The Incremental Role of Multiorgan Point-of-Care Ultrasounds in the Emergency Setting
Abstract
1. Introduction
2. Lung Ultrasounds in the Emergency Department
2.1. Technical Equipment and Methodology
2.2. Pleural Disorders
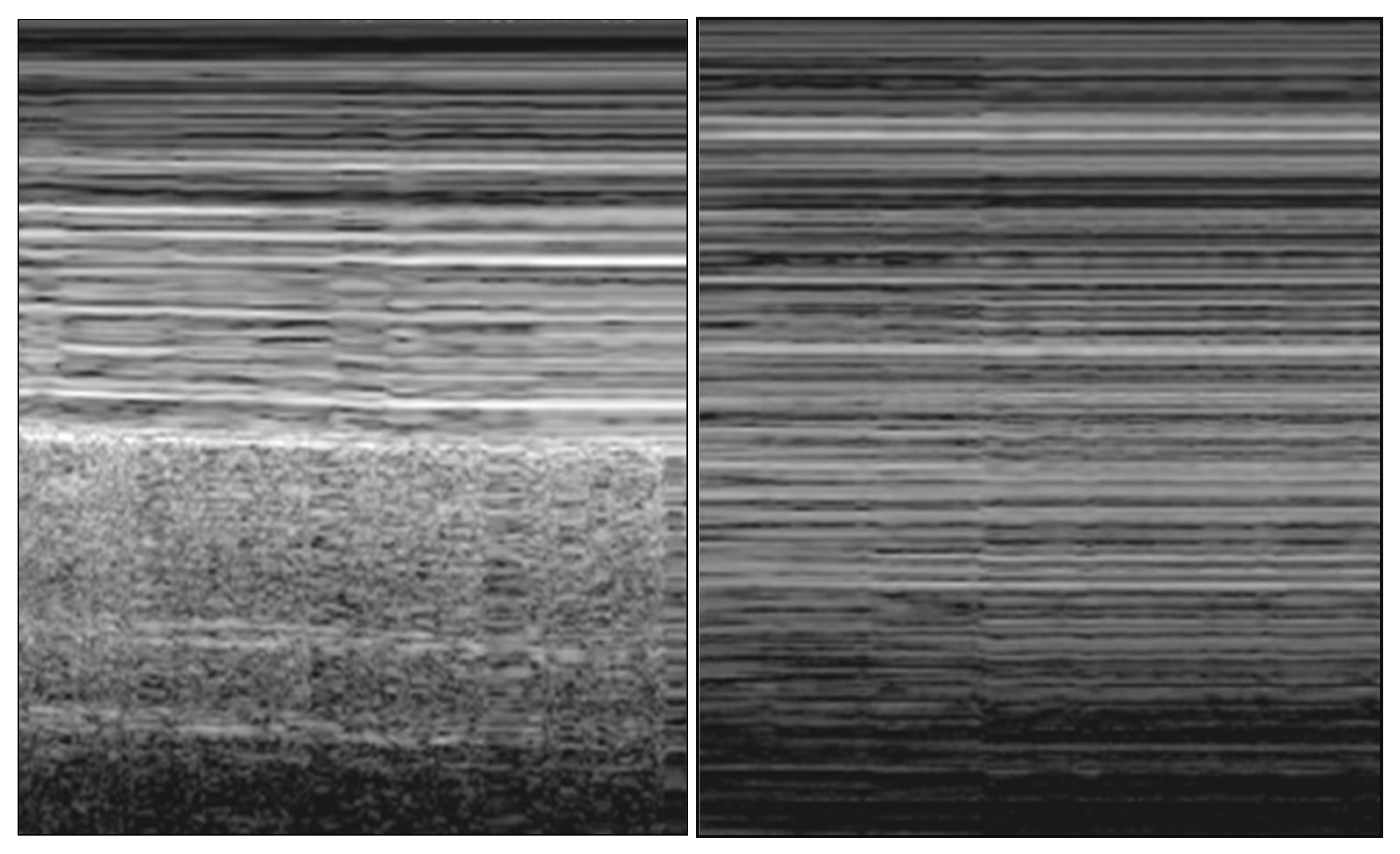
2.2.1. Pneumothorax
- (A)
- Abolition of lung sliding
- (B)
- A-lines
- (C)
- Lung point
2.2.2. Pleural Effusion
2.3. Lung Parenchymal Disorders
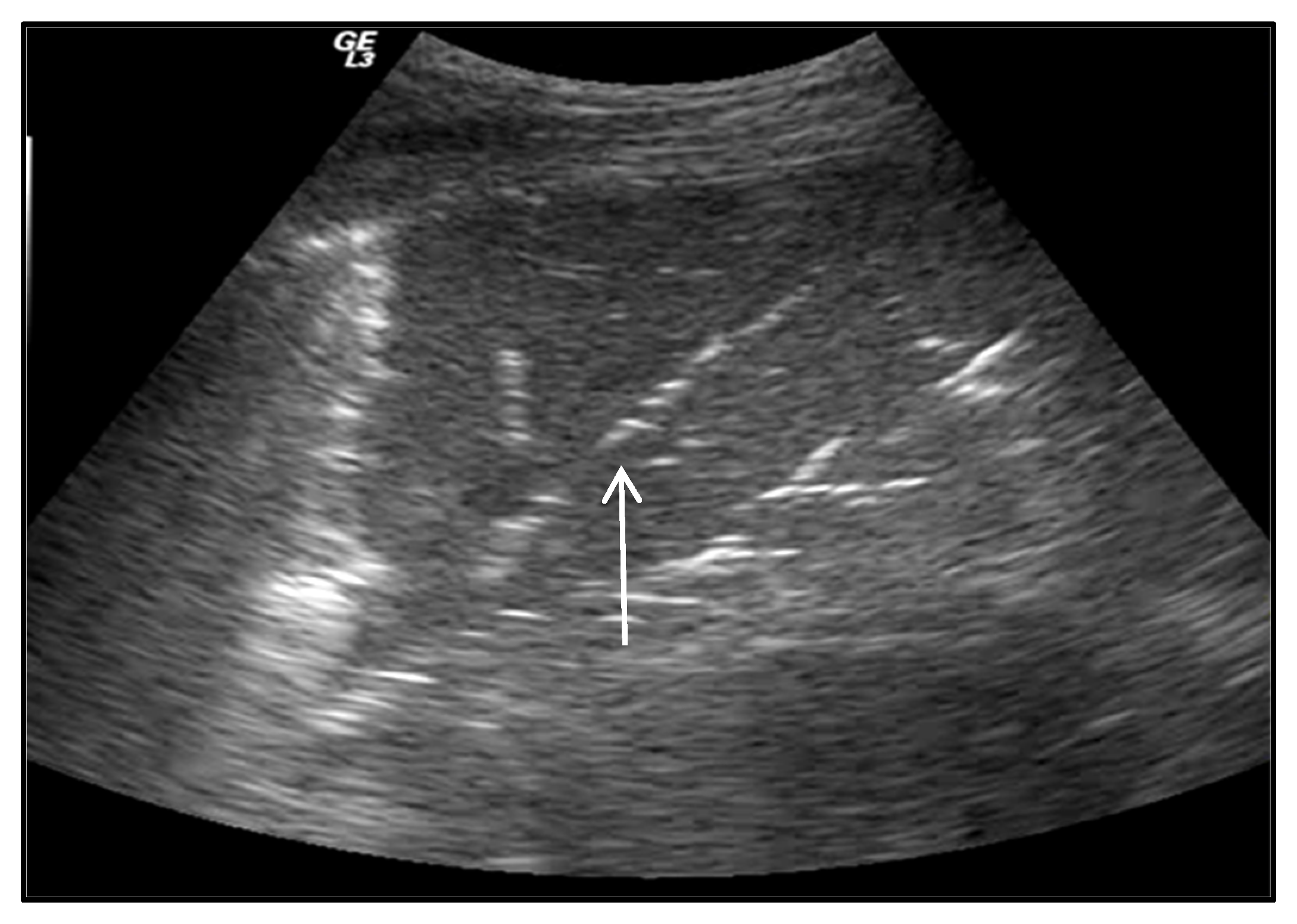
2.3.1. Interstitial Syndrome
2.3.2. Lung Consolidation
2.3.3. Pulmonary Embolism
3. Cardiac Ultrasounds in the Emergency Department
3.1. Rationale and Methodology
3.2. Shock
- Distributive.
- Hypovolemic.
- Obstructive.
- Cardiogenic.
