Association between Meteorological Factors and Outpatient Visits for Herpes Zoster in Hefei, China: A Time-Series Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Case of Outpatient Visits for HZ
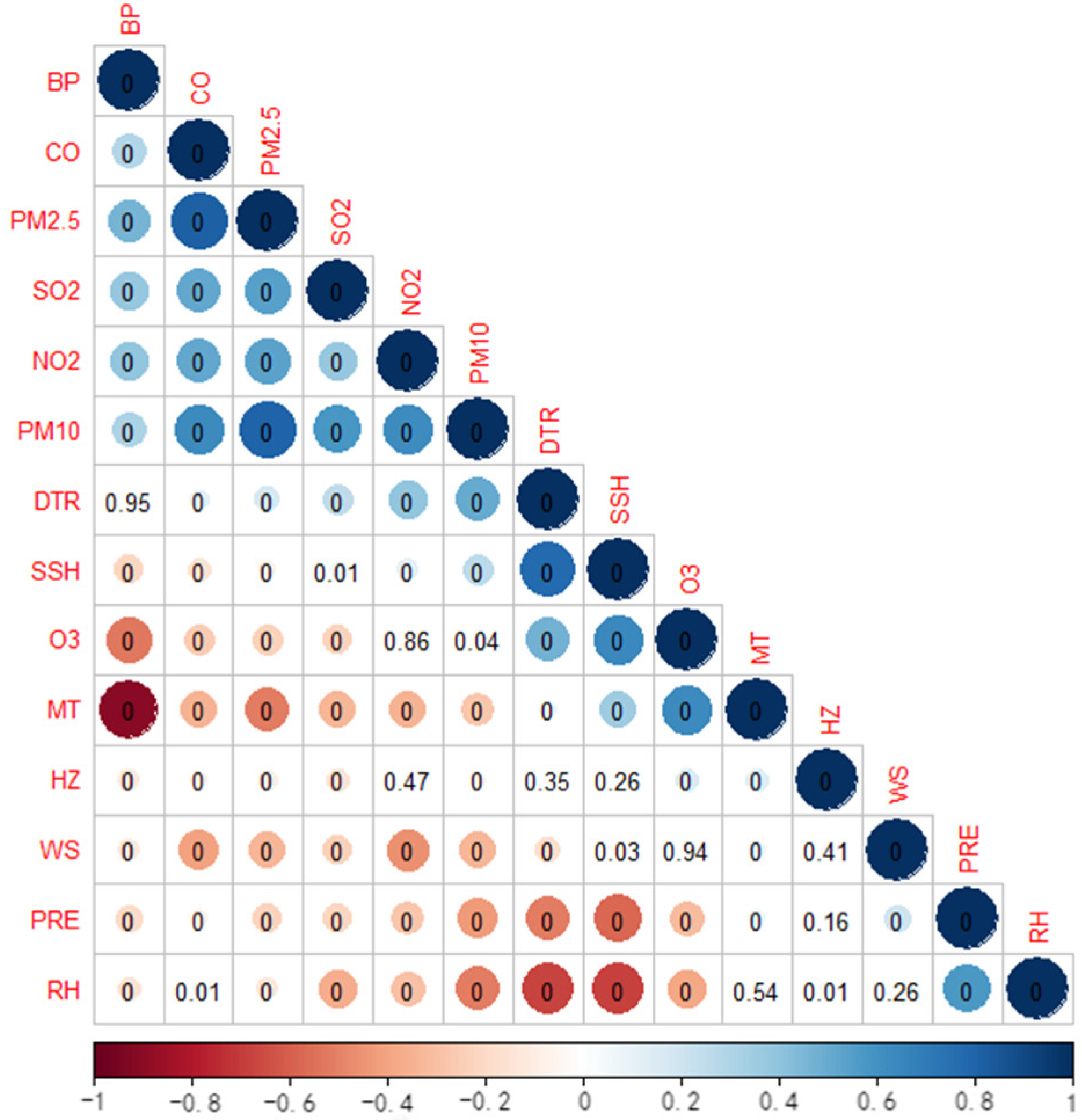
2.3. Environmental Variables
2.4. Statistical Analysis
3. Results
3.1. Data Description
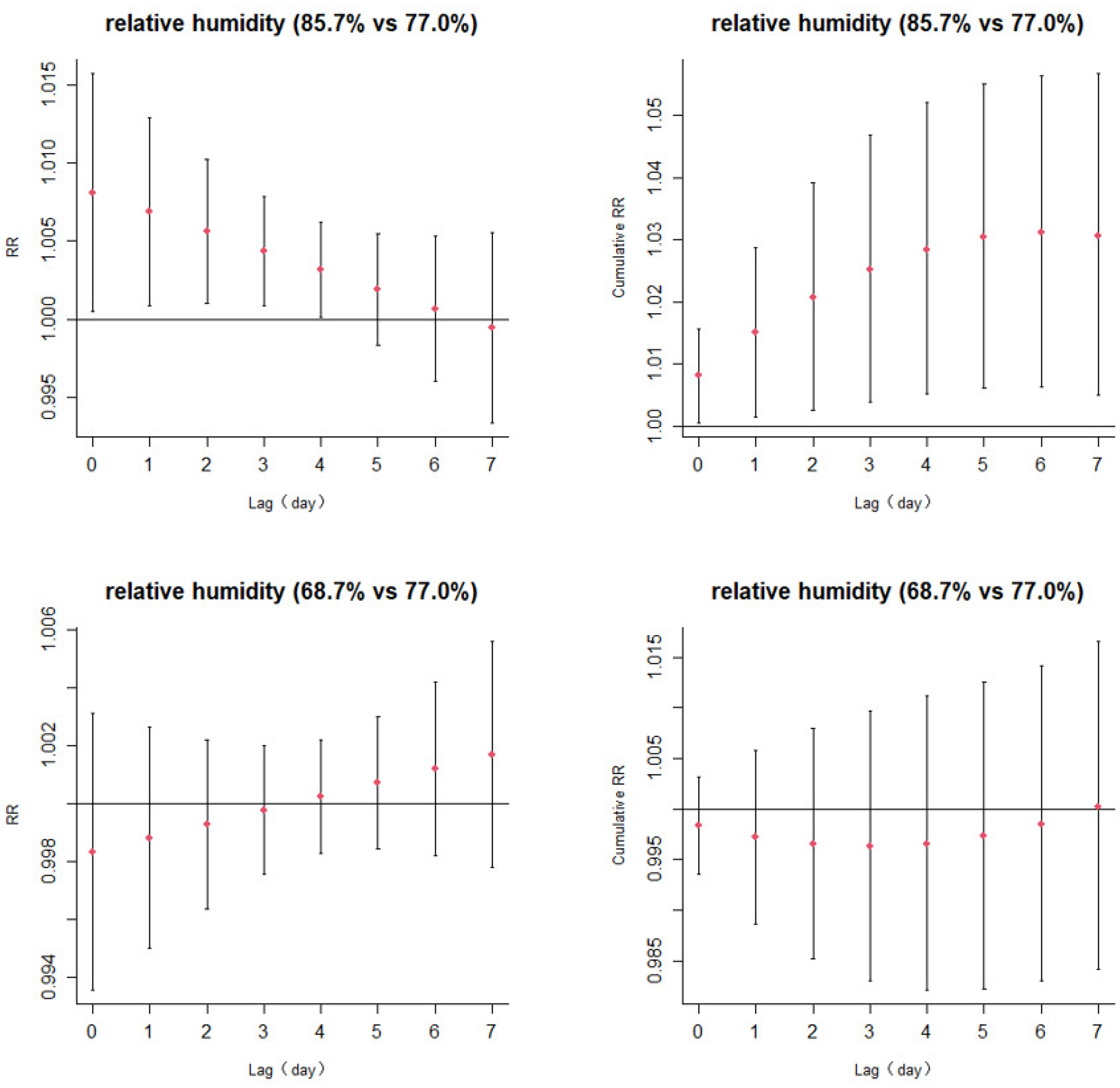
3.2. Lagged Effects of Temperature and Relative Humidity
3.3. Subgroup Analyses by Individual Characteristics
3.4. Sensitivity Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schmader, K. Herpes Zoster. Ann. Intern. Med. 2018, 169, ITC19–ITC31. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.-H.; Lin, Y.; Sun, P.-J. Segmental Zoster Abdominal Paresis Mimicking an Abdominal Hernia. Medicine 2019, 98, e15037. [Google Scholar] [CrossRef] [PubMed]
- Oster, G.; Harding, G.; Dukes, E.; Edelsberg, J.; Cleary, P.D. Pain, Medication Use, and Health-Related Quality of Life in Older Persons with Postherpetic Neuralgia: Results from a Population-Based Survey. J. Pain 2005, 6, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Jiang, W.; Jiang, J.; Hu, Y.; Wu, Y.; Li, Y.; Zhao, D.; Yin, D. The study on the relationship between herpes zoster and depression on Health Big Data-real word research examples. Chin. J. Dis. Control Prev. 2021, 25, 730–733. [Google Scholar] [CrossRef]
- Kawai, K.; Gebremeskel, B.G.; Acosta, C.J. Systematic Review of Incidence and Complications of Herpes Zoster: Towards a Global Perspective. BMJ Open 2014, 4, e004833. [Google Scholar] [CrossRef]
- Kawai, K.; Yawn, B.P.; Wollan, P.; Harpaz, R. Increasing Incidence of Herpes Zoster Over a 60-Year Period from a Population-Based Study. Clin. Infect. Dis. 2016, 63, 221–226. [Google Scholar] [CrossRef]
- Marra, F.; Chong, M.; Najafzadeh, M. Increasing Incidence Associated with Herpes Zoster Infection in British Columbia, Canada. BMC Infect. Dis. 2016, 16, 589. [Google Scholar] [CrossRef]
