J.; Bashir, S.; Spath, N.; Stelzle, D.; Mortimer, K.; Bularga, A.; Doudesis, D.; Joshi, S.S.; et al. Adverse health effects associated with household air pollution: A systematic review, meta-analysis, and burden estimation study. Lancet Glob. Health 2020, 8, e1427–e1434. [Google Scholar] [CrossRef]
- Lin, S.; Lawrence, W.R.; Lin, Z.; Francois, M.; Neamtiu, I.A.; Lin, Q.; Csobod, E.; Gurzau, E.S. Teacher respiratory health symptoms in relation to school and home environment. Int. Arch. Occup. Environ. Health 2017, 90, 725–739. [Google Scholar] [CrossRef]
- Turunen, M.; Iso-Markku, K.; Pekkonen, M.; Haverinen-Shaughnessy, U. Statistical associations between housing quality and health among Finnish households with children—Results from two (repeated) national surveys. Sci Total Environ. 2017, 574, 1580–1587. [Google Scholar] [CrossRef]
- Niemann, H.; Bonnefoy, X.; Braubach, M.; Hecht, K.; Maschke, C.; Rodrigues, C.; Robbel, N. Noise-induced annoyance and morbidity results from the pan-European LARES study. Noise Health 2006, 8, 63. [Google Scholar] [CrossRef]
- Aatamila, M.; Verkasalo, P.K.; Korhonen, M.J.; Suominen, A.L.; Hirvonen, M.-R.; Viluksela, M.K.; Nevalainen, A. Odour annoyance and physical symptoms among residents living near waste treatment centres. Environ. Res. 2011, 111, 164–170. [Google Scholar] [CrossRef]
- Beutel, M.E.; Jünger, C.; Klein, E.M.; Wild, P.; Lackner, K.; Blettner, M.; Binder, H.; Michal, M.; Wiltink, J.; Brähler, E. Noise annoyance is associated with depression and anxiety in the general population-the contribution of aircraft noise. PLoS ONE 2016, 11, e0155357. [Google Scholar] [CrossRef]
- Sucker, K.; Both, R.; Winneke, G. Review of adverse health effects of odours in field studies. Water Sci. Technol. 2009, 59, 1281–1289. [Google Scholar] [CrossRef]
- Freiberg, A.; Schefter, C.; Hegewald, J.; Seidler, A. The influence of wind turbine visibility on the health of local residents: A systematic review. Int. Arch. Occup. Environ. Health 2019, 92, 609–628. [Google Scholar] [CrossRef]
- Morawska, L. Droplet fate in indoor environments, or can we prevent the spread of infection? Indoor Air 2006, 16, 335–347. [Google Scholar] [CrossRef]
- Assane, D.; Makhtar, C.; Abdoulaye, D.; Amary, F.; Djibril, B.; Amadou, D.; Niokhor, D.J.B.; Amadou, D.; Cheikh, L.; Ndongo, D. Viral and bacterial etiologies of acute respiratory infections among children under 5 years in Senegal. Microbiol. Insights 2018, 11, 1178636118758651. [Google Scholar] [CrossRef]
- Prussin, A.J.; Marr, L.C. Sources of airborne microorganisms in the built environment. Microbiome 2015, 3, 1–10. [Google Scholar] [CrossRef]
- Fujiyoshi, S.; Tanaka, D.; Maruyama, F. Transmission of airborne bacteria across built environments and its measurement standards: A review. Front. Microbiol. 2017, 8, 2336. [Google Scholar] [CrossRef]
- Hakansson, A.; Orihuela, C.; Bogaert, D. Bacterial-host interactions: Physiology and pathophysiology of respiratory infection. Physiol. Rev. 2018, 98, 781–811. [Google Scholar] [CrossRef]
- Berman, S. Epidemiology of acute respiratory infections in children of developing countries. Rev. Infect. Dis. 1991, 13, S454–S462. [Google Scholar] [CrossRef]
- Klein, E.Y.; Monteforte, B.; Gupta, A.; Jiang, W.; May, L.; Hsieh, Y.; Dugas, A. The frequency of influenza and bacterial coinfection: A systematic review and meta-analysis. Influenza Other Respir. Viruses 2016, 10, 394–403. [Google Scholar] [CrossRef]
