Initial Studies on the Effect of the Rice–Duck–Crayfish Ecological Co-Culture System on Physical, Chemical, and Microbiological Properties of Soils: A Field Case Study in Chaohu Lake Basin, Southeast China
Abstract
:1. Introduction
2. Materials and Methods
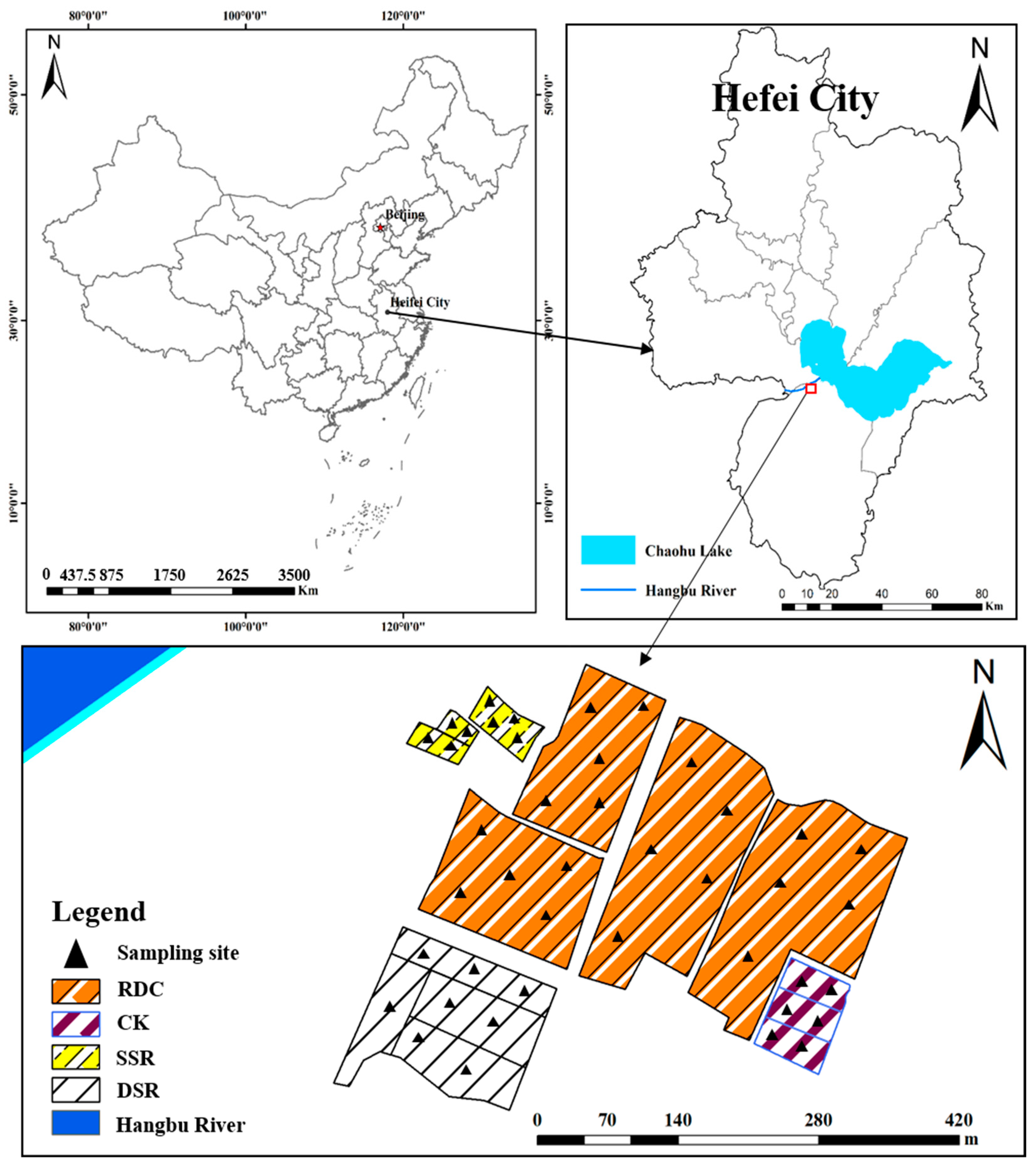
2.1. Location of the Study Area
2.2. Materials
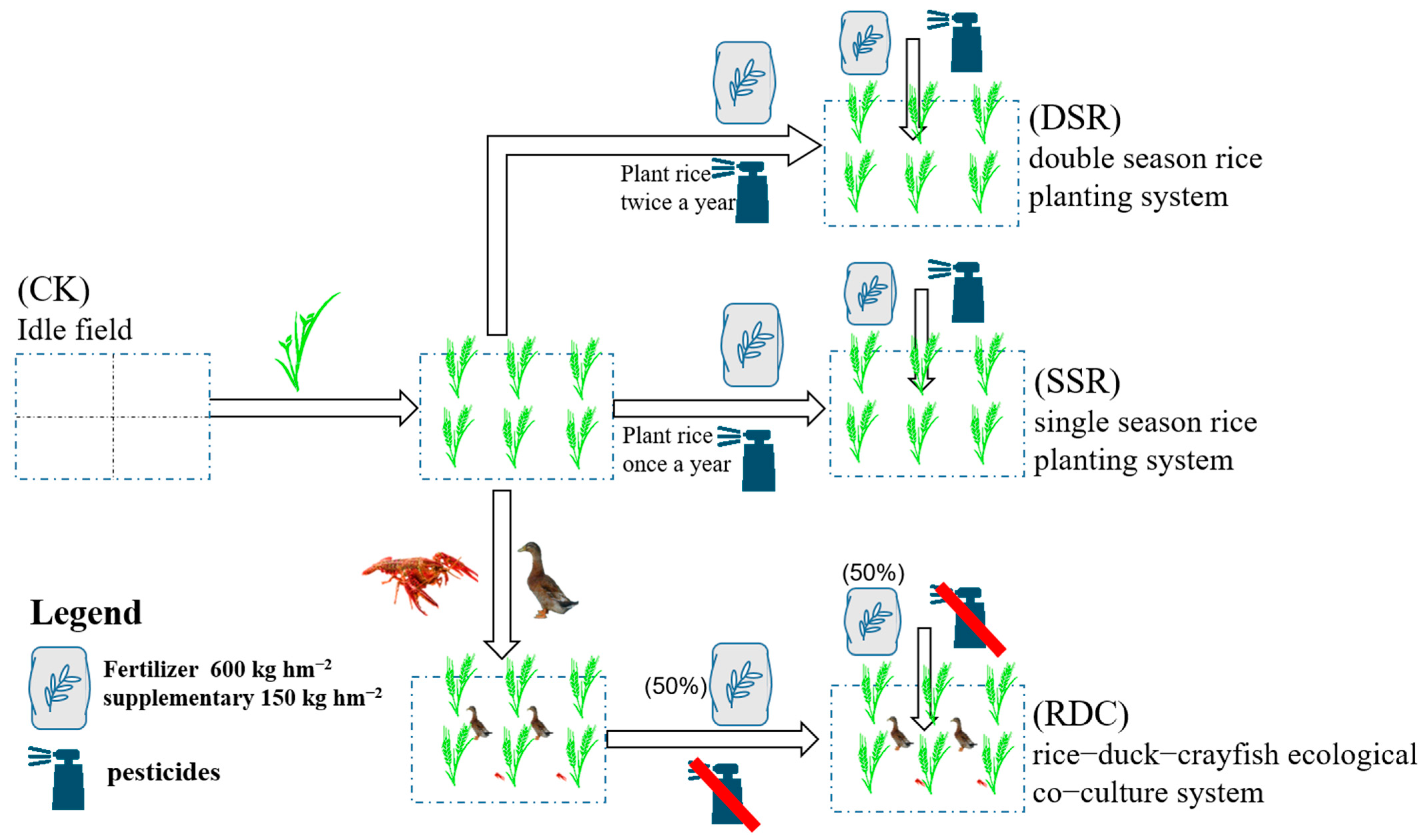
2.3. Design of Field Experiments
2.4. Field Management
2.4.1. RDC Group
2.4.2. Other Groups
2.5. Sample Collection
2.6. Chemical Analysis
2.7. QA/QC
2.8. Data Processing and Statistical Analysis
3. Results and Discussion
3.1. Improvements of the RDC Treatment on the Soil Quality
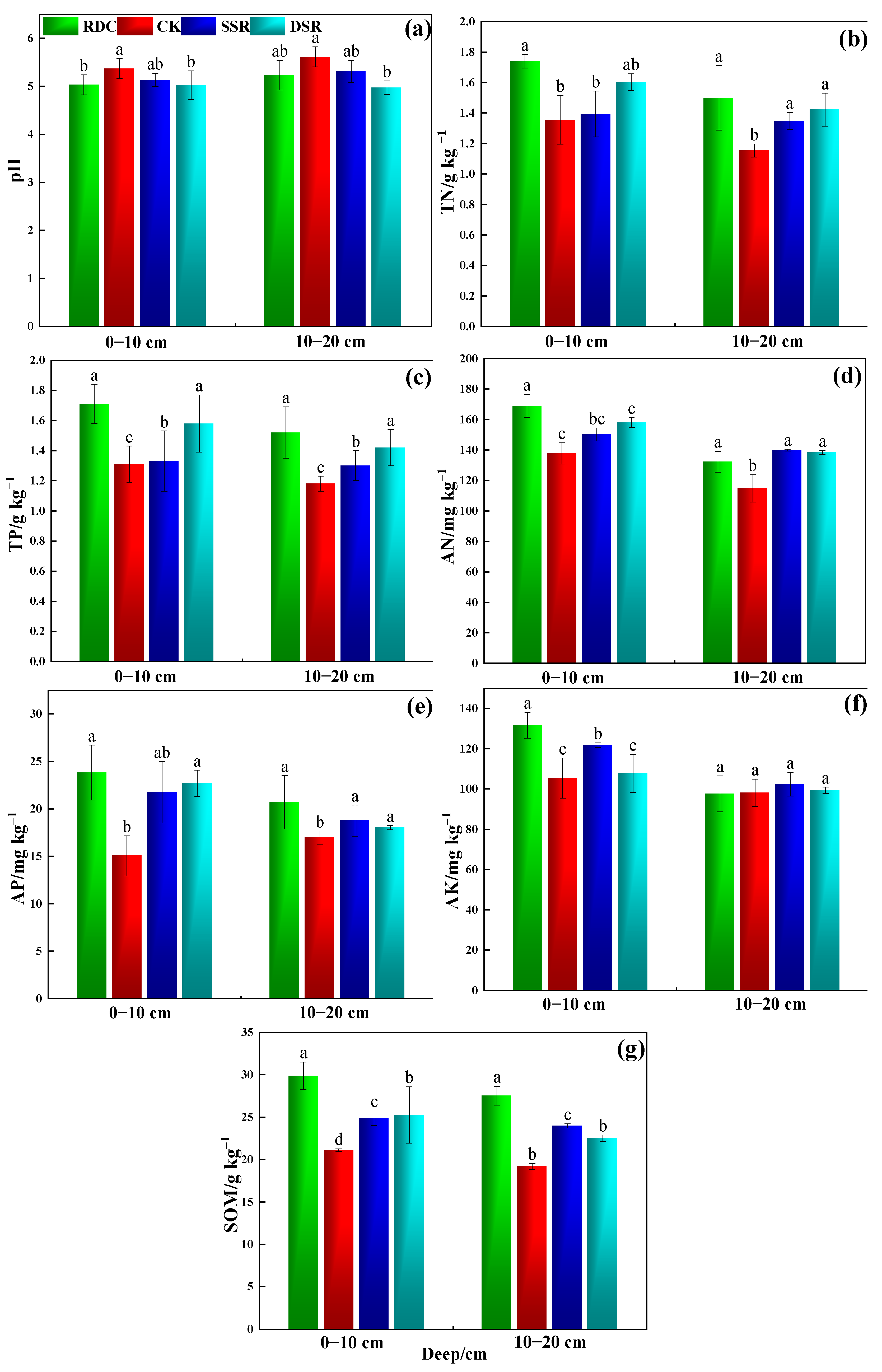
3.1.1. Physicochemical Properties
3.1.2. Soil Humic Substances
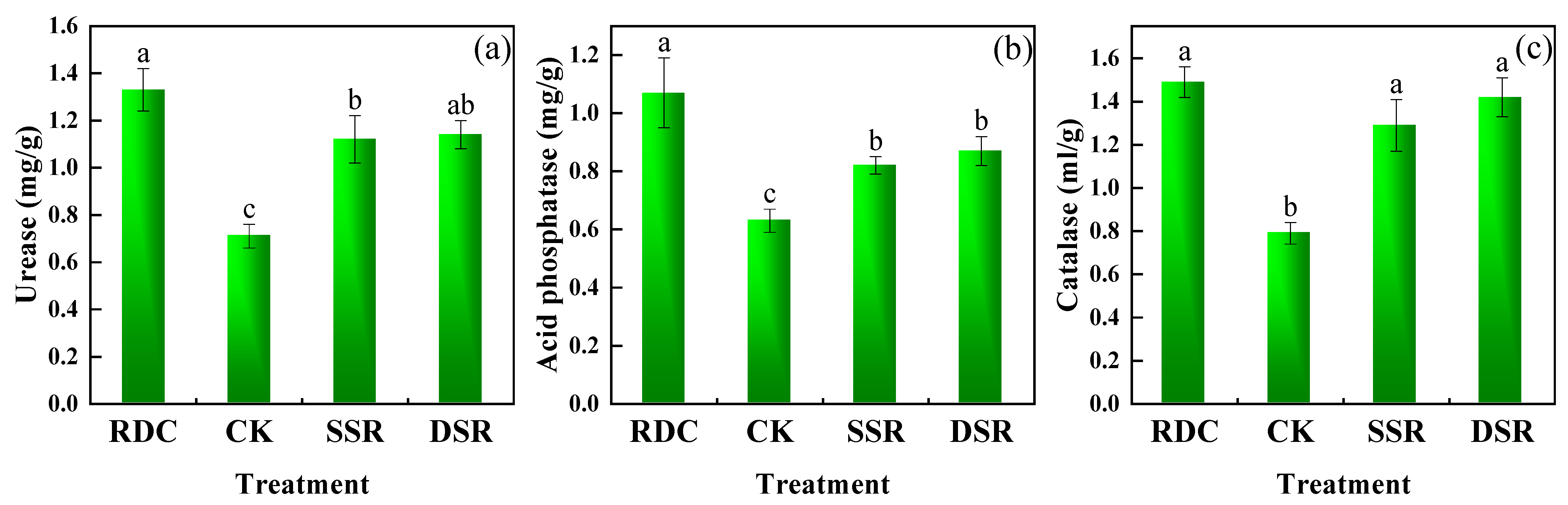
3.1.3. Soil Enzyme Activity
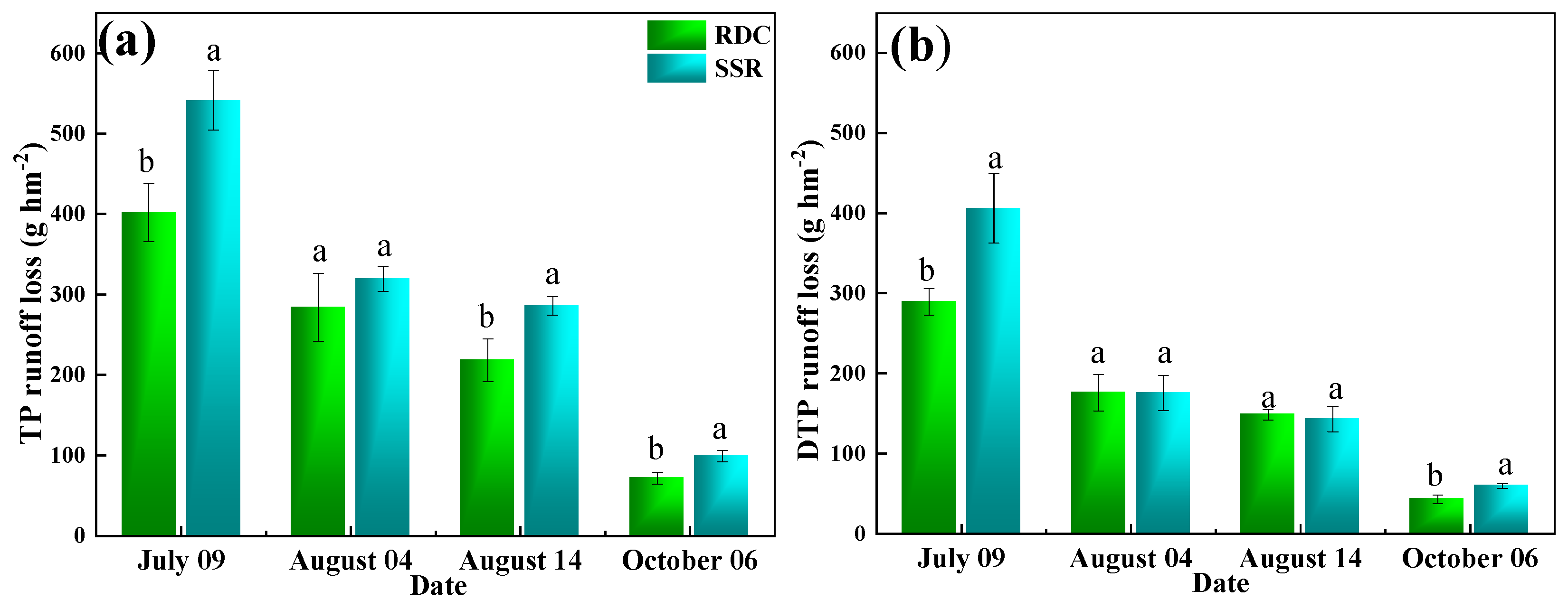
3.2. Effect of RDC and SSR Systems on Nitrogen and Phosphorus Loss via Runoff
3.3. Soil HMs in Different Groups
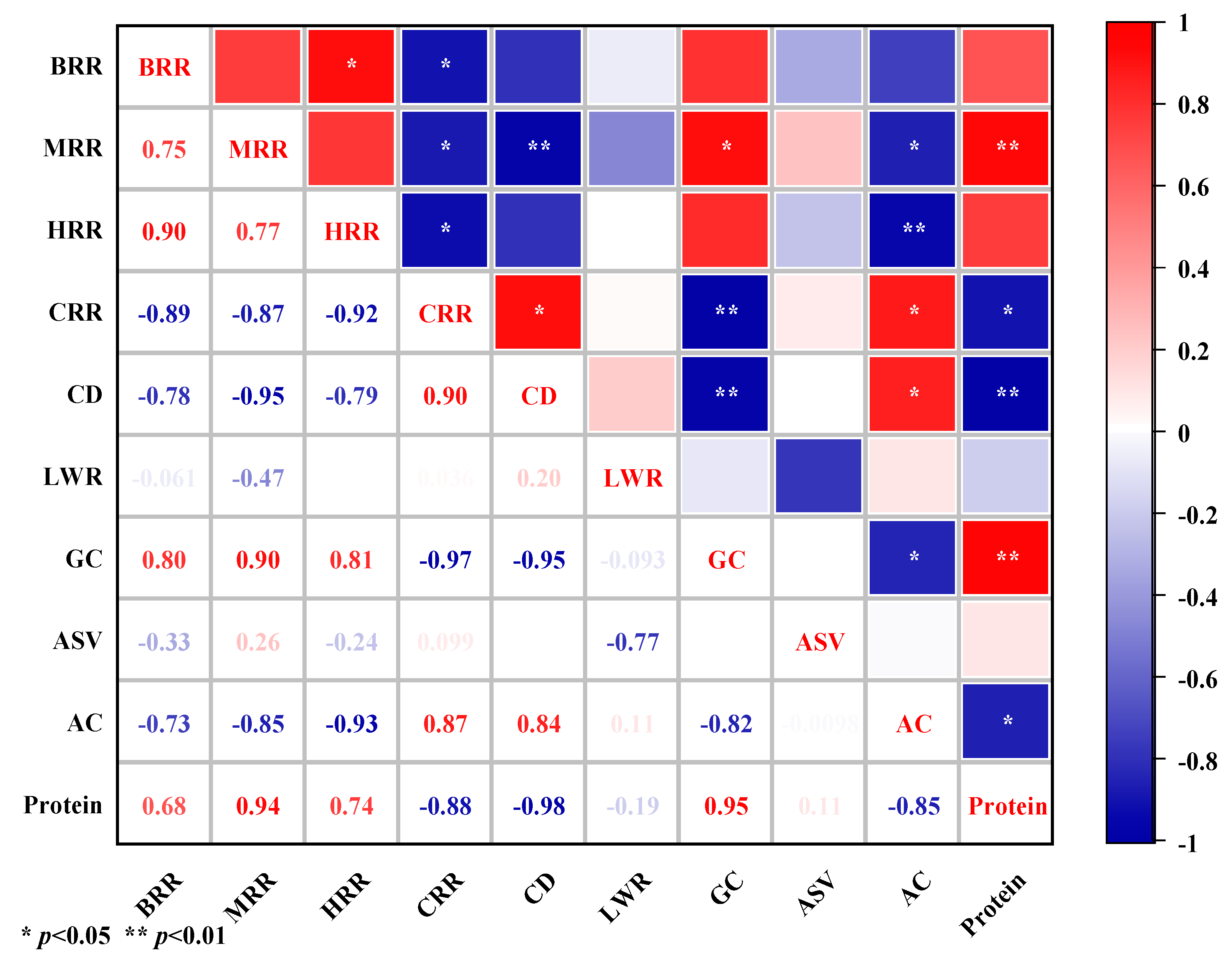
3.4. Rice Quality Promotion in the RDC System
3.5. Ecological, Environmental, and Economic Benefits of the RDC
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Frei, M.; Becker, K. Integrated rice-fish culture: Coupled production saves resources. Nat. Resour. Forum 2005, 29, 135–143. [Google Scholar] [CrossRef]
- Wu, W.; Ma, B.; Uphoff, N. A review of the system of rice intensification in China. Plant Soil 2015, 393, 361–381. [Google Scholar] [CrossRef]