- (A)
- Estimation of CVP and of blood volume
- (B)
- Fluid responsiveness and stroke volume evaluation
- (C)
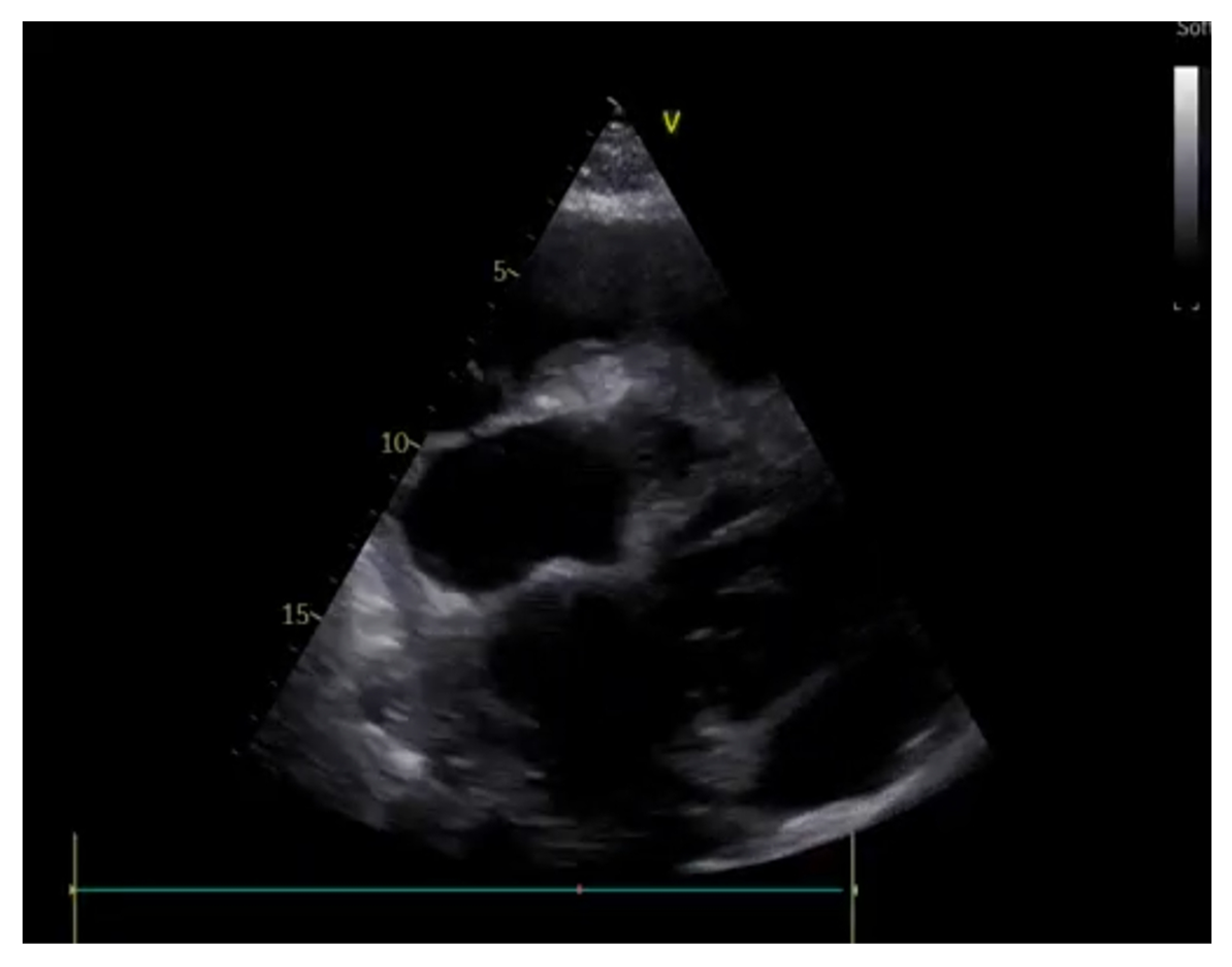
- Cardiac chambers size and systolic function
- (D)
- Diastolic function and Wedge pressure
- -
- An E/e’ < 8 is generally associated with a normal PWCP.
- -
- An E/e’ > 15 is associated with a high PWCP.
- -
- An E/e’ between 8 and 15 necessitates a multiparametric evaluation of diastolic function.
- (E)
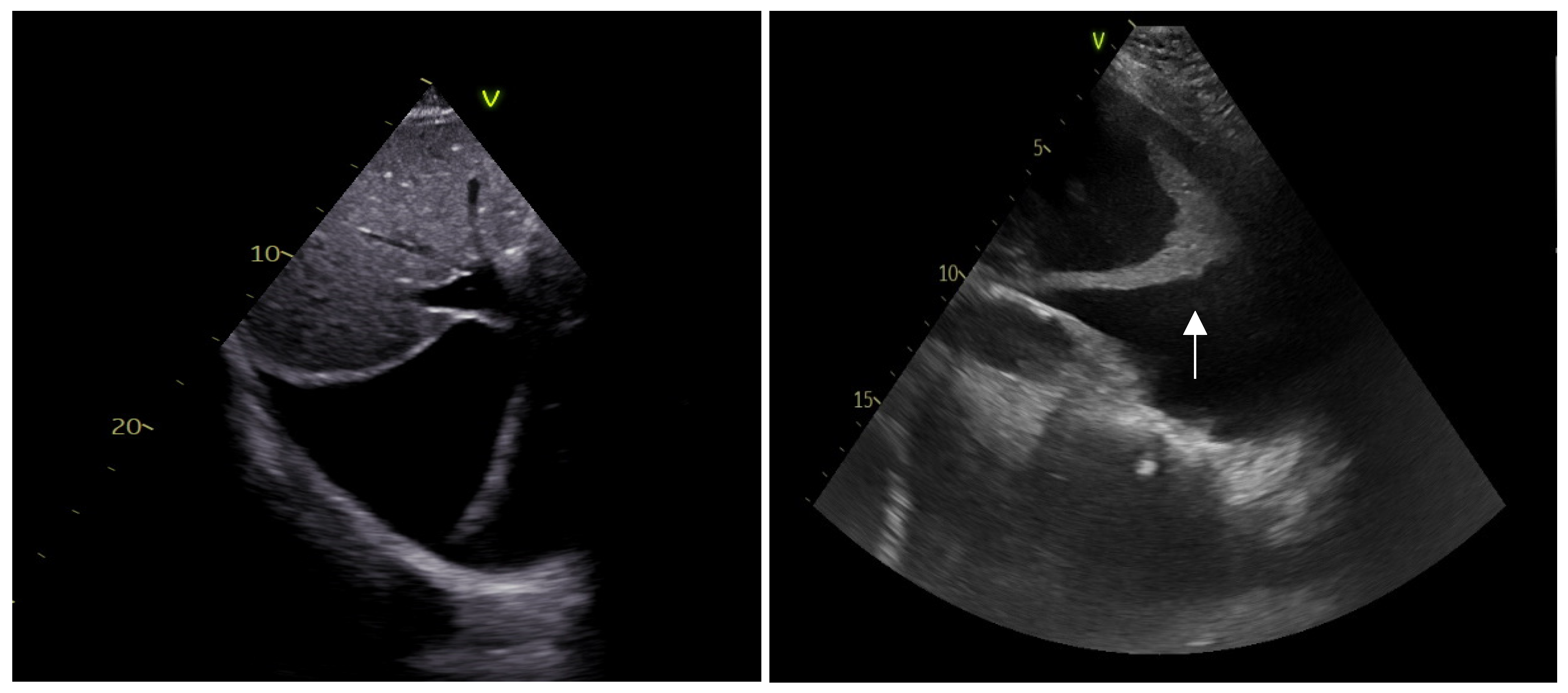
- Pericardium
3.3. Cardiac Arrest
4. Point-of-Care Abdominal Ultrasound
4.1. Acute Abdominal Pain
- (A)
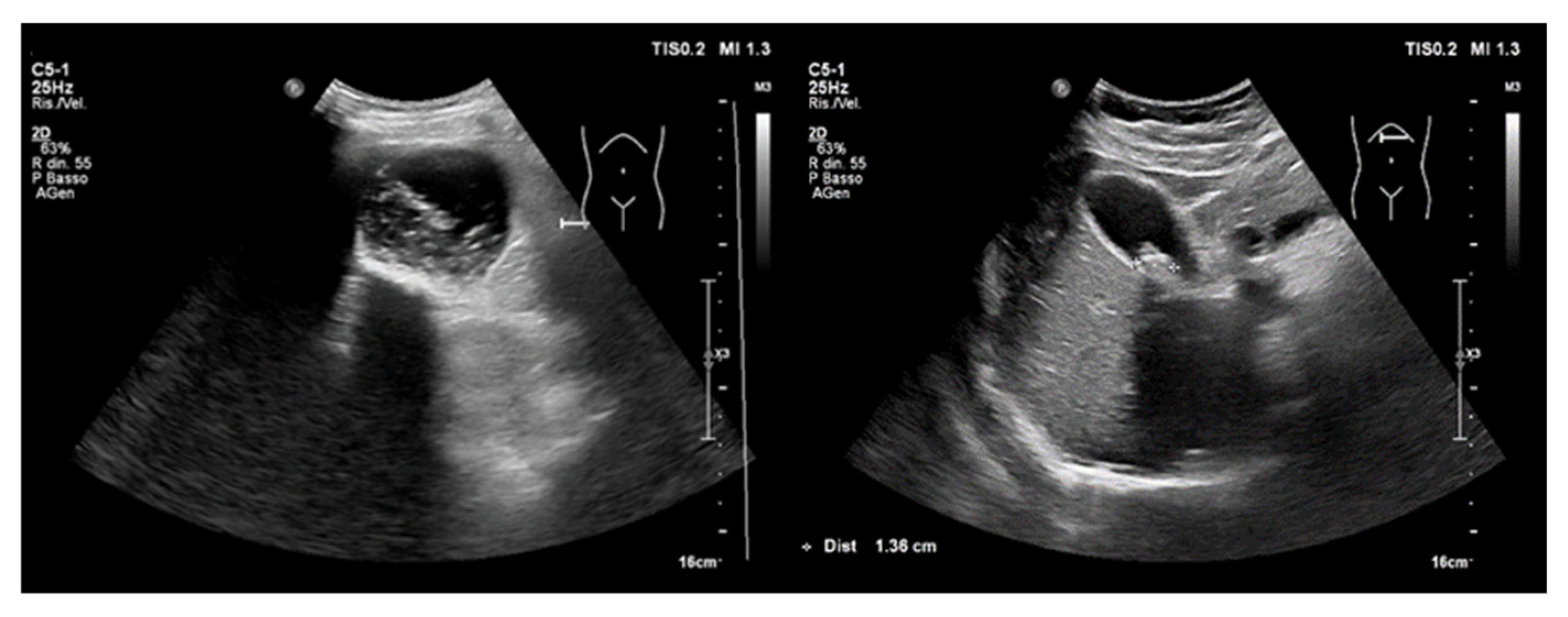
- Intraperitoneal free fluid
- (B)
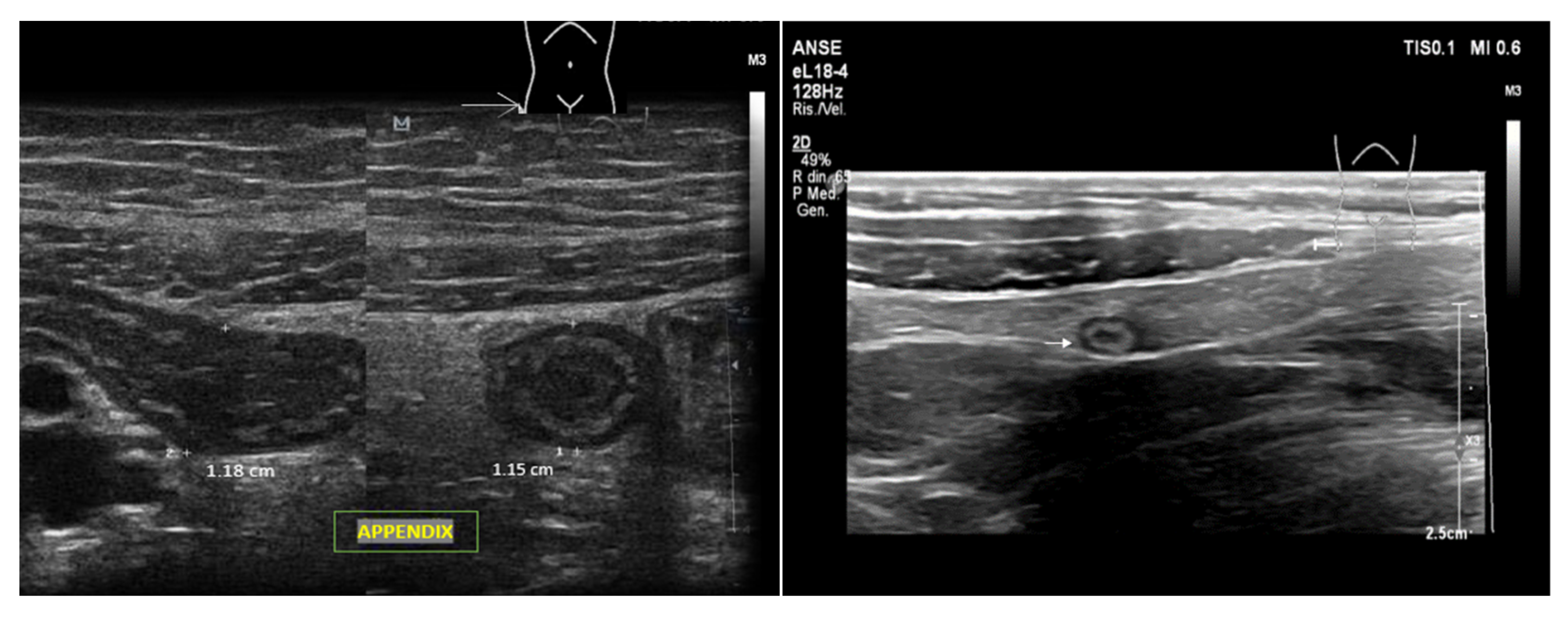
- Appendicitis and Cholecystitis
- (C)
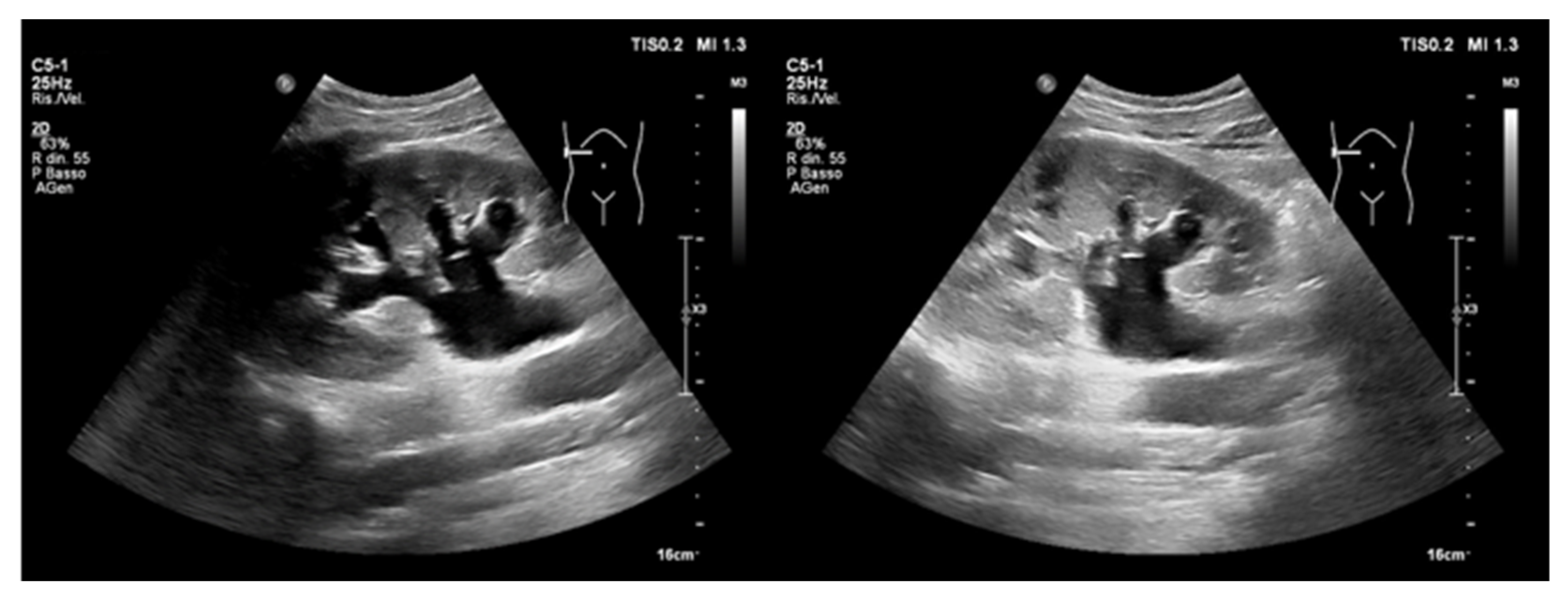
- Abdominal aortic aneurysm