- de Oliveira, T.B.; Klering, E.A.; da Veiga, A.B.G. Is recurrent respiratory infection associated with allergic respiratory disease? J. Asthma 2019, 56, 160–166. [Google Scholar] [CrossRef]
- Juhn, Y.J. Risks for infection in patients with asthma (or other atopic conditions): Is asthma more than a chronic airway disease? J. Allergy Clin. Immunol. 2014, 134, 247–257. [Google Scholar] [CrossRef]
- Sanchez-Ramirez, D.C.; Mackey, D. Underlying respiratory diseases, specifically COPD, and smoking are associated with severe COVID-19 outcomes: A systematic review and meta-analysis. Respir. Med. 2020, 171, 106096. [Google Scholar] [CrossRef]
- Quinn, A.; Shaman, J. Health symptoms in relation to temperature, humidity, and self-reported perceptions of climate in New York City residential environments. Int. J. Biometeorol. 2017, 61, 1209–1220. [Google Scholar] [CrossRef]
- Davidsen, M.; Kjøller, M. The Danish Health and Morbidity survey 2000—design and analysis. Stat. Transit. 2002, 5, 927–942. [Google Scholar]
- Ekholm, O.; Hesse, U.; Davidsen, M.; Kjøller, M. The study design and characteristics of the Danish national health interview surveys. Scand. J. Public Health 2009, 37, 758–765. [Google Scholar] [CrossRef]
- Jensen, H.A.R.; Ekholm, O.; Davidsen, M.; Christensen, A.I. The Danish health and morbidity surveys: Study design and participant characteristics. BMC Med. Res. Methodol. 2019, 19, 91. [Google Scholar] [CrossRef]
- Keiding, L.; Gunnarsen, L.; Rosdahl, N.; Machon, M.; Moller, R.; Valbjørn, O. Environmental Factors of Everyday Life in Denmark–with Special Focus on Housing Environment; National Institute of Public Health: Copenhagen, Denmark, 2003. [Google Scholar]
- World Health Organization. Environmental Health in Rural and Urban Development and Housing Unit. Indoor Environment: Health Aspects of Air Quality, Thermal Environment, Light and Noise. 1990; (WHO/EHE/RUD/90.2. Unpublished). Available online: https://apps.who.int/iris/handle/10665/62723 (accessed on 5 December 2022).
- Kloster, S.; Kirkegaard, A.M.; Davidsen, M.; Christensen, A.I.; Nielsen, N.S.; Gunnarsen, L.; Ersbøll, A.K. Patterns of Perceived Indoor Environment in Danish Homes. Int. J. Environ. Res. Public Health 2022, 19, 11498. [Google Scholar] [CrossRef]
- Schmidt, M.; Schmidt, S.A.J.; Sandegaard, J.L.; Ehrenstein, V.; Pedersen, L.; Sørensen, H.T. The Danish National Patient Registry: A review of content, data quality, and research potential. Clin. Epidemiol. 2015, 7, 449. [Google Scholar] [CrossRef]
- Pottegård, A.; Schmidt, S.A.J.; Wallach-Kildemoes, H.; Sørensen, H.T.; Hallas, J.; Schmidt, M. Data resource profile: The Danish national prescription registry. Int. J. Epidemiol. 2017, 46, 798–798f. [Google Scholar] [CrossRef]
- Textor, J.; van der Zander, B.; Gilthorpe, M.S.; Liśkiewicz, M.; Ellison, G.T. Robust causal inference using directed acyclic graphs: The R package ‘dagitty’. Int. J. Epidemiol. 2016, 45, 1887–1894. [Google Scholar] [CrossRef]
- Schmidt, M.; Pedersen, L.; Sørensen, H.T. The Danish Civil Registration System as a tool in epidemiology. Eur. J. Epidemiol. 2014, 29, 541–549. [Google Scholar] [CrossRef]
- Jensen, V.M.; Rasmussen, A.W. Danish education registers. Scand. J. Public Health 2011, 39, 91–94. [Google Scholar] [CrossRef]
- UNESCO. Institute for Statistics—2012—International Standard Classification of Education.pdf [Internet]. Available online: http://uis.unesco.org/sites/default/files/documents/international-standard-classification-of-education-isced-2011-en.pdf (accessed on 11 February 2022).