- Ge, Y.; Zhang, J.; Zhang, L.; Yang, M.; He, J. Long-term fertilization regimes affect bacterial community structure and diversity of an agricultural soil in northern China. J. Soils Sediments 2008, 8, 43–50. [Google Scholar] [CrossRef]
- van Groenigen, K.J.; van Kessel, C.; Hungate, B.A. Increased greenhouse-gas intensity of rice production under future atmospheric conditions. Nat. Clim. Chang. 2013, 3, 288–291. [Google Scholar] [CrossRef]
- Yang, B.; Ma, Y.; Zhang, C.; Jia, Y.; Li, B.; Zheng, X. Cleaner Production Technologies Increased Economic Benefits and Greenhouse Gas Intensity in an Eco-Rice System in China. Sustainability 2019, 11, 7090. [Google Scholar] [CrossRef] [Green Version]
- Cassman, K.G.; Dobermann, A.; Walters, D.T. Agroecosystems, Nitrogen-use Efficiency, and Nitrogen Management. AMBIO A J. Hum. Environ. 2002, 31, 132–140. [Google Scholar] [CrossRef]
- Liang, G.; Liangliang, H.; Lufeng, Z.; Xiaoyu, S.; Zijun, J.; Lilian, D.; Weizheng, R.; Jian, Z.; Jianjun, T.; Xin, C. Coupling Rice with Fish for Sustainable Yields and Soil Fertility in China. Rice Sci. 2020, 27, 175–179. [Google Scholar] [CrossRef]
- Si, G.; Yuan, J.; Xu, X.; Zhao, S.; Peng, C.; Wu, J.; Zhou, Z. Effects of an integrated rice-crayfish farming system on soil organic carbon, enzyme activity, and microbial diversity in waterlogged paddy soil. Acta Ecol. Sin. 2018, 38, 29–35. [Google Scholar] [CrossRef]