4.2. Acute Kidney Injury
4.2.1. Hemodynamic AKI
4.2.2. Obstructive AKI
4.2.3. Renal Resistive Index
4.2.4. Chronic Kidney Disease
4.3. Point-of-Care Venous Doppler Ultrasound
4.3.1. Inferior Vena Cava
4.3.2. Hepatic Vein Flow
4.3.3. Portal Vein Flow
4.3.4. Intrarenal Vein Flow
5. Deep-Vein Thrombosis
5.1. Protocol
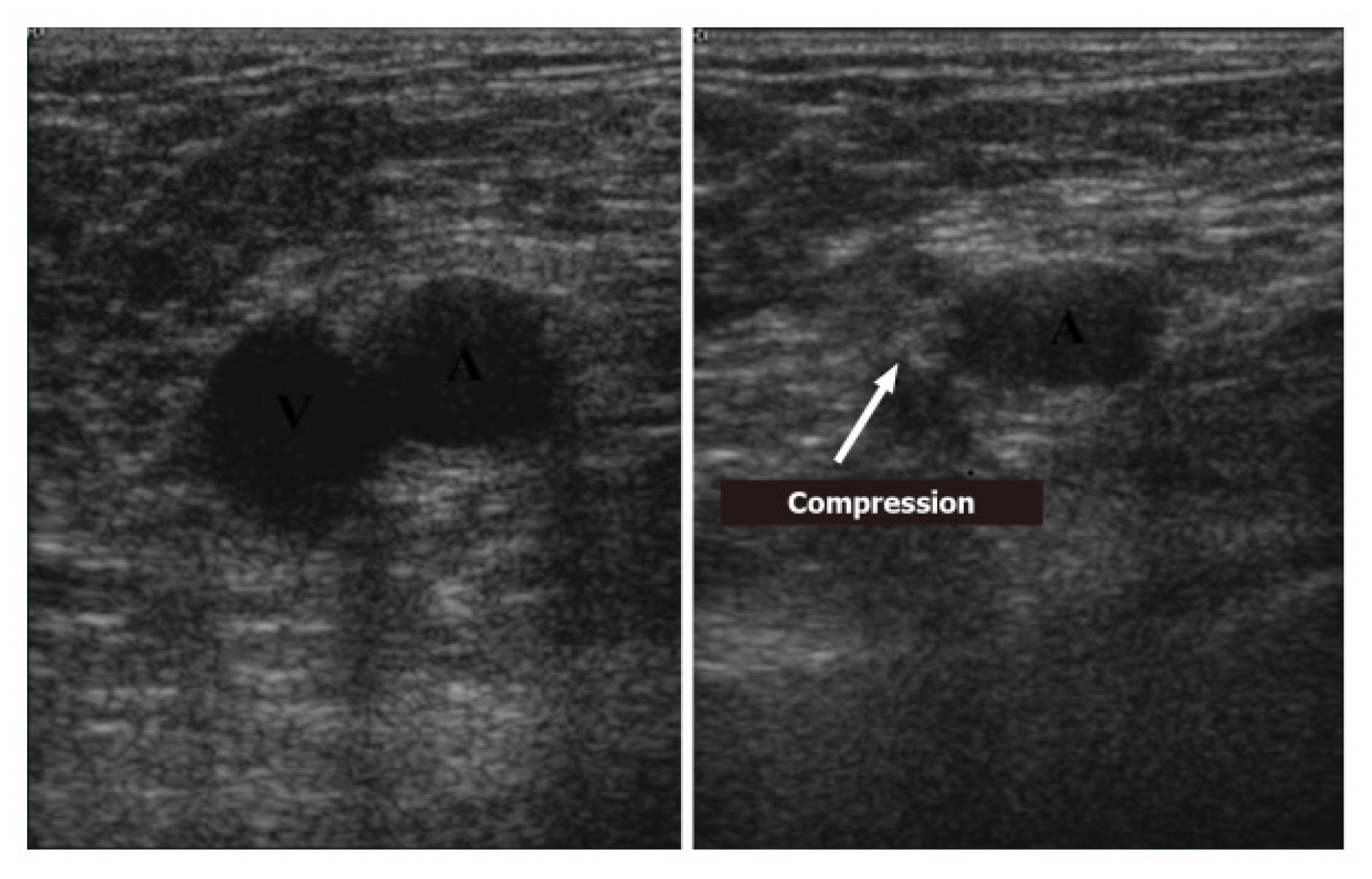
5.2. Imaging
- Acute: the thrombus itself may not be visible. If detected, it is deformable with the force applied on the probe and with a regular surface; it is a distended vein.
- Subacute thrombus: (before six months and after clot formation) intermediate morphological changes that cannot be included in the chronic phase.
- Chronic post-thrombotic change: not compressible nor deformable with an irregular surface; the vein caliber may be normal or reduced.