- Laird, N.; Olivier, D. Covariance analysis of censored survival data using log-linear analysis techniques. J. Am. Stat. Assoc. 1981, 76, 231–240. [Google Scholar] [CrossRef]
- Kirkwood, B.R.; Sterne, J.A. Essential Medical Statistics; John Wiley & Sons: Hoboken, NJ, USA, 2003; ISBN 1-4443-9284-0. [Google Scholar]
- Jensen, H.A.R.; Lau, C.J.; Davidsen, M.; Feveile, H.B.; Christensen, A.I.; Ekholm, O. The impact of non-response weighting in health surveys for estimates on primary health care utilization. Eur. J. Public Health 2022, 32, 450–455. [Google Scholar] [CrossRef]
- Fangel, S.; Linde, P.; Thorsted, B. Nye problemer med repræsentativitet i surveys, som opregning med registre kan reducere. Metode Og Data 2007, 93, 14–26. [Google Scholar]
- Kaspersen, K.A.; Pedersen, O.B.; Petersen, M.S.; Hjalgrim, H.; Rostgaard, K.; Møller, B.K.; Juul-Sørensen, C.; Kotzé, S.; Dinh, K.M.; Erikstrup, L.T. Obesity and risk of infection: Results from the Danish Blood Donor Study. Epidemiology 2015, 26, 580–589. [Google Scholar] [CrossRef]
- Bjerrum, L.; Gahrn-Hansen, B.; Hansen, M.P.; Cordoba, G.; Aabenhus, R.; Monrad, R.N. Luftvejsinfektioner—Diagnose og Behandling; Dansk Selskab for Almen Medicin: Copenhagen, Denmark, 2014; pp. 1–53. [Google Scholar]
- Schmidt, M.; Horvath-Puho, E.; Thomsen, R.; Smeeth, L.; Sørensen, H. Acute infections and venous thromboembolism. J. Intern. Med. 2012, 271, 608–618. [Google Scholar] [CrossRef]
- Yee, J.; Cho, Y.A.; Yoo, H.J.; Yun, H.; Gwak, H.S. Short-term exposure to air pollution and hospital admission for pneumonia: A systematic review and meta-analysis. Environ. Health 2021, 20, 1–10. [Google Scholar] [CrossRef]
- Laszlo, H.; McRobie, E.; Stansfeld, S.; Hansell, A. Annoyance and other reaction measures to changes in noise exposure—A review. Sci. Total Environ. 2012, 435, 551–562. [Google Scholar] [CrossRef]
- Guski, R. Personal and social variables as co-determinants of noise annoyance. Noise Health 1999, 1, 45. [Google Scholar]
- Heikkinen, T.; Järvinen, A. The common cold. Lancet 2003, 361, 51–59. [Google Scholar] [CrossRef]
- Filippini, M.; Ortiz, L.G.; Masiero, G. Assessing the impact of national antibiotic campaigns in Europe. Eur. J. Health Econ. 2013, 14, 587–599. [Google Scholar] [CrossRef]
- Little, P.; Stuart, B.; Francis, N.; Douglas, E.; Tonkin-Crine, S.; Anthierens, S.; Cals, J.W.; Melbye, H.; Santer, M.; Moore, M.; et al. Effects of internet-based training on antibiotic prescribing rates for acute respiratory-tract infections: A multinational, cluster, randomised, factorial, controlled trial. Lancet 2013, 382, 1175–1182. [Google Scholar] [CrossRef]
- Earnshaw, S.; Mancarella, G.; Mendez, A.; Todorova, B.; Magiorakos, A.; Possenti, E.; Stryk, M.; Gilbro, S.; Goossens, H.; Albiger, B. European Antibiotic Awareness Day: A five-year perspective of Europe-wide actions to promote prudent use of antibiotics. Eurosurveillance 2014, 19, 20928. [Google Scholar] [CrossRef]
