The Relationship between the Transmission of Different SARS-CoV-2 Strains and Air Quality: A Case Study in China
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Data Collection
2.2. Statistical Analysis
3. Results
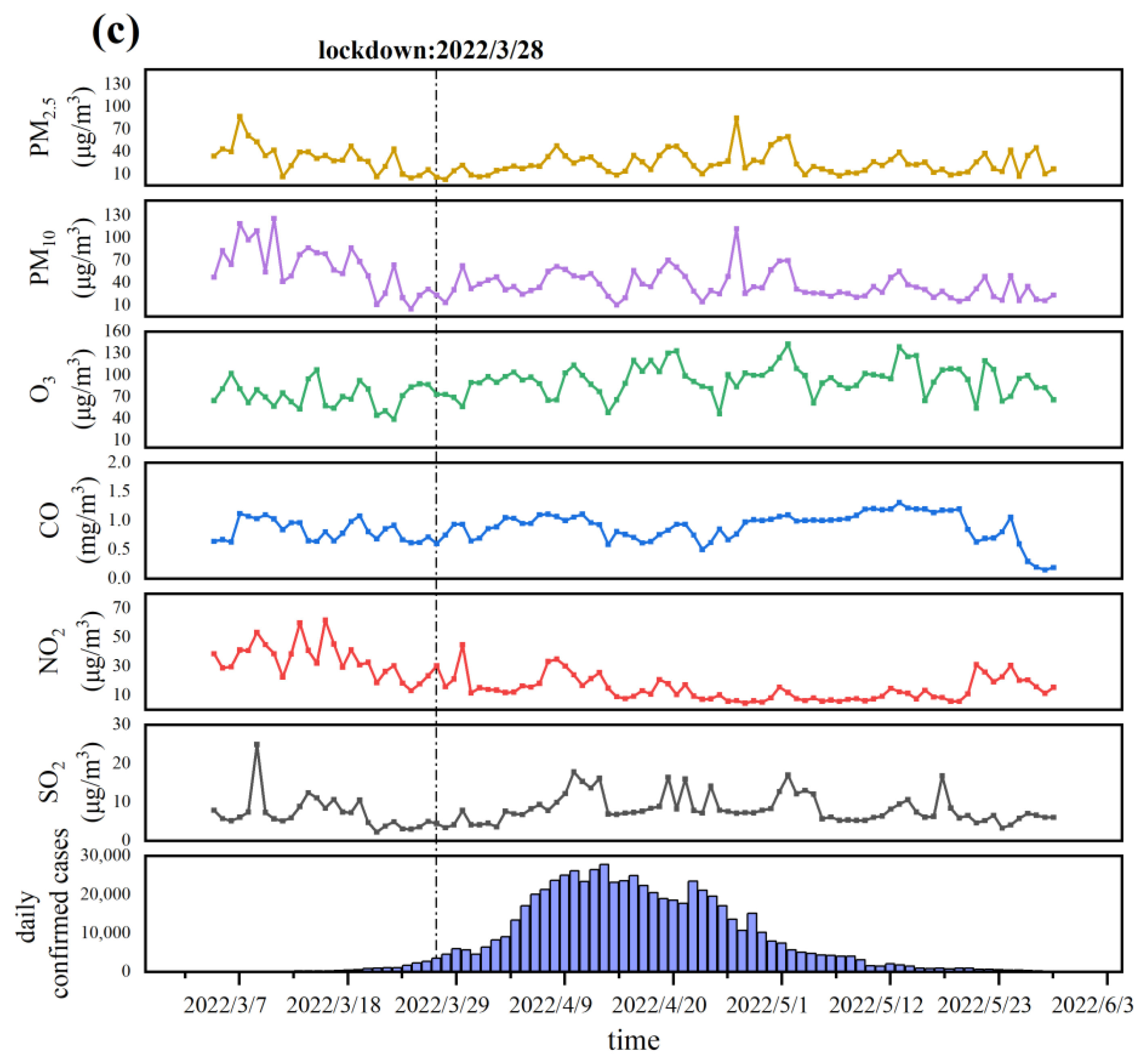
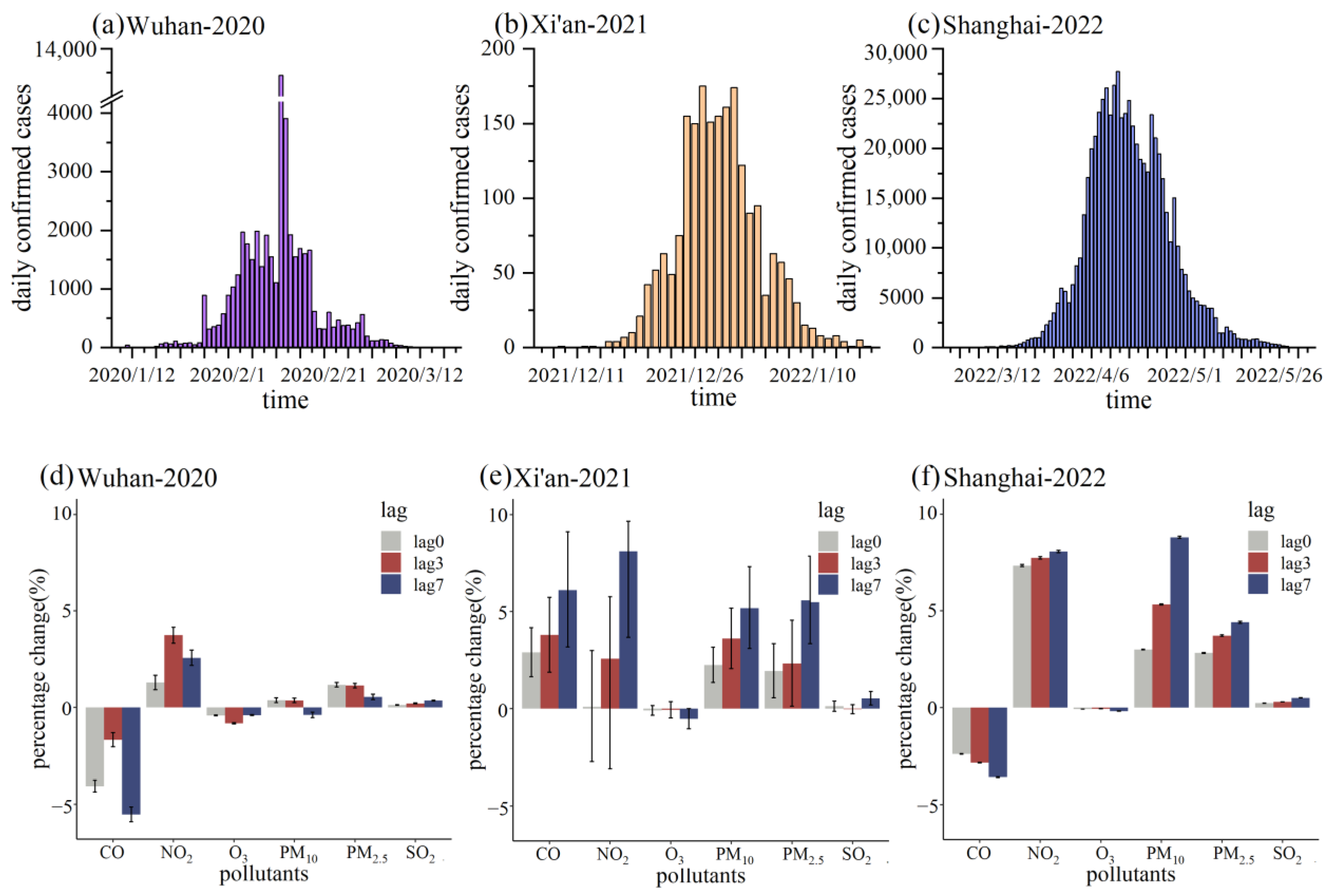
3.1. Descriptive Analysis
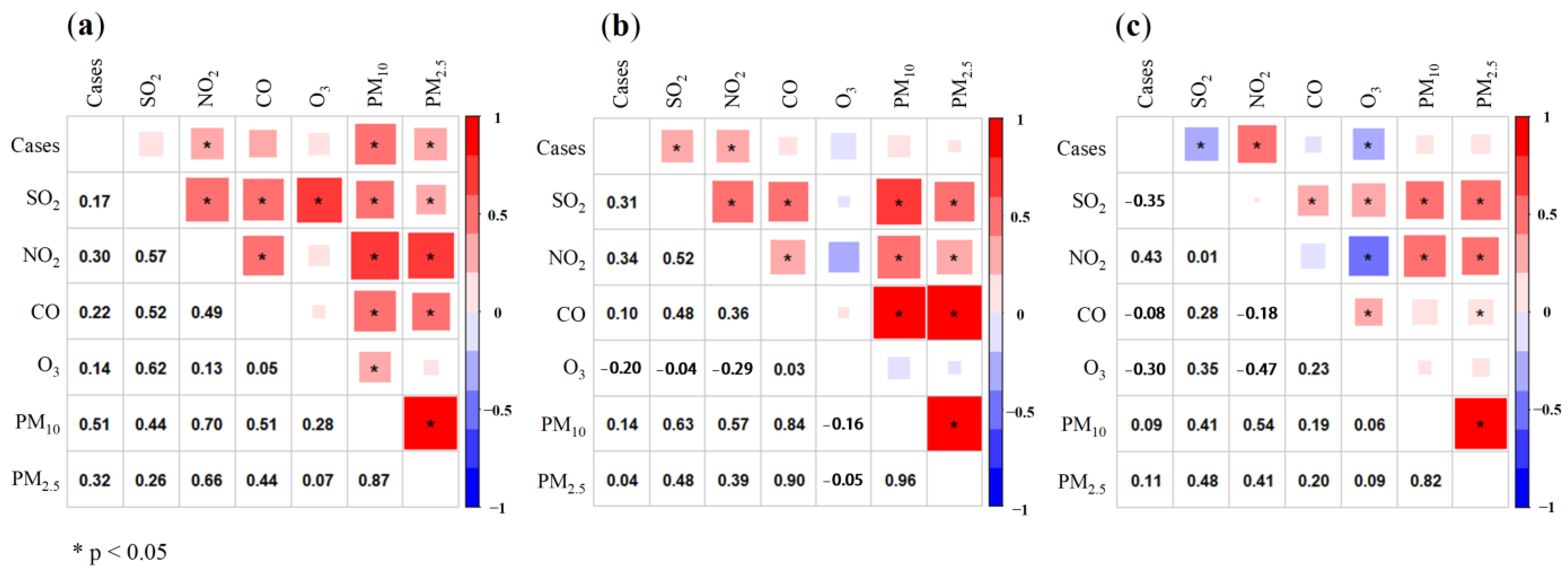
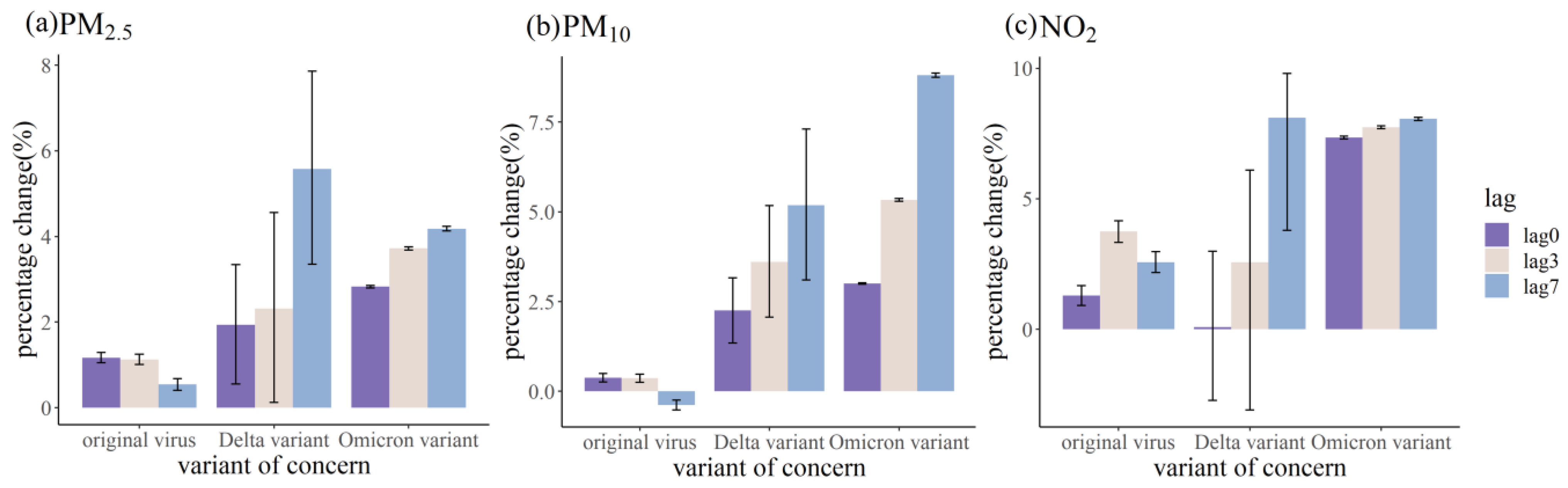
3.2. Relationship between Air Pollutants and COVID-19 Daily Confirmed Cases
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, N.S.; Zhou, M.; Dong, X.; Qu, J.M.; Gong, F.Y.; Han, Y.; Qiu, Y.; Wang, J.L.; Liu, Y.; Wei, Y.; et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet 2020, 395, 507–513. [Google Scholar] [CrossRef]
- Deng, X.; Garcia-Knight, M.; Khalid, M.M.; Servellita, V.; Wang, C.; Morris, M.K.; Sotomayor-González, A.; Glasner, D.R.; Reyes, K.R.; Gliwa, A.S.; et al. Transmission, infectivity, and neutralization of a spike L452R SARS-CoV-2 variant. Cell 2021, 184, 3426–3437.e8. [Google Scholar] [CrossRef] [PubMed]
- Pulliam, J.R.C.; van Schalkwyk, C.; Govender, N.; von Gottberg, A.; Cohen, C.; Groome, M.J.; Dushoff, J.; Mlisana, K.; Moultrie, H. Increased risk of SARS-CoV-2 reinfection associated with emergence of Omicron in South Africa. Science 2022, 376, eabn4947. [Google Scholar] [CrossRef] [PubMed]
- Yaniv, K.; Ozer, E.; Shagan, M.; Paitan, Y.; Granek, R.; Kushmaro, A. Managing an evolving pandemic: Cryptic circulation of the Delta variant during the Omicron rise. Sci. Total Environ. 2022, 836, 155599. [Google Scholar] [CrossRef] [PubMed]
- Davies, N.G.; Abbott, S.; Barnard, R.C.; Jarvis, C.I.; Kucharski, A.J.; Munday, J.D.; Pearson, C.A.B.; Russell, T.W.; Tully, D.C.; Washburne, A.D.; et al. Estimated transmissibility and impact of SARS-CoV-2 lineage B.1.1.7 in England. Science 2021, 372, eabg3055. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, R.; Hu, F.; Lan, Y.; Yang, Z.; Zhan, C.; Shi, J.; Deng, X.; Jiang, M.; Zhong, S.; et al. Transmission, viral kinetics and clinical characteristics of the emergent SARS-CoV-2 Delta VOC in Guangzhou, China. EClinicalMedicine 2021, 40, 101129. [Google Scholar] [CrossRef]
- Li, B.S.; Deng, A.; Li, K.B.; Hu, Y.; Li, Z.C.; Shi, Y.L.; Xiong, Q.L.; Liu, Z.; Guo, Q.F.; Zou, L.R.; et al. Viral infection and transmission in a large, well-traced outbreak caused by the SARS-CoV-2 Delta variant. Nat. Commun. 2022, 13, 460. [Google Scholar] [CrossRef]
- Twohig, K.A.; Nyberg, T.; Zaidi, A.; Thelwall, S.; Sinnathamby, M.A.; Aliabadi, S.; Seaman, S.R.; Harris, R.J.; Hope, R.; Lopez-Bernal, J.; et al. Hospital admission and emergency care attendance risk for SARS-CoV-2 delta (B.1.617.2) compared with alpha (B.1.1.7) variants of concern: A cohort study. Lancet Infect. Dis. 2022, 22, 35–42. [Google Scholar] [CrossRef]