5.3. Accuracy of POCUS in Detecting a Pulse during Cardiac Arrest
5.4. TIA/Stroke Clinical Setting
6. Emerging Settings for POCUS Employment in Clinical Practice
6.1. Intracranial Pressure Monitoring
6.2. Guidance for Invasive Procedures in Critically ill Patients
6.3. Chest Traumatology
6.4. The emerging Role of Artificial Intelligence in POCUS
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- American College of Emergency Physicians. ACEP emergency ultrasound guidelines 2001. Ann. Emerg. Med. 2001, 38, 470–481. [Google Scholar] [CrossRef]
- Whitson, M.R.; Mayo, P.H. Ultrasonography in the emergency department. Crit. Care 2016, 20, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Manson, W.C.; Kirksey, M.; Boublik, J.; Wu, C.L.; Haskins, S.C. Focused assessment with sonography in trauma (FAST) for the regional anesthesiologist and pain specialist. Reg. Anesth. Pain Med. 2019, 44, 540–548. [Google Scholar] [CrossRef] [PubMed]
- Bahner, D.; Blaivas, M.; Cohen, H.L.; Fox, J.C.; Hoffenberg, S.; Kendall, J.; Langer, J.; McGahan, J.P.; Sierzenski, P.; Tayal, V.S.; et al. AIUM Practice Guideline for the Performance of the Focused Assessment with Sonography for Trauma (FAST) Examination. J. Ultrasound Med. 2008, 27, 313–318. [Google Scholar] [CrossRef]
- Lichtenstein, D.A.; Mezière, G.A. Relevance of Lung Ultrasound in the Diagnosis of Acute Respiratory Failure: The BLUE Protocol. Chest 2008, 134, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Volpicelli, G.; Elbarbary, M.; Blaivas, M.; Lichtenstein, D.A.; Mathis, G.; Kirkpatrick, A.W.; Melniker, L.; Gargani, L.; Noble, V.E.; Via, G.; et al. International evidence-based recommendations for point-of-care lung ultrasound. Intensive Care Med. 2012, 38, 577–591. [Google Scholar] [CrossRef]
- Volpicelli, G.; Cardinale, L.; Garofalo, G.; Veltri, A. Usefulness of lung ultrasound in the bedside distinction between pulmonary edema and exacerbation of COPD. Emerg. Radiol. 2008, 15, 145–151. [Google Scholar] [CrossRef]
- Demi, L.; Wolfram, F.; Klersy, C.; De Silvestri, A.; Ferretti, V.V.; Muller, M.; Miller, D.; Feletti, F.; Wełnicki, M.; Buda, N.; et al. New International Guidelines and Consensus on the Use of Lung Ultrasound. J. Ultrasound Med. 2022, 42, 309–344. [Google Scholar] [CrossRef]
- Lichtenstein, D.; Meziere, G.; Biderman, P.; Gepner, A.; Barrè, O. The comet- tail artifact: An ultrasound sign of alveolar-interstitial syndrome. Am. J. Respir. Crit. Care Med. 1997, 156, 1640–1646. [Google Scholar] [CrossRef]
- Lichtenstein, D.A.; Mezière, G.; Lascols, N.; Biderman, P.; Courret, J.-P.; Gepner, A.; Goldstein, I.; Tenoudji-Cohen, M. Ultrasound diagnosis of occult pneumothorax. Crit. Care Med. 2005, 33, 1231–1238. [Google Scholar] [CrossRef]
- Lichtenstein, D.A.; Menu, Y. A bedside ultrasound sign ruling out pneumothorax in the critically ill: Lung sliding. Chest 1995, 108, 1345–1348. [Google Scholar] [CrossRef]
- Lichtenstein, D.; Mezière, G.; Biderman, P.; Gepner, A. The “lung point”: An ultrasound sign specific to pneumothorax. Intensive Care Med. 2000, 26, 1434–1440. [Google Scholar] [CrossRef]
- Chiumello, D.; Mongodi, S.; Algieri, I.; Vergani, G.L.; Orlando, A.; Via, G.; Crimella, F.; Cressoni, M.; Mojoli, F. Assessment of Lung Aeration and Recruitment by CT Scan and Ultrasound in Acute Respiratory Distress Syndrome Patients. Crit. Care Med. 2018, 46, 1761–1768. [Google Scholar] [CrossRef]
- Lichtenstein, D.; Mezière, G.; Biderman, P.; Gepner, A. The comet-tail artifact: An ultrasound sign ruling out pneumothorax. Intensive Care Med. 1999, 25, 383–388. [Google Scholar] [CrossRef]
- Blaivas, M.; Lyon, M.; Duggal, S. A prospective comparison of supine chest radiography and bedside ultrasound for the diagnosis of traumatic pneumothorax. Acad. Emerg. Med. 2005, 12, 844–849. [Google Scholar] [CrossRef]
- Lichtenstein, D.A.; Lascols, N.; Prin, S.; Mezière, G. The “lung pulse”: An early ultrasound sign of complete atelectasis. Intensive Care Med. 2003, 29, 2187–2192. [Google Scholar] [CrossRef]
- Volpicelli, G.; Boero, E.; Sverzellati, N.; Cardinale, L.; Busso, M.; Boccuzzi, F.; Tullio, M.; Lamorte, A.; Stefanone, V.; Ferrari, G.; et al. Semi-quantification of pneumothorax volume by lung ultrasound. Intensive Care Med. 2014, 40, 1460–1467. [Google Scholar] [CrossRef]
- Doust, B.D.; Baum, J.K.; Maklad, N.F.; Doust, V.L. Ultrasonic evaluation of pleural opacities. Radiology 1975, 114, 135–140. [Google Scholar] [CrossRef]
- Lichtenstein, D.A. Lung Ultrasound in the Critically Ill. Ann. Intensive Care 2014, 4, 1–12. [Google Scholar] [CrossRef]
- Lichtenstein, D.; Goldstein, I.; Mourgeon, E.; Cluzel, P.; Grenier, P.; Rouby, J.-J. Comparative Diagnostic Performances of Auscultation, Chest Radiography, and Lung Ultrasonography in Acute Respiratory Distress Syndrome. Anesthesiology 2004, 100, 9–15. [Google Scholar] [CrossRef]
- Lichtenstein, D. Should lung ultrasonography be more widely used in the assessment of acute respiratory disease? Expert Rev. Respir. Med. 2010, 4, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Yousefifard, M.; Baikpour, M.; Ghelichkhani, P.; Asady, H.; Nia, K.S.; Jafari, A.M.; Hosseini, M.; Safari, S. Screening Performance Characteristic of Ultrasonography and Radiography in Detection of Pleural Effusion; a Meta-Analysis. Emergency 2016, 4, 1–10. [Google Scholar] [PubMed]
- Bouhemad, B.; Zhang, M.; Lu, Q.; Rouby, J.-J. Clinical review: Bedside lung ultrasound in critical care practice. Crit. Care 2007, 11, 205. [Google Scholar] [CrossRef] [PubMed]
- Eibenberger, K.L.; Dock, W.I.; Ammann, M.E.; Dorffner, R.; Hormann, M.F.; Grabenwoger, F. Quantification of pleural effusions: Sonography versus radiography. Radiology 1994, 191, 681–684. [Google Scholar] [CrossRef] [PubMed]
- Roch, A.; Bojan, M.; Michelet, P.; Romain, F.; Bregeon, F.; Papazian, L.; Auffray, J.P. Usefulness of ultrasonography in predicting pleural effusions > 500 mL in patients receiving mechanical ventilation. Chest 2005, 127, 224–232. [Google Scholar] [CrossRef]
- Remerand, F.; Dellamonica, J.; Mao, Z.; Rouby, J.J. Direct bedside quantification of pleural effusion in ICU: A new sonographic method. Intensive Care Med. 2006, 32, S220. [Google Scholar]
- Lichtenstein, D.; Hulot, J.-S.; Rabiller, A.; Tostivint, I.; Mezière, G. Feasibility and safety of ultrasound-aided thoracentesis in mechanically ventilated patients. Intensive Care Med. 1999, 25, 955–958. [Google Scholar] [CrossRef]
- Lichtenstein, D. FALLS-protocol: Lung ultrasound in hemodynamic assessment of shock. Heart Lung Vessel. 2013, 5, 142–147. [Google Scholar]
- Lichtenstein, D.; Mezierè, G. A lung ultrasound sign allowing bedside distinction between pulmonary edema and COPD: The comet-tail artifact. Intensive Care Med. 1998, 24, 1331–1334. [Google Scholar] [CrossRef]
- Volpicelli, G.; Caramello, V.; Cardinale, L.; Mussa, A.; Bar, F.; Frascisco, M.F. Detection of sonographic B-lines in patients with normal lung or radiographic alveolar consolidation. J. Pharmacol. Exp. Ther. 2008, 14, 122–128. [Google Scholar]
- Copetti, R.; Soldati, G.; Copetti, P. Chest sonography: A useful tool to differentiate acute cardiogenic pulmonary edema from acute respiratory distress syndrome. Cardiovasc. Ultrasound 2008, 6, 16. [Google Scholar] [CrossRef]
- Yang, P.C.; Chang, D.B.; Yu, C.J.; Lee, Y.C.; Kuo, S.H.; Luh, K.T. Ultrasound guided percutaneous cutting biopsy for the diagnosis of pulmonary consolidations of unknown aetiology. Thorax 1992, 47, 457–460. [Google Scholar] [CrossRef]
- Reissig, A.; Copetti, R.; Mathis, G.; Mempel, C.; Schuler, A.; Zechner, P.; Aliberti, S.; Neumann, R.; Kroegel, C.; Hoyer, H. Lung ultrasound in the diagnosis and follow-up of community-acquired pneumonia: A prospective, multicenter, diagnostic accuracy study. Chest 2012, 142, 965–972. [Google Scholar] [CrossRef]
- Cortellaro, F.; Colombo, S.; Coen, D.; Duca, P. Lung ultrasound is an accurate diagnostic tool for the diagnosis of pneumonia in the emergency department. Emerg. Med. J. 2010, 29, 19–23. [Google Scholar] [CrossRef]