- Aabenhus, R.; Siersma, V.; Hansen, M.P.; Bjerrum, L. Antibiotic prescribing in Danish general practice 2004–13. J. Antimicrob. Chemother. 2016, 71, 2286–2294. [Google Scholar] [CrossRef]
- Twisk, J.W.; Smidt, N.; de Vente, W. Applied analysis of recurrent events: A practical overview. J. Epidemiol. Community Health 2005, 59, 706–710. [Google Scholar] [CrossRef]
- Schmidt, M.; Schmidt, S.A.J.; Adelborg, K.; Sundbøll, J.; Laugesen, K.; Ehrenstein, V.; Sørensen, H.T. The Danish health care system and epidemiological research: From health care contacts to database records. Clin. Epidemiol. 2019, 11, 563. [Google Scholar] [CrossRef]
- Ingeman, A.; Andersen, G.; Hundborg, H.H.; Johnsen, S.P. Medical complications in patients with stroke: Data validity in a stroke registry and a hospital discharge registry. Clin. Epidemiol. 2010, 2, 5. [Google Scholar] [CrossRef]
- Graff Stensballe, L.; Kristensen, K.; Nielsen, J.; Aaby, P. Diagnosis coding in The Danish National Patient Registry for respiratory syncytial virus infections. Scand. J. Infect. Dis. 2005, 37, 747–752. [Google Scholar] [CrossRef]
- Lynge, E.; Sandegaard, J.L.; Rebolj, M. The Danish national patient register. Scand. J. Public Health 2011, 39, 30–33. [Google Scholar] [CrossRef]
- Wang, J.; Norbäck, D. Subjective indoor air quality and thermal comfort among adults in relation to inspected and measured indoor environment factors in single-family houses in Sweden-the BETSI study. Sci. Total Environ. 2022, 802, 149804. [Google Scholar] [CrossRef]
- Ormandy, D.; Ezratty, V. Health and thermal comfort: From WHO guidance to housing strategies. Energy Policy 2012, 49, 116–121. [Google Scholar] [CrossRef]
- Ma, L.; Dill, J.; Mohr, C. The objective versus the perceived environment: What matters for bicycling? Transportation 2014, 41, 1135–1152. [Google Scholar] [CrossRef]
- Miles, R.; Jacobs, D.E. Future directions in housing and public health: Findings from Europe with broader implications for planners. J. Am. Plan. Assoc. 2008, 74, 77–89. [Google Scholar] [CrossRef]
- Justitsministeriet. Lov om Supplerende Bestemmelser til Forordning om Beskyttelse af Fysiske Personer i Forbindelse med Behandling af Personoplysninger og om fri Udveksling af Sådanne Oplysninger (databeskyttelsesloven) [Internet]. 23 May 2018. Available online: https://www.retsinformation.dk/eli/lta/2018/502 (accessed on 11 February 2022).
- Thygesen, L.C.; Daasnes, C.; Thaulow, I.; Brønnum-Hansen, H. Introduction to Danish (nationwide) registers on health and social issues: Structure, access, legislation, and archiving. Scand. J. Public Health 2011, 39, 12–16. [Google Scholar] [CrossRef]
| Baseline Characteristics | n (%) | Level of Annoyances, n (%) | |||
|---|---|---|---|---|---|
| Low (n = 14,820) | Moderate (n = 980) | High (n = 879) | |||