- Zhang, J.; Xiao, T.; Cai, Y.; Lavine, C.L.; Peng, H.; Zhu, H.; Anand, K.; Tong, P.; Gautam, A.; Mayer, M.L.; et al. Membrane fusion and immune evasion by the spike protein of SARS-CoV-2 Delta variant. Science 2021, 374, 1353–1360. [Google Scholar] [CrossRef]
- Zhu, Y.; Xie, J.; Huang, F.; Cao, L. Association between short-term exposure to air pollution and COVID-19 infection: Evidence from China. Sci. Total Environ. 2020, 727, 138704. [Google Scholar] [CrossRef]
- Xu, L.; Taylor, J.E.; Kaiser, J. Short-term air pollution exposure and COVID-19 infection in the United States. Environ. Pollut. 2022, 292, 118369. [Google Scholar] [CrossRef] [PubMed]
- Travaglio, M.; Yu, Y.; Popovic, R.; Selley, L.; Leal, N.S.; Martins, L.M. Links between air pollution and COVID-19 in England. Environ. Pollut. 2021, 268, 115859. [Google Scholar] [CrossRef] [PubMed]
- De Angelis, E.; Renzetti, S.; Volta, M.; Donato, F.; Calza, S.; Placidi, D.; Lucchini, R.G.; Rota, M. COVID-19 incidence and mortality in Lombardy, Italy: An ecological study on the role of air pollution, meteorological factors, demographic and socioeconomic variables. Environ. Res. 2021, 195, 110777. [Google Scholar] [CrossRef] [PubMed]
- Hutter, H.; Poteser, M.; Moshammer, H.; Lemmerer, K.; Mayer, M.; Weitensfelder, L.; Wallner, P.; Kundi, M. Air Pollution Is Associated with COVID-19 Incidence and Mortality in Vienna, Austria. Int. J. Environ. Res. Public Health 2020, 17, 9275. [Google Scholar] [CrossRef] [PubMed]
- Brunekreef, B.; Holgate, S. Air pollution and health. Lancet 2002, 360, 1233–1242. [Google Scholar] [CrossRef]
- Liu, C.; Chen, R.; Sera, F.; Vicedo-Cabrera, A.M.; Guo, Y.M.; Tong, S.L.; Coelho, M.; Saldiva, P.; Lavigne, E.; Matus, P.; et al. Ambient Particulate Air Pollution and Daily Mortality in 652 Cities. N. Engl. J. Med. 2019, 381, 705–715. [Google Scholar] [CrossRef]
- Pedersen, E.J.; Miller, D.; Simpson, G.L.; Ross, N. Hierarchical generalized additive models in ecology: An introduction with mgcv. PeerJ 2019, 7, e6876. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Li, J.; Wu, X.; Wolfson, J.M.; Lawrence, J.; Stern, R.A.; Koutrakis, P.; Wei, J.; Huang, S. The association between daily-diagnosed COVID-19 morbidity and short-term exposure to PM1 is larger than associations with PM2.5 and PM10. Environ. Res. 2022, 210, 113016. [Google Scholar] [CrossRef]
- Zhang, X.; Tang, M.; Guo, F.; Wei, F.; Yu, Z.; Gao, K.; Jin, M.; Wang, J.; Chen, K. Associations between air pollution and COVID-19 epidemic during quarantine period in China. Environ. Pollut. 2021, 268, 115897. [Google Scholar] [CrossRef]