- Lichtenstein, D.A.; Lascols, N.; Mezière, G.; Gepner, A. Ultrasound diagnosis of alveolar consolidation in the critically ill. Intensive Care Med. 2004, 30, 276–281. [Google Scholar] [CrossRef]
- Weinberg, E.D.B.; Diakoumakis, E.; Kassner, E.; Seife, B.; Zvi, Z.; Kurian, J.; Levin, T.L.; Han, B.K.; Taragin, B.H.; Weinstein, S.; et al. The air bronchogram: Sonographic demonstration. Am. J. Roentgenol. 1986, 147, 593–595. [Google Scholar] [CrossRef]
- Lichtenstein, D.; Mezière, G.; Seitz, J. The dynamic air bronchogram: A lung ultrasound sign of alveolar consolidation ruling out atelectasis. Chest 2009, 135, 1421–1425. [Google Scholar] [CrossRef]
- Lee, F.C.Y. Lung ultrasound—A primary survey of the acutely dyspneic patient. J. Intensive Care 2016, 4, 57. [Google Scholar] [CrossRef]
- Shrestha, G.S. Point-of-care Ultrasonography in Critically Ill Patients. Kathmandu Univ. Med. J. 2015, 13, 83–87. [Google Scholar] [CrossRef]
- Squizzato, A.; Rancan, E.; Dentali, F.; Bonzini, M.; Guasti, L.; Steidl, L.; Mathis, G.; Ageno, W. Diagnostic accuracy of lung ultrasound for pulmonary embolism: A systematic review and meta-analysis. J. Thromb. Haemost. 2013, 11, 1269–1278. [Google Scholar] [CrossRef]
- Labovitz, A.J.; Noble, V.E.; Bierig, M.; Goldstein, S.A.; Jones, R.; Kort, S.; Porter, T.R.; Spencer, K.T.; Tayal, V.S.; Wei, K. Focused Cardiac Ultrasound in the Emergent Setting: A Consensus Statement of the American Society of Echocardiography and American College of Emergency Physicians. J. Am. Soc. Echocardiogr. 2010, 23, 1225–1230. [Google Scholar] [CrossRef] [PubMed]
- Cibinel, G.A. POCUS shock management: Point-of-care ultrasound in the integrated management of shock. Ital. J. Emerg. Med. 2022, 11, 57–71. [Google Scholar] [CrossRef]
- Berg, I.; Walpot, K.; Lamprecht, H.; Valois, M.; Lanctôt, J.-F.; Srour, N.; Brand, C.V.D. A Systemic Review on the Diagnostic Accuracy of Point-of-Care Ultrasound in Patients with Undifferentiated Shock in the Emergency Department. Cureus 2022, 14, e23188. [Google Scholar] [CrossRef] [PubMed]
- Stickles, S.P.; Carpenter, C.R.; Gekle, R.; Kraus, C.K.; Scoville, C.; Theodoro, D.; Tran, V.H.; Ubiñas, G.; Raio, C. The diagnostic accuracy of a point-of-care ultrasound protocol for shock etiology: Asystematic review and meta-analysis. Can. J. Emerg. Med. 2019, 21, 406–417. [Google Scholar] [CrossRef] [PubMed]
- Perera, P.; Mailhot, T.; Riley, D.; Mandavia, D. The RUSH Exam: Rapid Ultrasound in Shock in the Evaluation of the Critically lll. Emerg. Med. Clin. 2010, 28, 29–56. [Google Scholar] [CrossRef]
- Porter, T.R.; Shillcutt, S.K.; Adams, M.S.; Desjardins, G.; Glas, K.E.; Olson, J.J.; Troughton, R.W. Guidelines for the Use of Echocardiography as a Monitor for Therapeutic Intervention in Adults: A Report from the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 2015, 28, 40–56. [Google Scholar] [CrossRef]
- Kim, D.-W.; Chung, S.; Kang, W.-S.; Kim, J. Diagnostic Accuracy of Ultrasonographic Respiratory Variation in the Inferior Vena Cava, Subclavian Vein, Internal Jugular Vein, and Femoral Vein Diameter to Predict Fluid Responsiveness: A Systematic Review and Meta-Analysis. Diagnostics 2021, 12, 49. [Google Scholar] [CrossRef]
- Monnet, X.; Shi, R.; Teboul, J.L. Prediction of fluid responsiveness. What’s new? Ann. Intensive Care 2022, 12, 46. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39. [Google Scholar] [CrossRef]
- Moore, C.L.; Rose, G.A.; Tayal, V.S.; Sullivan, D.M.; Arrowood, J.A.; Kline, J.A. Determination of Left Ventricular Function by Emergency Physician Echocardiography of Hypotensive Patients. Acad. Emerg. Med. 2002, 9, 186–193. [Google Scholar] [CrossRef]
- Lodato, J.A.; Ward, R.P.; Lang, R.M. Echocardiographic predictors of pulmonary embolism in patients referred for helical CT. Echocardiography 2008, 25, 584–590. [Google Scholar] [CrossRef]
- Konstantinides, S.; Meyer, G.; Becattini, C.; Bueno, H.; Geersing, G.-J.; Harjola, V.-P.; Huisman, M.; Humbert, M.; Jennings, C.S.; Jiménez, D.; et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur. Heart J. 2020, 41, 543–603. [Google Scholar] [CrossRef]
- Nagueh, S.F.; Appleton, C.P.; Gillebert, T.C.; Marino, P.N.; Oh, J.K.; Smiseth, O.A.; Waggoner, A.D.; Flachskampf, F.A.; Pellikka, P.A.; Evangelisa, A. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2016, 29, 277–314. [Google Scholar] [CrossRef]
- Saito, Y.; Donohue, A.; Attai, S.; Vahdat, A.; Brar, R.; Handapangoda, I.; Chandraratna, P.A. The syndrome of cardiac tamponade with “small” pericardial effusion. Echocardiography 2008, 25, 321–327. [Google Scholar] [CrossRef]
- Ainsworth, C.D.; Salehian, O. Echo-guided pericardiocentesis: Let the bubbles show the way. Circulation 2011, 123, e210–e211. [Google Scholar] [CrossRef]
- Flint, N.; Siegel, R.J. Echo-Guided Pericardiocentesis: When and How Should It Be Performed? Curr. Cardiol. Rep. 2020, 22, 1–9. [Google Scholar] [CrossRef]
- Lalande, E.; Burwash-Brennan, T.; Burns, K.; Atkinson, P.; Lambert, M.; Jarman, B.; Lamprecht, H.; Banerjee, A.; Woo, M.Y. Is point-of-care ultrasound a reliable predictor of outcome during atraumatic, non-shockable cardiac arrest? A systematic review and meta-analysis from the SHoC investigators. Resuscitation 2019, 139, 159–166. [Google Scholar] [CrossRef]
- Soar, J.; Böttiger, B.W.; Carli, P.; Couper, K.; Deakin, C.D.; Djärv, T.; Lott, C.; Olasveengen, T.; Paal, P.; Pellis, T.; et al. European Resuscitation Council Guidelines 2021: Adult advanced life support. Resuscitation 2021, 161, 115–151. [Google Scholar] [CrossRef]
- Reynolds, J.C.; Issa, M.S.; Nicholson, T.C.; Drennan, I.R.; Berg, K.M.; O’Neil, B.J.; Welsford, M.; Andersen, L.W.; Böttiger, B.W.; Callaway, C.W.; et al. Prognostication with point-of-care echocardiography during cardiac arrest: A systematic review. Resuscitation 2020, 152, 56–68. [Google Scholar] [CrossRef]
- Ávila-Reyes, D.; Acevedo-Cardona, A.O.; Gómez-González, J.F.; Echeverry-Piedrahita, D.R.; Aguirre-Flórez, M.; Giraldo-Diaconeasa, A. Point-of-care ultrasound in cardiorespiratory arrest (POCUS-CA): Narrative review article. Ultrasound J. 2021, 13, 46. [Google Scholar] [CrossRef]
- Aagaard, R.; Granfeldt, A.; Bøtker, M.T.; Mygind-Klausen, T.; Kirkegaard, H.; Løfgren, B. The Right Ventricle Is Dilated during Resuscitation from Cardiac Arrest Caused by Hypovolemia: A Porcine Ultrasound Study. Crit. Care Med. 2017, 45, e963–e970. [Google Scholar] [CrossRef] [PubMed]
- Radonjić, T.; Popović, M.; Zdravković, M.; Jovanović, I.; Popadić, V.; Crnokrak, B.; Klašnja, S.; Mandić, O.; Dukić, M.; Branković, M. Point-of-Care Abdominal Ultrasonography (POCUS) on the Way to the Right and Rapid Diagnosis. Diagnostics 2022, 12, 2052. [Google Scholar] [CrossRef] [PubMed]
- Sternbach, G. Abdominal ultrasound in emergency medicine. J. Emerg. Med. 1984, 1, 547–548. [Google Scholar] [CrossRef] [PubMed]
- Kameda, T.; Taniguchi, N. Overview of point-of-care abdominal ultrasound in emergency and critical care. J. Intensive Care 2016, 4, 1–9. [Google Scholar] [CrossRef]
- Mazzei, M.A.; Guerrini, S.; Squitieri, N.C.; Cagini, L.; Macarini, L.; Coppolino, F.; Giganti, M.; Volterrani, L. The role of US examination in the management of acute abdomen. Crit. Ultrasound J. 2013, 5, S6. [Google Scholar] [CrossRef]
- Abu-Zidan, F.M.; Khan, M.A.B. Point-of-care ultrasound for the acute abdomen in the primary health care. Turk. J. Emerg. Med. 2020, 20, 1–11. [Google Scholar] [CrossRef]
- Holmes, J.F.; Harris, D.; Battistella, F.D. Performance of abdominal ultrasonography in blunt trauma patients with out-of-hospital or emergency department hypotension. Ann. Emerg. Med. 2004, 43, 354–361. [Google Scholar] [CrossRef]
- Liu, R.B.; Donroe, J.H.; McNamara, R.L.; Forman, H.P.; Moore, C.L. The Practice and Implications of Finding Fluid during Point-of-Care Ultrasonography: A Review. JAMA Intern. Med. 2017, 177, 1818–1825. [Google Scholar] [CrossRef]
- Kendall, J.L.; Shimp, R.J. Performance and interpretation of focused right upper quadrant ultrasound by emergency physicians. J. Emerg. Med. 2001, 21, 7–13. [Google Scholar] [CrossRef]