| Participants | |||||
| Sex | Male | 8181 (49) | 7307 (49.3) | 503 (51.3) | 371 (42.2) |
| Female | 8498 (51) | 7513 (50.7) | 477 (48.7) | 508 (57.8) | |
| Age group, years | <20 | 876 (5.3) | 771 (5.2) | 35 (3.6) | 70 (8) |
| 20–24 | 1307 (7.8) | 1053 (7.1) | 104 (10.6) | 150 (17.1) | |
| 25–29 | 1366 (8.2) | 1107 (7.5) | 112 (11.4) | 147 (16.7) | |
| 30–34 | 1452 (8.7) | 1241 (8.4) | 92 (9.4) | 119 (13.5) | |
| 35–39 | 1485 (8.9) | 1311 (8.9) | 97 (9.9) | 77 (8.8) | |
| 40–44 | 1512 (9.1) | 1351 (9.1) | 106 (10.8) | 55 (6.3) | |
| 45–49 | 1554 (9.3) | 1408 (9.5) | 85 (8.7) | 61 (6.9) | |
| 50–54 | 1628 (9.8) | 1492 (10.1) | 86 (8.8) | 50 (5.7) | |
| 55–59 | 1425 (8.5) | 1317 (8.9) | 75 (7.7) | 33 (3.8) | |
| 60–64 | 1059 (6.3) | 967 (6.5) | 54 (5.5) | 38 (4.3) | |
| 65–69 | 866 (5.2) | 798 (5.4) | 42 (4.3) | 26 (3) | |
| 70–74 | 753 (4.5) | 708 (4.8) | 28 (2.9) | 17 (1.9) | |
| 75–79 | 636 (3.8) | 583 (3.9) | 34 (3.5) | 19 (2.2) | |
| 80–84 | 433 (2.6) | 404 (2.7) | 20 (2) | 9 (1) | |
| ≥85 | 327 (2.0) | 309 (2.1) | 10 (1) | 8 (0.9) | |
| Education a | Elementary | 6368 (38.2) | 5689 (38.4) | 321 (32.8) | 358 (40.7) |
| Short | 6997 (42) | 6185 (41.7) | 452 (46.1) | 360 (41) | |
| Medium/long | 3304 (19.8) | 2937 (19.8) | 206 (21) | 161 (18.3) | |
| Smoking status | Smoker | 6184 (37.1) | 5382 (36.3) | 377 (38.5) | 425 (48.4) |
| Non-smoker | 10,470 (62.8) | 9413 (63.5) | 603 (61.5) | 454 (51.7) | |
| Missing | 25 (0.1) | 25 (0.2) | 0 (0) | 0 (0) | |
| Country of origin | Danish | 16,349 (98) | 14,532 (98.1) | 959 (97.9) | 858 (97.6) |
| Non-Danish b | 330 (2) | 288 (1.9) | 21 (2.1) | 21 (2.4) | |
| Asthma or COPD diagnosis | Yes, n (%) | 1153 (6.9) | 1029 (6.9) | 52 (5.3) | 72 (8.2) |
| No, n (%) | 15,526 (93.1) | 13,791 (93.1) | 928 (94.7) | 807 (91.8) | |
| Household and Environment | |||||
| Type of dwelling | Detached house | 8523 (51.1) | 7867 (53.1) | 419 (42.8) | 237 (27) |
| Semi-detached and terrace houses | 2831 (17) | 2504 (16.9) | 157 (16) | 170 (19.3) | |
| Apartments | 3427 (20.5) | 2713 (18.3) | 344 (35) | 370 (42.1) | |
| Other house type | 1360 (8.2) | 1272 (8.6) | 30 (3.1) | 58 (6.6) | |
| Farms | 435 (2.6) | 375 (2.5) | 24 (2.5) | 36 (4.1) | |
| Missing | 103 (0.6) | 89 (0.6) | 6 (0.6) | 8 (0.9) | |
| Degree of urbanisation | <200 residents | 2631 (15.8) | 2431 (16.4) | 91 (9.3) | 109 (12.4) |
| 200–4900 residents | 4228 (25.3) | 3884 (26.2) | 203 (20.7) | 141 (16.04) | |
| 5–49,000 residents | 4984 (29.9) | 4449 (30) | 283 (28.9) | 252 (28.7) | |
| ≥50,000 residents | 4195 (25.2) | 3485 (23.5) | 366 (37.4) | 344 (39.1) | |
| Missing | 641 (3.8) | 571 (3.9) | 37 (3.8) | 33 (3.8) | |
| Heating source | Direct electricity | 1254 (7.5) | 1150 (7.8) | 49 (5) | 55 (6.3) |
| Central heating from liquid fuel | 3581 (21.5) | 3251 (21.9) | 169 (17.2) | 161 (18.3) | |
| Central heating from natural gas | 2614 (15.7) | 2373 (16) | 152 (15.5) | 89 (10.1) | |