- Romanello, M.; McGushin, A.; Di Napoli, C.; Drummond, P.; Hughes, N.; Jamart, L.; Kennard, H.; Lampard, P.; Solano Rodriguez, B.; Arnell, N.; et al. The 2021 Report of the Lancet Countdown on Health and Climate Change: Code Red for a Healthy Future. Lancet 2021, 398, 1619–1662. [Google Scholar] [CrossRef]
- Chen, R.; Yin, P.; Wang, L.; Liu, C.; Niu, Y.; Wang, W.; Jiang, Y.; Liu, Y.; Liu, J.; Qi, J.; et al. Association between Ambient Temperature and Mortality Risk and Burden: Time Series Study in 272 Main Chinese Cities. BMJ 2018, 363, k4306. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, A.; Zhai, J.; Tao, H.; Jiang, T.; Su, B.; Yang, J.; Wang, G.; Liu, Q.; Gao, C.; et al. Tens of Thousands Additional Deaths Annually in Cities of China between 1.5 °C and 2.0 °C Warming. Nat. Commun. 2019, 10, 3376. [Google Scholar] [CrossRef]
- Shi, X.M. Promoting research on air pollution, climate change and population health under the goal of carbon neutrality and at the peak carbon dioxide emissions. Chin. J. Dis. Control Prev. 2021, 25, 1117–1119, 1225. [Google Scholar] [CrossRef]
- Chen, Y.; Leng, R.X.; Pan, H.F. Research advance on the role of meteorolgical factors in inflammatory immune diseases. Chin. J. Dis. Control Prev. 2021, 25, 879–884+992. [Google Scholar] [CrossRef]
- Toyama, N.; Shiraki, K.; Society of the Miyazaki Prefecture Dermatologists. Epidemiology of Herpes Zoster and Its Relationship to Varicella in Japan: A 10-Year Survey of 48,388 Herpes Zoster Cases in Miyazaki Prefecture. J. Med. Virol. 2009, 81, 2053–2058. [Google Scholar] [CrossRef]