- Teng, Q.; Hu, X.-F.; Luo, F.; Cheng, C.; Ge, X.; Yang, M.; Liu, L. Influences of introducing frogs in the paddy fields on soil properties and rice growth. J. Soils Sediments 2016, 16, 51–61. [Google Scholar] [CrossRef]
- Bashir, M.A.; Wang, H.; Pan, J.; Khoshnevisan, B.; Sun, W.; Zhai, L.; Zhang, X.; Wang, N.; Rehim, A.; Liu, H. Variations in soil nutrient dynamics and their composition in rice under integrated rice-crab co-culture system. J. Clean. Prod. 2021, 281, 125222. [Google Scholar] [CrossRef]
- Teng, Q.; Hu, X.-F.; Cheng, C.; Luo, Z.; Luo, F.; Xue, Y.; Jiang, Y.; Mu, Z.; Liu, L.; Yang, M. Ecological effects of rice-duck integrated farming on soil fertility and weed and pest control. J. Soils Sediments 2016, 16, 2395–2407. [Google Scholar] [CrossRef]
- Liu, G.; Huang, H.; Zhou, J. Energy analysis and economic assessment of a rice-turtle-fish co-culture system. Agroecol. Sustain. Food Syst. 2019, 43, 299–309. [Google Scholar] [CrossRef]
- Yifan, L.; Tiaoyan, W.; Shaodong, W.; Xucan, K.; Zhaoman, Z.; Hongyan, L.; Jiaolong, L. Developing integrated rice-animal farming based on climate and farmers choices. Agric. Syst. 2023, 204, 103554. [Google Scholar] [CrossRef]
- Bashir, M.A.; Liu, J.; Geng, Y.; Wang, H.; Pan, J.; Zhang, D.; Rehim, A.; Aon, M.; Liu, H. Co-culture of rice and aquatic animals: An integrated system to achieve production and environmental sustainability. J. Clean. Prod. 2020, 249, 119310. [Google Scholar] [CrossRef]