- Xie, J.; Zhu, Y. Association between ambient temperature and COVID-19 infection in 122 cities from China. Sci. Total Environ. 2020, 724, 138201. [Google Scholar] [CrossRef]
- Hou, K.; Xu, X. Evaluation of the Influence between Local Meteorology and Air Quality in Beijing Using Generalized Additive Models. Atmosphere 2022, 13, 24. [Google Scholar] [CrossRef]
- Alene, M.; Yismaw, L.; Assemie, M.A.; Ketema, D.B.; Gietaneh, W.; Birhan, T.Y. Serial interval and incubation period of COVID-19: A systematic review and meta-analysis. BMC Infect. Dis. 2021, 21, 257. [Google Scholar] [CrossRef]
- Backer, J.A.; Klinkenberg, D.; Wallinga, J. Incubation period of 2019 novel coronavirus (2019-nCoV) infections among travellers from Wuhan, China, 20–28 January 2020. Euro Surveill. Bull. Eur. Mal. Transm. 2020, 25, 1. [Google Scholar] [CrossRef] [PubMed]
- Chun, J.Y.; Baek, G.; Kim, Y. Transmission onset distribution of COVID-19. Int. J. Infect. Dis. 2020, 99, 403–407. [Google Scholar] [CrossRef]
- Xie, S.; Zhang, G.; Yu, H.; Wang, J.; Wang, S.; Tang, G.; Guo, C.; Li, J.; Wei, S.; Wang, C.; et al. The epidemiologic and clinical features of suspected and confirmed cases of imported 2019 novel coronavirus pneumonia in north Shanghai, China. Ann. Transl. Med. 2020, 8, 637. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.; Xin, H.; Yuan, J.; Ali, S.T.; Liang, Z.; Zhang, J.; Hu, T.; Lau, E.H.; Zhang, Y.; Zhang, M.; et al. Transmission dynamics and epidemiological characteristics of SARS-CoV-2 Delta variant infections in Guangdong, China, May to June 2021. Eurosurveillance 2022, 27, 2100815. [Google Scholar] [CrossRef]
- Del Águila-Mejía, J.; Wallmann, R.; Calvo-Montes, J.; Rodríguez-Lozano, J.; Valle-Madrazo, T.; Aginagalde-Llorente, A. Secondary Attack Rate, Transmission and Incubation Periods, and Serial Interval of SARS-CoV-2 Omicron Variant, Spain. Emerg. Infect. Dis. 2022, 28, 1224–1228. [Google Scholar] [CrossRef]
- Phosri, A.; Ueda, K.; Phung, V.L.H.; Tawatsupa, B.; Honda, A.; Takano, H. Effects of ambient air pollution on daily hospital admissions for respiratory and cardiovascular diseases in Bangkok, Thailand. Sci. Total Environ. 2019, 651, 1144–1153. [Google Scholar] [CrossRef]
- Bao, R.; Zhang, A. Does lockdown reduce air pollution? Evidence from 44 cities in northern China. Sci. Total Environ. 2020, 731, 139052. [Google Scholar] [CrossRef]
- Zhang, K.; Liu, Z.; Zhang, X.; Li, Q.; Jensen, A.; Tan, W.; Huang, L.; Wang, Y.; de Gouw, J.; Li, L. Insights into the significant increase in ozone during COVID-19 in a typical urban city of China. Atmos. Chem. Phys. 2022, 22, 4853–4866. [Google Scholar] [CrossRef]