- Zenobii, M.F.; Accogli, E.; Domanico, A.; Arienti, V. Update on bedside ultrasound (US) diagnosis of acute cholecystitis (AC). Intern. Emerg. Med. 2015, 11, 261–264. [Google Scholar] [CrossRef]
- Summers, S.M.; Scruggs, W.; Menchine, M.D.; Lahham, S.; Anderson, C.; Amr, O.; Lotfipour, S.; Cusick, S.S.; Fox, J.C. A Prospective Evaluation of Emergency Department Bedside Ultrasonography for the Detection of Acute Cholecystitis. Ann. Emerg. Med. 2010, 56, 114–122. [Google Scholar] [CrossRef]
- Scruggs, W.; Fox, J.C.; Potts, B.; Zlidenny, A.; McDonough, J.; Anderson, C.L.; Larson, J.; Barajas, G.; Langdorf, M.I. Accuracy of ED Bedside Ultrasound for Identification of gallstones: Retrospective analysis of 575 studies. West. J. Emerg. Med. 2008, 9, 1–5. [Google Scholar]
- Benabbas, R.; Hanna, M.; Shah, J.; Sinert, R. Diagnostic Accuracy of History, Physical Examination, Laboratory Tests, and Point-of-Care Ultrasound for Pediatric Acute Appendicitis in the Emergency Department: A Systematic Review and Meta-Analysis. Acad. Emerg. Med. 2017, 24, 523–551. [Google Scholar] [CrossRef]
- Lee, S.H.; Seong, J.Y. Diagnostic performance of emergency physician-performed point-of-care ultrasonography for acute appendicitis: A meta-analysis. Am. J. Emerg. Med. 2019, 37, 696–705. [Google Scholar] [CrossRef]
- Fields, J.M.; Davis, J.; Alsup, C.; Bates, A.; Au, A.; Adhikari, S.; Farrell, I. Accuracy of Point-of-Care Ultrasonography for Diagnosing Acute Appendicitis: A Systematic Review and Meta-Analysis. Acad. Emerg. Med. 2017, 24, 1124–1136. [Google Scholar] [CrossRef]
- Mostbeck, G.; Adam, E.J.; Nielsen, M.B.; Claudon, M.; Clevert, D.A.; Nicolau, C.; Nyhsen, C.; Owens, C.M. How to diagnose acute appendicitis: Ultrasound first. Insights Imaging 2016, 7, 255–263. [Google Scholar] [CrossRef]
- Lam, S.H.; Grippo, A.; Kerwin, C.; Konicki, P.J.; Goodwine, D.; Lambert, M.J. Bedside Ultrasonography as an Adjunct to Routine Evaluation of Acute Appendicitis in the Emergency Department. West. J. Emerg. Med. 2014, 15, 808–815. [Google Scholar] [CrossRef]
- Costantino, T.G.; Bruno, E.C.; Handly, N.; Dean, A.J. Accuracy of Emergency Medicine Ultrasound in the Evaluation of Abdominal Aortic Aneurysm. J. Emerg. Med. 2005, 29, 455–460. [Google Scholar] [CrossRef]
- Moll, F.L.; Powell, J.T.; Fraedrich, G.; Verzini, F.; Haulon, S.; Waltham, M.; van Herwaarden, J.A.; Holt, P.J.E.; van Keulen, J.W.; Rantner, B.; et al. Management of Abdominal Aortic Aneurysms Clinical Practice Guidelines of the European Society for Vascular Surgery. Eur. J. Vasc. Endovasc. Surg. 2011, 41, S1–S58. [Google Scholar] [CrossRef]
- Rubano, E.; Mehta, N.; Caputo, W.; Paladino, L.; Sinert, R. Systematic Review: Emergency Department Bedside Ultrasonography for Diagnosing Suspected Abdominal Aortic Aneurysm. Acad. Emerg. Med. 2013, 20, 128–138. [Google Scholar] [CrossRef]
- Bravo-Merino, L.; González-Lozano, N.; Maroto-Salmón, R.; Meijide-Santos, G.; Suárez-Gil, P.; Fañanás-Mastral, A. Validity of the Abdominal Ecography in Primary Care for Detection of Aorta Abdominal Aneurism in Male between 65 and 75 Years. Aten. Primaria 2019, 51, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Hoste, E.A.J.; Bagshaw, S.M.; Bellomo, R.; Cely, C.M.; Colman, R.; Cruz, D.N.; Edipidis, K.; Forni, L.G.; Gomersall, C.D.; Govil, D.; et al. Epidemiology of acute kidney injury in critically ill patients: The multinational AKI-EPI study. Intensive Care Med. 2015, 41, 1411–1423. [Google Scholar] [CrossRef] [PubMed]
- Faubel, S.; Patel, N.U.; Lockhart, M.E.; Cadnapaphornchai, M.A. Renal Relevant Radiology: Use of Ultrasonography in Patients with AKI. Clin. J. Am. Soc. Nephrol. 2013, 9, 382–394. [Google Scholar] [CrossRef] [PubMed]
- Argaiz, E.R.; Koratala, A.; Reisinger, N. Comprehensive Assessment of Fluid Status by Point-of-Care Ultrasonography. Kidney360 2021, 2, 1326–1338. [Google Scholar] [CrossRef] [PubMed]
- Bissell, B.D.; Laine, M.E.; Thompson Bastin, M.L.; Flannery, A.H.; Kelly, A.; Riser, J.; Neyra, J.A.; Potter, J.; Morris, P.E. Impact of proto-colized diuresis for de-resuscitation in the intensive care unit. Crit. Care 2020, 24, 70. [Google Scholar] [CrossRef]
- Smith-Bindman, R.; Aubin, C.; Bailitz, J.; Bengiamin, R.N.; Camargo, C.A.; Corbo, J.; Dean, A.J.; Goldstein, R.B.; Griffey, R.T.; Jay, G.D.; et al. Ultrasonography versus Computed Tomography for Suspected Nephrolithiasis. N. Engl. J. Med. 2014, 371, 1100. [Google Scholar] [CrossRef]
- Wong, C.; Teitge, B.; Ross, M.; Young, P.; Robertson, H.L.; Lang, E. The Accuracy and Prognostic Value of Point-of-Care Ultrasound for Nephrolithiasis in the Emergency Department: A Systematic Review and Meta-Analysis. Acad. Emerg. Med. 2018, 25, 684–698. [Google Scholar] [CrossRef]
- Fields, J.M.; Fischer, J.I.; Anderson, K.L.; Mangili, A.; Panebianco, N.L.; Dean, A.J. The ability of renal ultrasound and ureteral jet evaluation to predict 30-day outcomes in patients with suspected nephrolithiasis. Am. J. Emerg. Med. 2015, 33, 1402–1406. [Google Scholar] [CrossRef]
- Capotondo, L.; Nicolai, G.A.; Garosi, G. The role of color Doppler in acute kidney injury. Arch. Ital. Urol. Androl. 2010, 82, 275–279. [Google Scholar]
- Schnell, D.; Deruddre, S.; Harrois, A.; Pottecher, J.; Cosson, C.; Adoui, N.; Benhamou, D.; Vicaut, E.; Azoulay, E.; Duranteau, J. Renal resistive indexbetter predicts the occurrence of acute kidney injury than cystatin C. Shock 2012, 38, 592–597. [Google Scholar] [CrossRef]
- Umgelter, A.; Reindl, W.; Franzen, M.; Lenhardt, C.; Huber, W.; Schmid, R.M. Renal resistive index and renal function before and after paracentesis in patients with hepatorenal syndrome and tense ascites. Intensive Care Med. 2008, 35, 152–156. [Google Scholar] [CrossRef]
- Iacoviello, M.; Monitillo, F.; Leone, M.; Citarelli, G.; Doronzo, A.; Antoncecchi, V.; Puzzovivo, A.; Rizzo, C.; Lattarulo, M.S.; Massari, F.; et al. The Renal Arterial Resistance Index Predicts Worsening Renal Function in Chronic Heart Failure Patients. Cardiorenal Med. 2016, 7, 42–49. [Google Scholar] [CrossRef]
- Goyal, S. Intrarenal resistance index (RI) as a predictor of early renal impairment in patients with liver cirrhosis. Trop. Gastroenterol. 2014, 34, 235–239. [Google Scholar] [CrossRef]
- Beland, M.D.; Walle, N.L.; Machan, J.T.; Cronan, J.J. Renal Cortical Thickness Measured at Ultrasound: Is It Better Than Renal Length as an Indicator of Renal Function in Chronic Kidney Disease? Am. J. Roentgenol. 2010, 195, W146–W149. [Google Scholar] [CrossRef]
- Yamashita, S.R.; Von Atzingen, A.C.; Iared, W.; Bezerra, A.S.D.A.; Ammirati, A.L.; Canziani, M.E.F.; D’Ippolito, G. Value of renal cortical thickness as a predictor of renal function impairment in chronic renal disease patients. Radiol. Bras. 2015, 48, 12–16. [Google Scholar] [CrossRef]
- Beaubien-Souligny, W.; Benkreira, A.; Robillard, P.; Bouabdallaoui, N.; Chasse, M.; Desjardins, G.; Lamarche, Y.; White, M.; Bouchard, J.; Denault, A. Alterations in portal vein flow and intrarenal venous flow are associated with acute kidney injury after cardiac surgery: A prospective observational cohort study. J. Am. Heart Assoc. 2018, 7, e009961. [Google Scholar] [CrossRef]
- Beaubien-Souligny, W.; Rola, P.; Haycock, K.; Bouchard, J.; Lamarche, Y.; Spiegel, R.; Denault, A.Y. Quantifying systemic congestion with Point-of-Care ultrasound: Development of the venous excess ultrasound grading system. Ultrasound J. 2020, 12, 1–12. [Google Scholar] [CrossRef]
- Abhilash, K.; Kazory, A. Point of Care Ultrasonography for Objective Assessment of Heart Failure: Integration of Cardiac, Vascular, and Extravascular Determinants of Volume Status. Cardiorenal Med. 2021, 11, 5–17. [Google Scholar]
- Saha, N.M.; Barbat, J.J.; Fedson, S.; Anderson, A.; Rich, J.D.; Spencer, K.T. Outpatient Use of Focused Cardiac Ultrasound to Assess the Inferior Vena Cava in Patients with Heart Failure. Am. J. Cardiol. 2015, 116, 1224–1228. [Google Scholar] [CrossRef]