- Cardinale, B.J.; Harvey, C.T.; Gross, K.; Ives, A.R. Biodiversity and biocontrol: Emergent impacts of a multi-enemy assemblage on pest suppression and crop yield in an agroecosystem. Ecol. Lett. 2003, 6, 857–865. [Google Scholar] [CrossRef]
- Yuan, P.; Wang, J.; Guo, C.; Guo, Z.; Guo, Y.; Cao, C. Sustainability of the rice–crayfish farming model in waterlogged land: A case study in Qianjiang County, Hubei Province, China. J. Integr. Agric. 2022, 21, 1203–1214. [Google Scholar] [CrossRef]
- Hu, Z.; Wu, S.; Ji, C.; Zou, J.; Zhou, Q.; Liu, S. A comparison of methane emissions following rice paddies conversion to crab-fish farming wetlands in southeast China. Environ. Sci. Pollut. Res. 2016, 23, 1505–1515. [Google Scholar] [CrossRef]
- Xu, Q.; Dai, L.; Gao, P.; Dou, Z. The environmental, nutritional, and economic benefits of rice-aquaculture animal coculture in China. Energy 2022, 249, 123723. [Google Scholar] [CrossRef]
- Sheng, F.; Cao, C.; Li, C. Integrated rice-duck farming decreases global warming potential and increases net ecosystem economic budget in central China. Environ. Sci. Pollut. Res. 2018, 25, 22744–22753. [Google Scholar] [CrossRef]
- Zheng, H.; Huang, H.; Chen, C.; Fu, Z.; Xu, H.; Tan, S.; She, W.; Liao, X.; Tang, J. Traditional symbiotic farming technology in China promotes the sustainability of a flooded rice production system. Sustain. Sci. 2017, 12, 155–161. [Google Scholar] [CrossRef]