| Other, gas furnace, solid fuel stove | 455 (2.7) | 408 (2.8) | 22 (2.2) | 25 (2.8) | |
| Undisclosed | 8775 (52.6) | 7638 (51.5) | 588 (60) | 549 (62.5) | |
| Residential density, m2/resident | <40 | 2748 (16.5) | 2501 (16.9) | 164 (16.7) | 83 (9.4) |
| 40–79 | 7691 (46.1) | 6948 (46.9) | 413 (42.1) | 330 (37.5) | |
| ≥80 | 5541 (33.2) | 4751 (32.1) | 364 (37.1) | 426 (48.5) | |
| Missing m2 | 699 (4.2) | 620 (4.2) | 39 (4) | 40 (4.6) | |
| Number of residents in household | 1 | 3349 (20.1) | 2916 (19.7) | 225 (23) | 208 (23.7) |
| 2 | 6358 (38.1) | 5644 (38.1) | 405 (41.3) | 309 (35.2) | |
| 3 | 2829 (17) | 2526 (17) | 150 (15.3) | 153 (17.4) | |
| 4 | 2723 (16.3) | 2442 (16.5) | 136 (13.9) | 145 (16.5) | |
| ≥5 | 1420 (8.5) | 1292 (8.7) | 64 (6.5) | 64 (7.3) | |
| Years lived in the dwelling | <3 | 4841 (29) | 4054 (27.4) | 383 (39.1) | 404 (46) |
| 3–7 | 3691 (22.1) | 3245 (21.9) | 222 (22.7) | 224 (25.5) | |
| 8–12 | 2035 (12.2) | 1850 (12.5) | 93 (9.5) | 92 (10.5) | |
| 13–20 | 2595 (15.6) | 2397 (16.2) | 107 (10.9) | 91 (10.4) | |
| ≥21 | 3517 (21.1) | 3274 (22.1) | 175 (17.9) | 68 (7.7) | |
| Interview Details | |||||
| Season of interview | Spring | 4404 (26.4) | 3951 (26.7) | 286 (29.2) | 167 (19) |
| Summer | 3166 (19) | 2856 (19.3) | 189 (19.3) | 121 (13.8) | |
| Autumn | 4309 (25.8) | 3781 (25.5) | 289 (29.5) | 239 (27.2) | |
| Winter | 4800 (28.8) | 4232 (28.6) | 216 (22) | 352 (40.1) | |
| Outcome | Level of Perceived Annoyances | n | Number of Events | PYs at Risk | IR Per 100 PY | IRR (95% CI) a | Adjusted IRR (95% CI) a,b |
|---|---|---|---|---|---|---|---|
| Respiratory infections of all causes c | |||||||
| Low | 13,787 | 13,949 | 124,602 | 11.19 | 1 (reference) | 1 (reference) | |
| Moderate | 928 | 875 | 7046 | 12.42 | 1.09 (0.98, 1.23) | 1.15 (1.02, 1.31) | |
| High | 806 | 584 | 4675 | 12.49 | 1.16 (1.02, 1.32) | 1.16 (1.01, 1.34) | |
| Bacterial respiratory infections d | |||||||
| Low | 13,787 | 13,074 | 124,645 | 10.49 | 1 (reference) | 1 (reference) | |
| Moderate | 928 | 826 | 7049 | 11.72 | 1.11 (0.99, 1.25) | 1.17 (1.03, 1.33) | |
| High | 806 | 559 | 4676 | 11.96 | 1.19 (1.04, 1.36) | 1.18 (1.02, 1.37) | |
| Viral respiratory infections e | |||||||
| Low | 13,787 | 1179 | 125,267 | 0.94 | 1 (reference) | 1 (reference) | |
| Moderate | 928 | 60 | 7089 | 0.85 | 0.84 (0.60, 1.17) | 0.96 (0.66, 1.39) | |
| High | 806 | 32 | 4704 | 0.68 | 0.65 (0.42, 0.99) | 0.82 (0.50, 1.33) | |
| Hospital admissions caused by respiratory infections f | |||||||
| Low | 13,787 | 855 | 125,593 | 0.68 | 1 (reference) | 1 (reference) | |
| Moderate | 928 | 40 | 7110 | 0.56 | 0.76 (0.50, 1.15) | 0.93 (0.58, 1.50) | |
| High | 806 | 20 | 4748 | 0.42 | 0.55 (0.32, 0.95) | 0.74 (0.39, 1.40) | |
| Outcome | Level of Perceived Annoyances | n | Number of Events | PYs at Risk | IR Per 100 PY | IRR (95% CI) a | Adjusted IRR (95% CI) a,b,c |