- Feissel, M.; Michard, F.; Faller, J.-P.; Teboul, J.-L. The respiratory variation in inferior vena cava diameter as a guide to fluid therapy. Intensive Care Med. 2004, 30, 1834–1837. [Google Scholar] [CrossRef]
- Lombardi, A.; Gambardella, M.; Palermi, S.; Frecentese, F.; Serio, A.; Sperlongano, S.; Tavarozzi, R.; D’andrea, A.; De Luca, M.; Politi, C. Liver and heart failure: An ultrasound relationship. J. Basic Clin. Physiol. Pharmacol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Koratala, A. Utility of Doppler ultrasound derived hepatic and portal venous waveforms in the management of heart failure exacerbation. Clin. Case Rep. 2020, 8, 1489–1493. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Himura, Y.; Kumada, T. The characteristics of hepatic venous flow velocity pattern in patients with pulmonary hypertension by pulsed Doppler echocardiography. Jpn. Circ. J. 1992, 56, 317–324. [Google Scholar]
- Catalano, D.; Caruso, G.; Di Fazzio, S.; Carpinteri, G.; Scalisi, N.; Trovato, G.M. Portal vein pulsatility ratio and heart failure. J. Clin. Ultrasound 1998, 26, 27–31. [Google Scholar] [CrossRef]
- Shih, C.Y.; Yang, S.S.; Hu, J.T.; Lin, C.L.; Lai, Y.C.; Chang, C.W. Portal vein pulsatility index is a more important indicator than congestion index in the clinical evaluation of right heart function. World J. Gastroenterol. 2006, 12, 768–771. [Google Scholar] [CrossRef]
- Tang, W.H.W.; Kitai, T. Intrarenal venous flow: A window into the congestive kidney failure phenotype of heart failure? JACC Heart Fail. 2016, 4, 683–686. [Google Scholar] [CrossRef]
- Di Nisio, M.; Van Es, N.; Büller, H.R. Deep vein thrombosis and pulmonary embolism. Lancet 2016, 388, 3060–3073. [Google Scholar] [CrossRef]
- De Valois, J.C.; Schaik, C.C.; Verzijlbergen, F.; Ramshorst, B.; Eikelboom, B.C.; Meuwissen, O.J.A.T. Contrast venography: From gold standard to ‘golden backup’ in clinically suspected deep vein thrombosis. Eur. J. Radiol. 1990, 11, 131–137. [Google Scholar] [CrossRef]
- Pomero, F.; Dentali, F.; Borretta, V.; Bonzini, M.; Melchio, R.; Douketis, J.D.; Fenoglio, L.M. Accuracy of emergency physician-performed ultrasonography in the diagnosis of deep-vein thrombosis: A systematic review and meta-analysis. Thromb. Haemost. 2013, 109, 137–145. [Google Scholar] [CrossRef]
- Di Vilio, A.; Vergara, A.; Desiderio, A.; Iodice, F.; Serio, A.; Palermi, S.; Gambardella, F.; Sperlongano, S.; Gioia, R.; Acitorio, M.; et al. Incremental value of compression ultrasound sonography in the emergency department. World J. Crit. Care. Med. 2021, 10, 194–203. [Google Scholar] [CrossRef]
- Magazzini, S.; Vanni, S.; Toccafondi, S.; Paladini, B.; Zanobetti, M.; Giannazzo, G.; Federico, R.; Grifoni, S. Duplex ultrasound in the emergency department for the diagnostic management of clinically suspected deep vein thrombosis. Acad. Emerg. Med. 2007, 14, 216–220. [Google Scholar] [CrossRef]
- Burnside, P.R.; Brown, M.D.; Kline, J.A. Systematic review of emergency physician-performed ultrasonography for lower-extremity deep vein thrombosis. Acad. Emerg. Med. 2008, 15, 493–498. [Google Scholar] [CrossRef]
- Canakci, M.E.; Acar, N.; Bilgin, M.; Kuas, C. Diagnostic value of point-of-care ultrasound in deep vein thrombosis in the emergency department. J. Clin. Ultrasound 2020, 48, 527–531. [Google Scholar] [CrossRef]
- Varrias, D.; Palaiodimos, L.; Balasubramanian, P.; Barrera, C.A.; Nauka, P.; Arfaras-Melainis, A.; Zamora, C.; Zavras, P.; Napolitano, M.; Gulani, P.; et al. The Use of Point-of-Care Ultrasound (POCUS) in the Diagnosis of Deep Vein Thrombosis. J. Clin. Med. 2021, 10, 3903. [Google Scholar] [CrossRef]
- Kline, J.A.; O’Malley, P.M.; Tayal, V.S.; Snead, G.R.; Mitchell, A.M. Emergency Clinician–Performed Compression Ultrasonography for Deep Venous Thrombosis of the Lower Extremity. Ann. Emerg. Med. 2008, 52, 437–445. [Google Scholar] [CrossRef]
- Adhikari, S.; Zeger, W.; Thom, C.; Fields, J.M. Isolated Deep Venous Thrombosis: Implications for 2-Point Compression Ultra-sonography of the Lower Extremity. Ann. Emerg. Med. 2015, 66, 262–266. [Google Scholar] [CrossRef]
- Sanchez, S.; Miller, M.; Asha, S. Assessing the validity of two-dimensional carotid ultrasound to detect the presence and absence of a pulse. Resuscitation 2020, 157, 67–73. [Google Scholar] [CrossRef]
- Cohen, A.L.; Li, T.; Becker, L.B.; Owens, C.; Singh, N.; Gold, A.; Nelson, M.J.; Jafari, D.; Haddad, G.; Nello, A.V.; et al. Femoral artery Doppler ultrasound is more accurate than manual palpation for pulse detection in cardiac arrest. Resuscitation 2022, 173, 156–165. [Google Scholar] [CrossRef]
- Kang, S.Y.; Jo, I.J.; Lee, G.; Park, J.E.; Kim, T.; Lee, S.U.; Hwang, S.Y.; Shin, T.G.; Kim, K.; Shim, J.S.; et al. Point-of-care ultrasound compression of the carotid artery for pulse determination in cardiopulmonary resuscitation. Resuscitation 2022, 179, 206–213. [Google Scholar] [CrossRef]
- Suttie, R.; Woo, M.Y.; Park, L.; Nemnom, M.-J.; Stotts, G.; Perry, J.J. Can Emergency Physicians Perform Carotid Artery Point-of-Care Ultrasound to Detect Stenosis in Patients with TIA and Stroke? A Pilot Study. West. J. Emerg. Med. 2020, 21, 626–632. [Google Scholar] [CrossRef]
- Saxhaug, L.M.; Graven, T.; Olsen, O.; Kleinau, J.O.; Skjetne, K.; Ellekjær, H.; Dalen, H. Reliability and agreement of point-of-care carotid artery examinations by experts using hand-held ultrasound devices in patients with ischaemic stroke or transitory ischaemic attack. Open Heart 2022, 9, e001917. [Google Scholar] [CrossRef]
- Caldas, J.; Rynkowski, C.B.; Robba, C. POCUS, how can we include the brain? An overview. J. Anesth. Analg. Crit. Care 2022, 2, 1–16. [Google Scholar] [CrossRef]
- Meyfroidt, G.; Bouzat, P.; Casaer, M.P.; Chesnut, R.; Hamada, S.R.; Helbok, R.; Hutchinson, P.; Maas, A.I.R.; Manley, G.; Menon, D.K. Management of moderate to severe traumatic brain injury: An update for the intensivist. Intensive Care Med. 2022, 48, 649–666. [Google Scholar] [CrossRef]
- Cannata, G.; Pezzato, S.; Esposito, S.; Moscatelli, A. Optic Nerve Sheath Diameter Ultrasound: A Non-Invasive Approach to Evaluate Increased Intracranial Pressure in Critically Ill Pediatric Patients. Diagnostics 2022, 12, 767. [Google Scholar] [CrossRef] [PubMed]
- Richards, E.; Munakomi, S.; Mathew, D. Optic Nerve Sheath Ultrasound. In StatPearls; StatPearls Publishing: Tampa, FL, USA, 2022. [Google Scholar]
- Aslan, N.; Yıldızdaş, D.; Horoz, Ö.Ö.; Özsoy, M.; Yöntem, A.; Çetinalp, E.; Mert, G.G. Evaluation of ultrasonographic optic nerve sheath diameter and central retinal artery Doppler indices by point-of-care ultrasound in pediatric patients with increased intracranial pressure. Turk. J. Pediatr. 2021, 63, 300–306. [Google Scholar] [CrossRef] [PubMed]
- D’Andrea, A.; Fabiani, D.; Cante, L.; Caputo, A.; Sabatella, F.; Riegler, L.; Alfano, G.; Russo, V. Transcranial Doppler ultrasound: Clinical applications from neurological to cardiological setting. J. Clin. Ultrasound 2022, 50, 1212–1223. [Google Scholar] [CrossRef] [PubMed]
- Bouzat, P.; Oddo, M.; Payen, J.F. Transcranial Doppler after traumatic brain injury: Is there a role? Curr. Opin. Crit. Care 2014, 20, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Robba, C.; Pozzebon, S.; Moro, B.; Vincent, J.-L.; Creteur, J.; Taccone, F.S. Multimodal non-invasive assessment of intracranial hypertension: An observational study. Crit. Care 2020, 24, 1–10. [Google Scholar] [CrossRef]
- Duran-Gehring, P.E.; Guirgis, F.W.; McKee, K.C.; Goggans, S.; Tran, H.; Kalynych, C.J.; Wears, R.L. The bubble study: Ultrasound confirmation of central venous catheter placement. Am. J. Emerg. Med. 2015, 33, 315–319. [Google Scholar] [CrossRef]
- Montrief, T.; Alerhand, S.; Denault, A.; Scott, J. Point-of-care echocardiography for the evaluation of right-to-left cardiopulmonary shunts: A narrative review. Can. J. Anesth. 2020, 67, 1824–1838. [Google Scholar] [CrossRef]
- Amir, R.; Knio, Z.O.; Mahmood, F.; Oren-Grinberg, A.; Leibowitz, A.; Bose, R.; Shaefi, S.; Mitchell, J.D.; Ahmed, M.; Bardia, A.; et al. Ultrasound as a Screening Tool for Central Venous Catheter Positioning and Exclusion of Pneumothorax. Crit. Care Med. 2017, 45, 1192–1198. [Google Scholar] [CrossRef]