- Dai, Q.; Hou, L.; Liu, B.; Zhang, Y.; Song, C.; Shi, Z.; Hopke, P.K.; Feng, Y. Spring Festival and COVID-19 Lockdown: Disentangling PM Sources in Major Chinese Cities. Geophys. Res. Lett. 2021, 48, e2021GL093403. [Google Scholar] [CrossRef] [PubMed]
- Skirienė, A.F.; Stasiškienė, Ž. COVID-19 and Air Pollution: Measuring Pandemic Impact to Air Quality in Five European Countries. Atmosphere 2021, 12, 290. [Google Scholar] [CrossRef]
- Zangari, S.; Hill, D.; Charette, A.T.; Mirowsky, J.E. Air quality changes in New York City during the COVID-19 pandemic. Sci. Total Environ. 2020, 742, 140496. [Google Scholar] [CrossRef]
- Guo, B.; Wang, X.; Zhang, D.; Pei, L.; Zhang, D.; Wang, X. A Land Use Regression Application into Simulating Spatial Distribution Characteristics of Particulate Matter (PM2.5) Concentration in City of Xi’an, China. Pol. J. Environ. Stud. 2020, 29, 4065–4076. [Google Scholar] [CrossRef]
- Fuller, C.H.; Feeser, K.; Sarnat, J.A.; O Neill, M.S. Air pollution, cardiovascular endpoints and susceptibility by stress and material resources: A systematic review of the evidence. Environ. Health 2017, 16, 58. [Google Scholar] [CrossRef] [PubMed]
- Flanders, W.D.; Klein, M.; Tolbert, P. A new variance estimator for parameters of semiparametric generalized additive models. J. Agric. Biol. Environ. Stat. 2005, 10, 246–257. [Google Scholar] [CrossRef]
- Troeger, C.; Blacker, B.; Khalil, I.A.; Rao, P.C.; Cao, J.; Zimsen, S.R.M.; Albertson, S.B.; Deshpande, A.; Farag, T.; Abebe, Z.; et al. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory infections in 195 countries, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect. Dis. 2018, 18, 1191–1210. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Wu, N.; Jiang, J.; Li, X. Associations of acute exposure to airborne pollutants with COVID-19 infection: Evidence from China. Environ. Sci. Pollut. Res. 2021, 28, 50554–50564. [Google Scholar] [CrossRef]
- Munir, S.; Coskuner, G.; Jassim, M.S.; Aina, Y.A.; Ali, A.; Mayfield, M. Changes in Air Quality Associated with Mobility Trends and Meteorological Conditions during COVID-19 Lockdown in Northern England, UK. Atmosphere 2021, 12, 504. [Google Scholar] [CrossRef]
- Bourdrel, T.; Annesi-Maesano, I.; Alahmad, B.; Maesano, C.N.; Bind, M. The impact of outdoor air pollution on COVID-19: A review of evidence from in vitro, animal, and human studies. Eur. Respir. Rev. 2021, 30, 200242. [Google Scholar] [CrossRef]
- Adhikari, A.; Yin, J. Short-Term Effects of Ambient Ozone, PM2.5, and Meteorological Factors on COVID-19 Confirmed Cases and Deaths in Queens, New York. Int. J. Environ. Res. Public Health 2020, 17, 4047. [Google Scholar] [CrossRef] [PubMed]