- Li, M.; Li, R.; Zhang, J.; Wu, T.; Liu, S.; Hei, Z.; Qiu, S. Effects of the integration of mixed-cropping and rice–duck co-culture on rice yield and soil nutrients in southern China. J. Sci. Food Agric. 2020, 100, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Zhang, J.; Ren, W.; Guo, L.; Cheng, Y.; Li, J.; Li, K.; Zhu, Z.; Zhang, J.; Luo, S.; et al. Can the co-cultivation of rice and fish help sustain rice production? Sci. Rep. 2016, 6, 28728. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Yu, D.; Zhou, J.; Zhai, S.; Bian, X.; Weih, M. Rice-duck co-culture for reducing negative impacts of biogas slurry application in rice production systems. J. Environ. Manag. 2018, 213, 142–150. [Google Scholar] [CrossRef]
- Yang, C.; Yang, P.; Geng, J.; Yin, H.; Chen, K. Sediment internal nutrient loading in the most polluted area of a shallow eutrophic lake (Lake Chaohu, China) and its contribution to lake eutrophication. Environ. Pollut. 2020, 262, 114292. [Google Scholar] [CrossRef]
- Liu, E.; Shen, J.; Birch, G.F.; Yang, X.; Wu, Y.; Xue, B. Human-induced change in sedimentary trace metals and phosphorus in Chaohu Lake, China, over the past half-millennium. J. Paleolimnol. 2012, 47, 677–691. [Google Scholar] [CrossRef]
- GB 3838-2002; Environmental Quality Standards for Surface Water. Ministry of Ecology and Environment of China: Beijing, China, 2002.