|---|---|---|---|---|---|---|---|
| Respiratory infections of all causes d | |||||||
| Low | 2100 | 1476 | 16,297 | 9.06 | 1 (reference) | 1 (reference) | |
| Moderate | 105 | 63 | 782 | 8.06 | 0.80 (0.55, 1.18) | 0.84 (0.59, 1.21) | |
| High | 110 | 80 | 745 | 10.74 | 1.20 (0.85, 1.71) | 1.20 (0.85, 1.69) | |
| Bacterial respiratory infections e | |||||||
| Low | 2100 | 901 | 16,323 | 5.52 | 1 (reference) | 1 (reference) | |
| Moderate | 105 | 38 | 783 | 4.85 | 0.88 (0.64, 1.22) | 0.80 (0.52, 1.23) | |
| High | 110 | 48 | 746 | 6.43 | 1.17 (0.87, 1.56) | 1.22 (0.82, 1.83) | |
| Viral respiratory infections f | |||||||
| Low | 2100 | 634 | 16,343 | 3.88 | 1 (reference) | 1 (reference) | |
| Moderate | 105 | 25 | 784 | 3.19 | 0.70 (0.55, 1.40) | 0.76 (0.38, 1.49) | |
| High | 110 | 33 | 747 | 4.41 | 1.11 (0.59, 2.06) | 1.28 (0.68, 2.42) | |
| Hospital admissions caused by respiratory infections e | |||||||
| Low | 2100 | 547 | 16,367 | 3.34 | 1 (reference) | 1 (reference) | |
| Moderate | 105 | 22 | 785 | 2.80 | 0.70 (0.33, 1.45) | 0.79 (0.39, 1.61) | |
| High | 110 | 28 | 749 | 3.74 | 1.14 (0.59, 2.19) | 1.31 (0.68, 2.53) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kirkegaard, A.M.; Kloster, S.; Davidsen, M.; Christensen, A.I.; Vestbo, J.; Nielsen, N.S.; Ersbøll, A.K.; Gunnarsen, L. The Association between Perceived Annoyances in the Indoor Home Environment and Respiratory Infections: A Danish Cohort Study with up to 19 Years of Follow-Up. Int. J. Environ. Res. Public Health 2023, 20, 1911. https://doi.org/10.3390/ijerph20031911
Kirkegaard AM, Kloster S, Davidsen M, Christensen AI, Vestbo J, Nielsen NS, Ersbøll AK, Gunnarsen L. The Association between Perceived Annoyances in the Indoor Home Environment and Respiratory Infections: A Danish Cohort Study with up to 19 Years of Follow-Up. International Journal of Environmental Research and Public Health. 2023; 20(3):1911. https://doi.org/10.3390/ijerph20031911
Chicago/Turabian StyleKirkegaard, Anne Marie, Stine Kloster, Michael Davidsen, Anne Illemann Christensen, Jørgen Vestbo, Niss Skov Nielsen, Annette Kjær Ersbøll, and Lars Gunnarsen. 2023. "The Association between Perceived Annoyances in the Indoor Home Environment and Respiratory Infections: A Danish Cohort Study with up to 19 Years of Follow-Up" International Journal of Environmental Research and Public Health 20, no. 3: 1911. https://doi.org/10.3390/ijerph20031911
APA StyleKirkegaard, A. M., Kloster, S., Davidsen, M., Christensen, A. I., Vestbo, J., Nielsen, N. S., Ersbøll, A. K., & Gunnarsen, L. (2023). The Association between Perceived Annoyances in the Indoor Home Environment and Respiratory Infections: A Danish Cohort Study with up to 19 Years of Follow-Up. International Journal of Environmental Research and Public Health, 20(3), 1911. https://doi.org/10.3390/ijerph20031911