- Rebuli, M.E.; Brocke, S.; Jaspers, I. Impact of inhaled pollutants on response to viral infection in controlled exposures. J. Allergy Clin. Immunol. 2021, 148, 1420–1429. [Google Scholar] [CrossRef] [PubMed]
- Saygin, M.; Gonca, T.; Ozturk, O.; Has, M.; Caliskan, S.; Has, Z.G. To Investigate the Effects of Air Pollution (PM10 and SO2) on the Respiratory Diseases Asthma and Chronic Obstructive Pulmonary Disease. Turk. Thorac. J. 2017, 18, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Wu, S.; Kou, H.; Chen, K.; Chuang, H.; Feng, P.; Chung, K.F.; Ito, K.; Chen, T.; Sun, W.; et al. Association of air pollution exposure with exercise-induced oxygen desaturation in COPD. Respir. Res. 2022, 23, 77. [Google Scholar] [CrossRef]
- Wu, X.; Nethery, R.; Sabath, B.M.; Braun, D.; Dominici, F. Exposure to air pollution and COVID-19 mortality in the United States: A nationwide cross-sectional study. medRxiv 2020. [Google Scholar] [CrossRef]
- Bayarri, B.; Cruz-Alcalde, A.; López-Vinent, N.; Micó, M.M.; Sans, C. Can ozone inactivate SARS-CoV-2? A review of mechanisms and performance on viruses. J. Hazard. Mater. 2021, 415, 125658. [Google Scholar] [CrossRef]
- Huang, R.J.; Zhang, Y.; Bozzetti, C.; Ho, K.F.; Cao, J.J.; Han, Y.M.; Daellenbach, K.R.; Slowik, J.G.; Platt, S.M.; Canonaco, F.; et al. High secondary aerosol contribution to particulate pollution during haze events in China. Nature 2014, 514, 218–222. [Google Scholar] [CrossRef]
- Comunian, S.; Dongo, D.; Milani, C.; Palestini, P. Air Pollution and COVID-19: The Role of Particulate Matter in the Spread and Increase of COVID-19′s Morbidity and Mortality. Int. J. Environ. Res. Public Health 2020, 17, 4487. [Google Scholar] [CrossRef]
- Hsiao, T.; Cheng, P.; Chi, K.H.; Wang, H.; Pan, S.; Kao, C.; Lee, Y.; Kuo, H.; Chung, K.F.; Chuang, H. Interactions of chemical components in ambient PM2.5 with influenza viruses. J. Hazard. Mater. 2022, 423, 127243. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Chen, Y.; Song, Y.Y.; Dong, C.; Cai, Z.W. Atmospheric pressure gas chromatography-tandem mass spectrometry analysis of fourteen emerging polycyclic aromatic sulfur heterocycles in PM2.5. Chin. Chem. Lett. 2021, 32, 801–804. [Google Scholar] [CrossRef]
- Setti, L.; Passarini, F.; De Gennaro, G.; Barbieri, P.; Perrone, M.G.; Borelli, M.; Palmisani, J.; Di Gilio, A.; Torboli, V.; Fontana, F.; et al. SARS-Cov-2RNA found on particulate matter of Bergamo in Northern Italy: First evidence. Environ. Res. 2020, 188, 109754. [Google Scholar] [CrossRef] [PubMed]
- Zaim, S.; Chong, J.; Sankaranarayanan, V.; Harky, A. COVID-19 and Multiorgan Response. COVID-19 and Multiorgan Response. Curr. Probl. Cardiol. 2020, 45, 100618. [Google Scholar] [CrossRef] [PubMed]