- Min, M.; Duan, X.; Yan, W.; Miao, C. Quantitative simulation of the relationships between cultivated land-use patterns and non-point source pollutant loads at a township scale in Chaohu Lake Basin, China. CATENA 2022, 208, 105776. [Google Scholar] [CrossRef]
- Shu, Q. Land use patterns of different scale units in Chaohu Lake Basin and their impact on river water quality. Ph.D. Thesis, Nanjing Agricultural University, Nanjing, China, 2016. [Google Scholar]
- Chen, Y.; Liu, D.; Ma, J.; Jin, B.; Peng, J.; He, X. Assessing the influence of immobilization remediation of heavy metal contaminated farmland on the physical properties of soil. Sci. Total Environ. 2021, 781, 146773. [Google Scholar] [CrossRef]
- Bao, S.D. Soil and Agricultural Chemistry Analysis; China Agricultural Press: Beijing, China, 2000. [Google Scholar]
- Kumada, K.; Sato, O.; Ohsumi, Y.; Ohta, S. Humus composition of mountain soils in Central Japan with special reference to the distribution of P type humic acid. Soil Sci. Plant Nutr. 1967, 13, 151–158. [Google Scholar] [CrossRef] [Green Version]
- Guan, S.Y.; Zhang, D.; Zhang, Z. Soil enzyme and its research methods; Agricultural Press: Beijing, China, 1986. [Google Scholar]
- Pueyo, M.; Mateu, J.; Rigol, A.; Vidal, M.; López-Sánchez, J.F.; Rauret, G. Use of the modified BCR three-step sequential extraction procedure for the study of trace element dynamics in contaminated soils. Environ. Pollut. 2008, 152, 330–341. [Google Scholar] [CrossRef]
- GB 11893-89; Water Quality—Determination of Total Phosphorus—Ammonium Molybdate Spectrophotometric Method. Ministry of Ecology and Environment of China: Beijing, China, 1989.
- GB 11894-89; Water Quality—Determination of Total Nitrogen—Alkaline Potassium Persulfate digestion-UV spectrophotometric Method. Ministry of Ecology and Environment of China: Beijing, China, 1989.
- HJ 535-2009; Water Quality Determination of Ammonia Nitrogen. Nessler’s Reagent Spectrophotometry. Ministry of Ecology and Environment of China: Beijing, China, 2009.
- NY/T 83-2017; Determination of Rice Quality. Ministry of Agriculture of the People’s Republic of China: Beijing, China, 2017.
- Eshky, A.; Atkinson, R.; Taylor, A. Physiological ecology of crabs from Saudi Arabian mangrove. Mar. Ecol. Prog. 1995, 126, 83–95. [Google Scholar] [CrossRef]
- Sarr, M.; Agbogba, C.; Russell-Smith, A.; Masse, D. Effects of soil faunal activity and woody shrubs on water infiltration rates in a semi-arid fallow of Senegal. Appl. Soil Ecol. 2001, 16, 283–290. [Google Scholar] [CrossRef]
- Yu, S.M.; Zhu, L.F.; Ouyang, Y.N.; Xu, J.Y.; Zhang, J.H.; Xu, D.H.; Jin, Q.Y. Influence of rice-duck farming system on soil physical and chemical properties, fertility factors and yield in paddy field. Chin. J. Soil Sci. 2014, 45, 6. [Google Scholar]
- Xu, G.; Liu, X.; Wang, Q.; Yu, X.; Hang, Y. Integrated rice-duck farming mitigates the global warming potential in rice season. Sci. Total Environ. 2017, 575, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Yuan, P.; Wang, J.; Li, C.; Xiao, Q.; Liu, Q.; Sun, Z.; Wang, J.; Cao, C. Soil quality indicators of integrated rice-crayfish farming in the Jianghan Plain, China using a minimum data set. Soil Tillage Res. 2020, 204, 104732. [Google Scholar] [CrossRef]
- Adani, F.; Genevini, P.; Ricca, G.; Tambone, F.; Montoneri, E. Modification of soil humic matter after 4 years of compost application. Waste Manag. 2007, 27, 319–324. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, Y.; Zong, Y.; Hu, Z.; Wu, S.; Zhou, J.; Jin, Y.; Zou, J. Response of soil carbon dioxide fluxes, soil organic carbon and microbial biomass carbon to biochar amendment: A meta-analysis. GCB Bioenergy 2016, 8, 392–406. [Google Scholar] [CrossRef]
- Li, F.; Kong, Q.; Zhang, Q.; Wang, H.; Wang, L.; Luo, T. Spent mushroom substrates affect soil humus composition, microbial biomass and functional diversity in paddy fields. Appl. Soil Ecol. 2020, 149, 103489. [Google Scholar] [CrossRef]
- Zhang, J.; Hu, F.; Li, H.; Gao, Q.; Song, X.; Ke, X.; Wang, L. Effects of earthworm activity on humus composition and humic acid characteristics of soil in a maize residue amended rice–wheat rotation agroecosystem. Appl. Soil Ecol. 2011, 51, 1–8. [Google Scholar] [CrossRef]
- Mi, W.; Sun, Y.; Gao, Q.; Liu, M.; Wu, L. Changes in humus carbon fractions in paddy soil given different organic amendments and mineral fertilizers. Soil Tillage Res. 2019, 195, 104421. [Google Scholar] [CrossRef]
- Chen, X.; Wu, J.; Opoku-Kwanowaa, Y. Effects of Returning Granular Corn Straw on Soil Humus Composition and Humic Acid Structure Characteristics in Saline-Alkali Soil. Sustainability 2020, 12, 1005. [Google Scholar] [CrossRef] [Green Version]