- Osman, A.; Sum, K.M. Role of upper airway ultrasound in airway management. J. Intensive Care 2016, 4, 1–7. [Google Scholar] [CrossRef]
- Hurley, M.E.; Keye, G.D.; Hamilton, S. Is ultrasound really helpful in the detection of rib fractures? Injury 2004, 35, 562–566. [Google Scholar] [CrossRef]
- Cameron, P.; Dziukas, L.; Hadj, A.; Clark, P.; Hooper, S. Rib Fractures in Major Trauma. Aust. N. Z. J. Surg. 1996, 66, 530–534. [Google Scholar] [CrossRef]
- Barnea, Y.; Kashtan, H.; Skornick, Y.; Werbin, N. Isolated rib fractures in elderly patients: Mortality and morbidity. Can. J. Surg. 2002, 45, 43–46. [Google Scholar]
- Kara, M.; Dikmen, E.; Erdal, H.H.; Simsir, I.; Kara, S.A. Disclosure of unnoticed rib fractures with the use of ultrasonography in minor blunt chest trauma. Eur. J. Cardio-Thorac. Surg. 2003, 24, 608–613. [Google Scholar] [CrossRef]
- Mathis, G. Thoraxsonography—Part I: Chest wall and pleura. Ultrasound Med. Biol. 1997, 23, 1131–1139. [Google Scholar] [CrossRef]
- Chan, S.S. Emergency bedside ultrasound to detect pneumothorax. Acad. Emerg. Med. 2003, 10, 91–94. [Google Scholar] [CrossRef]
- Ma, O.; Mateer, J.R. Trauma Ultrasound Examination Versus Chest Radiography in the Detection of Hemothorax. Ann. Emerg. Med. 1997, 29, 312–316. [Google Scholar] [CrossRef]
- Turk, F.; Kurt, A.B.; Saglam, S. Evaluation by ultrasound of traumatic rib fractures missed by radiography. Emerg. Radiol. 2010, 17, 473–477. [Google Scholar] [CrossRef]
- Crandall, J.; Kent, R.; Patrie, J.; Fertile, J.; Martin, P. Rib fracture patterns and radiologic detection—A restraint-based comparison. Annu. Proc. Assoc. Adv. Automot. Med. 2000, 44, 235–259. [Google Scholar] [PubMed]
- Çelik, A.; Akoglu, H.; Omercikoglu, S.; Bugdayci, O.; Karacabey, S.; Kabaroglu, K.A.; Onur, O.; Denizbasi, A. The Diagnostic Accuracy of Ultrasonography for the Diagnosis of Rib Fractures in Patients Presenting to Emergency Department with Blunt Chest Trauma. J. Emerg. Med. 2021, 60, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Lalande, É.; Guimont, C.; Émond, M.; Parent, M.C.; Topping, C.; Kuimi, B.L.B.; Boucher, V.; Le Sage, N. Feasibility of emergency department point-of-care ultrasound for rib fracture diagnosis in minor thoracic injury. Can. J. Emerg. Med. 2017, 19, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Griffith, J.; Rainer, T.; Ching, A.S.; Law, K.L.; Cocks, R.A.; Metreweli, C. Sonography compared with radiography in revealing acute rib fracture. Am. J. Roentgenol. 1999, 173, 1603–1609. [Google Scholar] [CrossRef]
- Narang, A.; Bae, R.; Hong, H.; Thomas, Y.; Surette, S.; Cadieu, C.; Chaudhry, A.; Martin, R.P.; McCarthy, P.M.; Rubenson, D.S.; et al. Utility of a Deep-Learning Algorithm to Guide Novices to Acquire Echocardiograms for Limited Diagnostic Use. JAMA Cardiol. 2021, 6, 624–632. [Google Scholar] [CrossRef]
- Chen, X.; Owen, C.A.; Huang, E.C.; Maggard, B.D.; Latif, R.K.; Clifford, S.P.; Li, J.; Huang, J. Artificial Intelligence in Echocardiography for Anesthesiologists. J. Cardiothorac. Vasc. Anesth. 2021, 35, 251–261. [Google Scholar] [CrossRef]
- Asch, F.M.; Abraham, T.; Jankowski, M.; Cleve, J.; Adams, M.; Romano, N.; Polivert, N.; Hong, H.; Lang, R. Accuracy and Reproducibility of a Novel Artificial Intelligence Deep Learning-Based Algorithm for Automated Calculation of Ejection Fraction in Echocardiography. J. Am. Coll. Cardiol. 2019, 73, 1447. [Google Scholar] [CrossRef]
- Bobbia, X.; Muller, L.; Claret, P.-G.; Vigouroux, L.; Perez-Martin, A.; de La Coussaye, J.E.; Lefrant, J.Y.; Louart, G.; Roger, C.; Markarian, T. A New Echocardiographic Tool for Cardiac Output Evaluation: An Experimental Study. Shock 2019, 52, 449–455. [Google Scholar] [CrossRef]
- Zhang, J.; Gajjala, S.; Agrawal, P.; Tison, G.H.; Hallock, L.A.; Beussink-Nelson, L.; Lassen, M.H.; Fan, E.; Aras, M.A.; Jordan, C.; et al. Fully Automated Echocardiogram Interpretation in Clinical Practice. Circulation 2018, 138, 1623–1635. [Google Scholar] [CrossRef]
- Camacho, J.; Muñoz, M.; Genovés, V.; Herraiz, J.L.; Ortega, I.; Belarra, A.; González, R.; Sánchez, D.; Giacchetta, R.C.; Trueba-Vicente, A.; et al. Artificial Intelligence and Democratization of the Use of Lung Ultrasound in COVID-19: On the Feasibility of Automatic Calculation of Lung Ultrasound Score. Int. J. Transl. Med. 2022, 2, 17–25. [Google Scholar] [CrossRef]
- Cho, Y.-J.; Song, K.-H.; Lee, Y.; Yoon, J.H.; Park, J.Y.; Jung, J.; Lim, S.Y.; Lee, H.; Yoon, H.I.; Park, K.U.; et al. Lung ultrasound for early diagnosis and severity assessment of pneumonia in patients with corona virus disease 2019. Korean J. Intern. Med. 2020, 35, 771–781. [Google Scholar] [CrossRef]
- Russell, F.M.; Ehrman, R.R.; Barton, A.; Sarmiento, E.; Ottenhoff, J.E.; Nti, B.K. B-line quantification: Comparing learners novice to lung ultrasound assisted by machine artificial intelligence technology to expert review. Ultrasound J. 2021, 13, 1–7. [Google Scholar] [CrossRef]






| Types of Shock | ||
|---|---|---|
| Type | Etiology | Hemodynamics |
| Distributive | Septic, neurogenic, anaphylactic. | ↓ SVR ↓CVP ↓ PWCP ↑ CO (Hyperdynamic state) ↓ CO (Hypodynamic state) |
| Hypovolemic | Hemorrhagic, dehydration, third space sequestration. | ↑ SVR ↓ CVP ↓ PWCP ↓ CO |
| Cardiogenic | Myocardial infarction, advanced heart failure, brady/tachyarrhythmias, valvular disease. | ↑ SVR ↑ CVP ↑ PCWP (LV disfunction) ↓ PCWP (RV disfunction) ↓ CO |
| Obstructive | Heart tamponade, pulmonary embolism, tensive pneumothorax, constrictive pericarditis, restrictive cardiomyopathy. | ↑ SVR ↓ CVP ↓ PCWP (RV disfunction) ↑ PCWP ↓ CO |
| RUSH Protocol | ||||
|---|---|---|---|---|
| Hypovolemic | Distributive | Cardiogenic | Obstructive | |
| Pump | - Hypercontractility. - Small dimensions of LV. - “Kissing” LV walls in systole. | - Hyper- or hypocontractility. | - Hypocontractility. - Normal dimensions or dilation of LV. | - Dyskinetic RV (McConnell’s sign). - Intraventricular thrombus. - Cardiac tamponade. |
| Tank | - Small or virtual IVC. - Look for pleural and peritoneal fluid. | - Normal or small IVC. - Look for causes of sepsis. | - Dilated and hypocollapsing IVC. - Look for ascites, pleural effusion, and pulmonary congestion. | - Dilated and hypocollapsing IVC. - Look for PNX (Lung point). |
| Pipes | - Aortic aneurism. - Aortic dissection. | - Normal. | - Normal. | - DVT. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Andrea, A.; Del Giudice, C.; Fabiani, D.; Caputo, A.; Sabatella, F.; Cante, L.; Palermi, S.; Desiderio, A.; Tagliamonte, E.; Liccardo, B.; et al. The Incremental Role of Multiorgan Point-of-Care Ultrasounds in the Emergency Setting. Int. J. Environ. Res. Public Health 2023, 20, 2088. https://doi.org/10.3390/ijerph20032088
D’Andrea A, Del Giudice C, Fabiani D, Caputo A, Sabatella F, Cante L, Palermi S, Desiderio A, Tagliamonte E, Liccardo B, et al. The Incremental Role of Multiorgan Point-of-Care Ultrasounds in the Emergency Setting. International Journal of Environmental Research and Public Health. 2023; 20(3):2088. https://doi.org/10.3390/ijerph20032088
Chicago/Turabian StyleD’Andrea, Antonello, Carmen Del Giudice, Dario Fabiani, Adriano Caputo, Francesco Sabatella, Luigi Cante, Stefano Palermi, Alfonso Desiderio, Ercole Tagliamonte, Biagio Liccardo, and et al. 2023. "The Incremental Role of Multiorgan Point-of-Care Ultrasounds in the Emergency Setting" International Journal of Environmental Research and Public Health 20, no. 3: 2088. https://doi.org/10.3390/ijerph20032088
APA StyleD’Andrea, A., Del Giudice, C., Fabiani, D., Caputo, A., Sabatella, F., Cante, L., Palermi, S., Desiderio, A., Tagliamonte, E., Liccardo, B., & Russo, V. (2023). The Incremental Role of Multiorgan Point-of-Care Ultrasounds in the Emergency Setting. International Journal of Environmental Research and Public Health, 20(3), 2088. https://doi.org/10.3390/ijerph20032088