- Wegmann, M.; Fehrenbach, A.; Heimann, S.; Fehrenbach, H.; Renz, H.; Garn, H.; Herz, U. NO2-induced airway inflammation is associated with progressive airflow limitation and development of emphysema-like lesions in C57BL/6 mice. Exp. Toxicol. Pathol. 2005, 56, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Sannigrahi, S.; Pilla, F.; Basu, B.; Basu, A.S.; Sarkar, K.; Chakraborti, S.; Joshi, P.K.; Zhang, Q.; Wang, Y.; Bhatt, S.; et al. Examining the effects of forest fire on terrestrial carbon emission and ecosystem production in India using remote sensing approaches. Sci. Total Environ. 2020, 725, 138331. [Google Scholar] [CrossRef]
- Liang, D.; Shi, L.; Zhao, J.; Liu, P.; Schwartz, J.; Gao, S.; Sarnat, J.; Liu, Y.; Ebelt, S.; Scovronick, N.; et al. Urban Air Pollution May Enhance COVID-19 Case-Fatality and Mortality Rates in the United States. medRxiv 2020, 1, 100047. [Google Scholar] [CrossRef]
- Di Ciaula, A.; Bonfrate, L.; Portincasa, P.; Appice, C.; Belfiore, A.; Binetti, M.; Cafagna, G.; Campanale, G.; Carrieri, A.; Cascella, G.; et al. Nitrogen dioxide pollution increases vulnerability to COVID-19 through altered immune function. Environ. Sci. Pollut. Res. 2022, 29, 44404–44412. [Google Scholar] [CrossRef]
- Domingo, J.L.; Rovira, J. Effects of air pollutants on the transmission and severity of respiratory viral infections. Environ. Res. 2020, 187, 109650. [Google Scholar] [CrossRef]
- Obermeyer, F.; Jankowiak, M.; Barkas, N.; Schaffner, S.F.; Pyle, J.D.; Yurkovetskiy, L.; Bosso, M.; Park, D.J.; Babadi, M.; MacInnis, B.L.; et al. Analysis of 6.4 million SARS-CoV-2 genomes identifies mutations associated with fitness. Science 2022, 376, 1327–1332. [Google Scholar] [CrossRef]
- Choi, B.; Choudhary, M.C.; Regan, J.; Sparks, J.A.; Padera, R.F.; Qiu, X.; Solomon, I.H.; Kuo, H.; Boucau, J.; Bowman, K.; et al. Persistence and Evolution of SARS-CoV-2 in an Immunocompromised Host. N. Engl. J. Med. 2020, 383, 2291–2293. [Google Scholar] [CrossRef]
- Guo, D.; Duan, H.; Cheng, Y.; Wang, Y.; Hu, J.; Shi, H. Omicron-included mutation-induced changes in epitopes of SARS-CoV-2 spike protein and effectiveness assessments of current antibodies. Mol. Biomed. 2022, 3, 12. [Google Scholar] [CrossRef]
- Liu, Y.; Rocklöv, J. The reproductive number of the Delta variant of SARS-CoV-2 is far higher compared to the ancestral SARS-CoV-2 virus. J. Travel Med. 2021, 28, taab124. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, R.; Gilby, N.B.; Wei, G. Omicron Variant (B.1.1.529): Infectivity, Vaccine Breakthrough, and Antibody Resistance. J. Chem. Inf. Model. 2022, 62, 412–422. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Gayle, A.; Wilder-Smith, A.; Rocklöv, J. The reproductive number of COVID-19 is higher compared to SARS coronavirus. J. Travel Med. 2020, 27, taaa021. [Google Scholar] [CrossRef]