- Lazcano, C.; Gómez-Brandón, M.; Revilla, P.; Domínguez, J. Short-term effects of organic and inorganic fertilizers on soil microbial community structure and function: A field study with sweet corn. Biol. Fertil. Soils 2013, 49, 723–733. [Google Scholar] [CrossRef]
- Xu, C.; Li, Y.; Hu, X.; Zang, Q.; Zhuang, H.; Huang, L. The Influence of Organic and Conventional Cultivation Patterns on Physicochemical Property, Enzyme Activity and Microbial Community Characteristics of Paddy Soil. Agriculture 2022, 12, 121. [Google Scholar] [CrossRef]
- Fu, J.; Jian, Y.; Wu, Y.; Chen, D.; Zhao, X.; Ma, Y.; Niu, S.; Wang, Y.; Zhang, F.; Xu, C.; et al. Nationwide estimates of nitrogen and phosphorus losses via runoff from rice paddies using data-constrained model simulations. J. Clean. Prod. 2021, 279, 123642. [Google Scholar] [CrossRef]
- Liu, J.; Ouyang, X.; Shen, J.; Li, Y.; Sun, W.; Jiang, W.; Wu, J. Nitrogen and phosphorus runoff losses were influenced by chemical fertilization but not by pesticide application in a double rice-cropping system in the subtropical hilly region of China. Sci. Total Environ. 2020, 715, 136852. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.-W.; Lim, S.-S.; Kwak, J.-H.; Park, H.-J.; Yoon, K.-S.; Kim, H.-Y.; Baek, W.-J.; Choi, W.-J. Further Understanding of the Impacts of Rainfall and Agricultural Management Practices on Nutrient Loss from Rice Paddies in a Monsoon Area. Water Air Soil Pollut. 2015, 11, 283. [Google Scholar] [CrossRef]
- Krupa, M.; Tate, K.W.; van Kessel, C.; Sarwar, N.; Linquist, B.A. Water quality in rice-growing watersheds in a Mediterranean climate. Agric. Ecosyst. Environ. 2011, 144, 290–301. [Google Scholar] [CrossRef]
- Zhao, Z.; Yue, Y.; Sha, Z.; Li, C.; Deng, J.; Zhang, H.; Gao, M.; Cao, L. Assessing impacts of alternative fertilizer management practices on both nitrogen loading and greenhouse gas emissions in rice cultivation. Atmos. Environ. 2015, 119, 393–401. [Google Scholar] [CrossRef]
- Cao, Y.; Sun, H.; Liu, Y.; Fu, Z.; Chen, G.; Zou, G.; Zhou, S. Reducing N losses through surface runoff from rice-wheat rotation by improving fertilizer management. Environ. Sci. Pollut. Res. 2017, 24, 4841–4850. [Google Scholar] [CrossRef]
- GB 15618-2018; Soil Environment Quality Risk Control Standard for Soil Contamination of Agriculture Land. Ministry of Ecology and Environment of China: Beijing, China, 2018.
- Qiutong, X.; Mingkui, Z. Source identification and exchangeability of heavy metals accumulated in vegetable soils in the coastal plain of eastern Zhejiang province, China. Ecotoxicol. Environ. Saf. 2017, 142, 410–416. [Google Scholar] [CrossRef]
- Ning, C.; Gao, P.; Wang, B.; Lin, W.; Jiang, N.; Cai, K. Impacts of chemical fertilizer reduction and organic amendments supplementation on soil nutrient, enzyme activity and heavy metal content. J. Integr. Agric. 2017, 16, 1819–1831. [Google Scholar] [CrossRef] [Green Version]
- Yu, H.-Y.; Liu, C.; Zhu, J.; Li, F.; Deng, D.-M.; Wang, Q.; Chengshuai, L. Cadmium availability in rice paddy fields from a mining area: The effects of soil properties highlighting iron fractions and pH value. Environ. Pollut. 2016, 209, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Huang, Q.; Wang, Q.; Yu, Y.; Su, D.; Qiao, Y.; Li, H. Accumulation and bioavailability of heavy metals in an acid soil and their uptake by paddy rice under continuous application of chicken and swine manure. J. Hazard. Mater. 2020, 384, 121293. [Google Scholar] [CrossRef] [PubMed]
- Halim, M.A.; Majumder, R.K.; Zaman, M.N. Paddy soil heavy metal contamination and uptake in rice plants from the adjacent area of Barapukuria coal mine, northwest Bangladesh. Arab. J. Geosci. 2015, 8, 3391–3401. [Google Scholar] [CrossRef]
- Shaheen, S.M.; Rinklebe, J. Impact of emerging and low cost alternative amendments on the (im)mobilization and phytoavailability of Cd and Pb in a contaminated floodplain soil. Ecol. Eng. 2015, 74, 319–326. [Google Scholar] [CrossRef]
- Yu, Y.; Wan, Y.; Camara, A.Y.; Li, H. Effects of the addition and aging of humic acid-based amendments on the solubility of Cd in soil solution and its accumulation in rice. Chemosphere 2018, 196, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.M.; Zhong, L.J. Variation of Rice Quality Traits under Different Climate Conditions and Its Main Affected Factors. Chin. J. Rice Sci. 2001, 15, 187–191. [Google Scholar]
- Pernollet, C.A.; Simpson, D.; Gauthier-Clerc, M.; Guillemain, M. Rice and duck, a good combination? Identifying the incentives and triggers for joint rice farming and wild duck conservation. Agric. Ecosyst. Environ. 2015, 214, 118–132. [Google Scholar] [CrossRef]
- Wang, J.L.; Li, J.; Cao, Y.Y. Effects of rice-duck mutualistic organic farming on the rice quality in the Yellow River Delta, China. Chin. J. Appl. Ecol. 2016, 27, 6. [Google Scholar]
- GB/T 17891-2017; High Quality Paddy; Standardization Administration of the People’s Republic of China: Beijing, China, 2017.