- Campbell, F.; Archer, B.; Laurenson-Schafer, H.; Jinnai, Y.; Konings, F.; Batra, N.; Pavlin, B.; Vandemaele, K.; Van Kerkhove, M.D.; Jombart, T.; et al. Increased transmissibility and global spread of SARS-CoV-2 variants of concern as at June 2021. Eurosurveillance 2021, 26, 2100509. [Google Scholar] [CrossRef] [PubMed]
- Hoteit, R.; Yassine, H.M. Biological Properties of SARS-CoV-2 Variants: Epidemiological Impact and Clinical Consequences. Vaccines 2022, 10, 919. [Google Scholar] [CrossRef]
- Wolter, N.; Jassat, W.; Walaza, S.; Welch, R.; Moultrie, H.; Groome, M.; Amoako, D.G.; Everatt, J.; Bhiman, J.N.; Scheepers, C.; et al. Early assessment of the clinical severity of the SARS-CoV-2 omicron variant in South Africa: A data linkage study. Lancet 2022, 399, 437–446. [Google Scholar] [CrossRef]
- Pascarella, S.; Ciccozzi, M.; Bianchi, M.; Benvenuto, D.; Cauda, R.; Cassone, A. The electrostatic potential of the Omicron variant spike is higher than in Delta and Delta-plus variants: A hint to higher transmissibility? J. Med. Virol. 2022, 94, 1277–1280. [Google Scholar] [CrossRef]
- Starr, T.N.; Greaney, A.J.; Hilton, S.K.; Ellis, D.; Crawford, K.H.D.; Dingens, A.S.; Navarro, M.J.; Bowen, J.E.; Tortorici, M.A.; Walls, A.C.; et al. Deep Mutational Scanning of SARS-CoV-2 Receptor Binding Domain Reveals Constraints on Folding and ACE2 Binding. Cell 2020, 182, 1295–1310.e20. [Google Scholar] [CrossRef]
- Glocker, M.O.; Opuni, K.; Thiesen, H. From Free Binding Energy Calculations of SARS-CoV-2—Receptor Interactions to Cellular Immune Responses. Medicina 2022, 58, 226. [Google Scholar] [CrossRef]
- Alexey, V.; Krasheninnikov, D.N.L.S. Atmospheric electric field in megacity aerosol pollution conditions. Proc. SPIE 2018, 10833, 1485–1493. [Google Scholar] [CrossRef]
- Li, J.; Wu, J.; Long, Q.; Wu, Y.A.; Hu, X.; He, Y.; Jiang, M.; Li, J.; Zhao, L.; Yang, S.; et al. Comprehensive Humoral and Cellular Immune Responses to SARS-CoV-2 Variants in Diverse Chinese Population. Research 2022, 2022, 1–9. [Google Scholar] [CrossRef]
- Wang, L.; Liu, L. The present condition of China’s medical and health facilities in the epidemic situation of COVID-19,and a comparison with other countries. Sci. Technol. Rev. 2020, 38, 29–38. [Google Scholar]
- Kong, Z.; Sandhu, H.; Qiu, L.; Wu, J.; Tian, W.; Chi, X.; Tao, Z.; Yang, C.J.; Wang, X. Virus Dynamics and Decay in Evaporating Human Saliva Droplets on Fomites. Environ. Sci. Technol. 2022. [Google Scholar] [CrossRef]