- Hu, X.; Chen Yang, Y.; Zhou, K.; Tian, G.; Liu, B.; He, H.; Zhang, L.; Cao, Y.; Bian, B. Verification of agricultural cleaner production through rice-duck farming system and two-stage aerobic composting of typical organic waste. J. Clean. Prod. 2022, 337, 130576. [Google Scholar] [CrossRef]
- Wang, J.Q.; Zhang, X.D.; Jiang, L.F.; Bertness, M.D.; Fang, C.M.; Chen, J.K.; Hara, T.; Li, B. Bioturbation of Burrowing Crabs Promotes Sediment Turnover and Carbon and Nitrogen Movements in an Estuarine Salt Marsh. Ecosystems 2010, 13, 586–599. [Google Scholar] [CrossRef]
- Costanza, R.; de Groot, R.; Braat, L.; Kubiszewski, I.; Fioramonti, L.; Sutton, P.; Farber, S.; Grasso, M. Twenty years of ecosystem services: How far have we come and how far do we still need to go? Ecosyst. Serv. 2017, 28, 1–16. [Google Scholar] [CrossRef]
- Droste, N.; D’Amato, D.; Goddard, J.J. Where communities intermingle, diversity grows—The evolution of topics in ecosystem service research. PLoS ONE 2018, 13, e0204749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Q.; Liu, T.; Guo, H.; Dou, Z.; Gao, H.; Zhang, H. Conversion from rice–wheat rotation to rice–crayfish coculture increases net ecosystem service values in Hung-tse Lake area, east China. J. Clean. Prod. 2021, 319, 128883. [Google Scholar] [CrossRef]





| Groups | Processing | Fertilizer Application (kg hm−2) | Pesticide Application | Total Planting Area (hm2) |
|---|---|---|---|---|
| Rice–duck–crayfish ecological co-culture system (RDC) | First stage: paddy field (crayfish: 375 kg hm−2); later stage: paddy field (195 ducks hm−2). | Base fertilizer: 300; supplementary fertilizer: 75 | - | 5.34 |
| Idle field (CK) | - | - | - | 0.45 |
| Single-season rice planting system (SSR) | Inactive in the first period; late-season rice planting. | Base fertilizer: 600; supplementary fertilizer: 150 | 3 kg hm−2 | 0.67 |
| Double-season rice planting system (DSR) | Planting rice at the first stage; later planting of late rice | Base fertilizer: 600; supplementary fertilizer: 150 (one season) | 3 kg hm−2 | 1.34 |
| Indicators | P-Cu | P-Zn | P-Cd | P-Pb | P-As | P-Cr | Nemerow Index (PN) |
|---|---|---|---|---|---|---|---|
| RDC | 0.28 | 0.58 | 0.75 | 0.90 | 0.75 | 0.09 | 0.75 |
| CK | 0.25 | 0.60 | 0.63 | 0.85 | 0.72 | 0.11 | 0.71 |
| SSR | 0.29 | 0.81 | 0.83 | 0.98 | 0.69 | 0.09 | 0.82 |
| DSR | 0.23 | 0.62 | 0.76 | 1.03 | 0.67 | 0.14 | 0.83 |
| Treatment | Rice | Procambarus clarkii. Duck | Total Output Value (¥ hm−2) | Total Input Value (¥ hm−2) | Profit (¥ hm−2) | |||
|---|---|---|---|---|---|---|---|---|
| Yield (t hm−2) | Output Value (¥ hm−2) | Input Value (¥ hm−2) | Output Value (Million hm−2) | Input Value (¥ hm−2) | ||||
| RDC | 8.39 ± 0.17 | 2.07 × 104 | 1.30 × 104 | 3.38 × 104 | 1.67 × 104 | 5.45 × 104 | 3.00 × 104 | 2.45 × 104 |
| SSR | 7.96 ± 0.11 | 2.19 × 104 | 1.16 × 104 | - | - | 2.19 × 104 | 1.16 × 104 | 1.03 × 104 |
| DSR | 8.16 ± 0.20 | 2.13 × 104 | 1.21 × 104 | - | - | 4.49 × 104 | 2.42 × 104 | 2.07 × 104 |
| Ecosystem Service (¥ hm−2 Year−1) | RDC | SSR | DSR |
|---|---|---|---|
| Food supply | 5.45 × 104 | 2.19 × 104 | 4.49 × 104 |
| Gas regulation | 2.99 × 103 | 2.83 × 103 | 5.81 × 103 |
| Temperature regulation | 3.30 × 104 | 3.30 × 104 | 3.30 × 104 |
| Flood-control storage | 3.87 × 103 | 3.78 × 103 | 3.78 × 103 |
| Soil maintenance | 1.00 × 104 | 9.82 × 103 | 1.13 × 104 |
| Waste disposal | 4.87 × 102 | 3.83 × 102 | 3.83 × 102 |
| Destruction of arable land | −1.83 × 103 | 0.00 | 0.00 |
| GHG emissions | −1.50 × 103 | −1.70 × 103 | −3.4 × 103 |
| Total value | 1.02 × 105 | 7.00 × 104 | 9.58 × 104 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, J.; Yu, J.; Huang, W.; Pan, X.; Li, Y.; Li, S.; Tao, Y.; Zhang, K.; Zhang, X. Initial Studies on the Effect of the Rice–Duck–Crayfish Ecological Co-Culture System on Physical, Chemical, and Microbiological Properties of Soils: A Field Case Study in Chaohu Lake Basin, Southeast China. Int. J. Environ. Res. Public Health 2023, 20, 2006. https://doi.org/10.3390/ijerph20032006
Yan J, Yu J, Huang W, Pan X, Li Y, Li S, Tao Y, Zhang K, Zhang X. Initial Studies on the Effect of the Rice–Duck–Crayfish Ecological Co-Culture System on Physical, Chemical, and Microbiological Properties of Soils: A Field Case Study in Chaohu Lake Basin, Southeast China. International Journal of Environmental Research and Public Health. 2023; 20(3):2006. https://doi.org/10.3390/ijerph20032006
Chicago/Turabian StyleYan, Jun, Jingwei Yu, Wei Huang, Xiaoxue Pan, Yucheng Li, Shunyao Li, Yalu Tao, Kang Zhang, and Xuesheng Zhang. 2023. "Initial Studies on the Effect of the Rice–Duck–Crayfish Ecological Co-Culture System on Physical, Chemical, and Microbiological Properties of Soils: A Field Case Study in Chaohu Lake Basin, Southeast China" International Journal of Environmental Research and Public Health 20, no. 3: 2006. https://doi.org/10.3390/ijerph20032006