- Rittweger, J.; Gilardi, L.; Baltruweit, M.; Dally, S.; Erbertseder, T.; Mittag, U.; Naeem, M.; Schmid, M.; Schmitz, M.; Wüst, S.; et al. Temperature and particulate matter as environmental factors associated with seasonality of influenza incidence—An approach using Earth observation-based modeling in a health insurance cohort study from Baden-Württemberg (Germany). Environ. Health 2022, 21, 131. [Google Scholar] [CrossRef] [PubMed]


| Wuhan (68 Days) | Xi’an (44 Days) | Shanghai (99 Days) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Max | Min | Average | Median | Max | Min | Average | Median | Max | Min | Average | Median | |
| Daily confirmed cases | 3910 | 0 | 543.2 | 131 | 175 | 0 | 46.6 | 14 | 27,719 | 0 | 6559.4 | 1487 |
| Relative humidity (%) | 94.3 | 42.7 | 73.1 | 75.0 | 86.5 | 28.0 | 52.5 | 49.5 | 95.3 | 8.9 | 60.9 | 63 |
| Precipitation (mm) | 36.3 | 0 | 2.4 | 0 | 2.8 | 0 | 0.1 | 0 | 82.9 | 0 | 9.4 | 0 |
| Wind speed (m/s) | 5.6 | 1.1 | 2.4 | 2.2 | 3.3 | 0.8 | 1.8 | 1.85 | 4.4 | 0.7 | 2.5 | 2.5 |
| Average temperature (°C) | 21.5 | 0.7 | 9.1 | 8.6 | 8 | −2.3 | 3.7 | 3.4 | 25.5 | 7.5 | 17.3 | 18.3 |
| PM2.5 (μg/m3) | 108 | 9 | 46.1 | 43 | 224 | 19 | 87.3 | 80 | 86.8 | 2.7 | 25.9 | 22.7 |
| PM10 (μg/m3) | 122 | 12 | 58.1 | 57 | 276 | 43 | 124.0 | 120.5 | 125.8 | 5.1 | 42.9 | 34.7 |
| SO2 (μg/m3) | 17 | 5 | 7.7 | 7 | 16 | 6 | 8.9 | 8 | 24.8 | 2.2 | 7.9 | 7.1 |
| NO2 (μg/m3) | 76 | 10 | 25.3 | 22 | 87 | 26 | 53.3 | 57 | 61.7 | 4.3 | 19.5 | 15.5 |
| O3 (μg/m3) | 135 | 28 | 77.5 | 78 | 113 | 4 | 63.4 | 60.5 | 142.5 | 38.9 | 87.7 | 88.8 |
| CO (mg/m3) | 1.4 | 0.5 | 0.9 | 0.9 | 1.6 | 0.1 | 0.8 | 0.8 | 1.3 | 0.1 | 0.9 | 0.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, R.; Zhang, Y.; Zhang, Y.; Li, X.; Ji, Z. The Relationship between the Transmission of Different SARS-CoV-2 Strains and Air Quality: A Case Study in China. Int. J. Environ. Res. Public Health 2023, 20, 1943. https://doi.org/10.3390/ijerph20031943
Ma R, Zhang Y, Zhang Y, Li X, Ji Z. The Relationship between the Transmission of Different SARS-CoV-2 Strains and Air Quality: A Case Study in China. International Journal of Environmental Research and Public Health. 2023; 20(3):1943. https://doi.org/10.3390/ijerph20031943
Chicago/Turabian StyleMa, Ruiqing, Yeyue Zhang, Yini Zhang, Xi Li, and Zheng Ji. 2023. "The Relationship between the Transmission of Different SARS-CoV-2 Strains and Air Quality: A Case Study in China" International Journal of Environmental Research and Public Health 20, no. 3: 1943. https://doi.org/10.3390/ijerph20031943
APA StyleMa, R., Zhang, Y., Zhang, Y., Li, X., & Ji, Z. (2023). The Relationship between the Transmission of Different SARS-CoV-2 Strains and Air Quality: A Case Study in China. International Journal of Environmental Research and Public Health, 20(3), 1943. https://doi.org/10.3390/ijerph20031943