- Korostil, I.A.; Regan, D.G. Varicella-Zoster Virus in Perth, Western Australia: Seasonality and Reactivation. PLoS ONE 2016, 11, e0151319. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, Y.-J.; Shen, X.-P.; Ning, G.-C.; Wei, Y.-J.; Zeng, W.; Yu, D.-H.; Lu, H.-G. Effects of Meteorological Factors on Daily Outpatient Visits for Skin Diseases: A Time Series Study in a Chinese Population. Chin. Med. J. 2021, 134, 1122–1124. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, R.; Xu, J.; Li, Q.; Xu, X.; Ha, S.; Song, W.; Tan, J.; Xu, F.; Kan, H. The Effects of Ambient Temperature on Outpatient Visits for Varicella and Herpes Zoster in Shanghai, China: A Time-Series Study. J. Am. Acad. Dermatol. 2015, 73, 660–665. [Google Scholar] [CrossRef]
- Choi, Y.-J.; Lim, Y.-H.; Lee, K.-S.; Hong, Y.-C. Elevation of Ambient Temperature Is Associated with an Increased Risk of Herpes Zoster: A Time-Series Analysis. Sci. Rep. 2019, 9, 12254. [Google Scholar] [CrossRef]
- Lai, S.-W.; Liao, K.-F.; Kuo, Y.-H.; Lin, C.-L.; Liu, C.-S.; Hwang, B.-F.; Lai, Y.-J. The Impacts of Ambient Temperature and Ultraviolet Radiation on the Incidence of Herpes Zoster: An Ecological Study in Taiwan. Int. J. Clin. Pract. 2021, 75, e13854. [Google Scholar] [CrossRef]
- Gasparrini, A.; Armstrong, B.; Kenward, M.G. Distributed Lag Non-Linear Models. Stat. Med. 2010, 29, 2224–2234. [Google Scholar] [CrossRef]
- Berlinberg, E.J.; Kim, E.; Deiner, M.S.; Patterson, C.; Porco, T.C.; Acharya, N.R. Seasonality of Herpes Zoster and Herpes Zoster Ophthalmicus. J. Clin. Virol. 2020, 126, 104306. [Google Scholar] [CrossRef]
- Jung, H.S.; Kang, J.K.; Yoo, S.H. Epidemiological Study on the Incidence of Herpes Zoster in Nearby Cheonan. Korean J. Pain 2015, 28, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Abendroth, A.; Arvin, A.M. Immune Evasion as a Pathogenic Mechanism of Varicella Zoster Virus. Semin. Immunol. 2001, 13, 27–39. [Google Scholar] [CrossRef]
- Gershon, A.A.; Breuer, J.; Cohen, J.I.; Cohrs, R.J.; Gershon, M.D.; Gilden, D.; Grose, C.; Hambleton, S.; Kennedy, P.G.E.; Oxman, M.N.; et al. Varicella Zoster Virus Infection. Nat. Rev. Dis. Prim. 2015, 1, 15016. [Google Scholar] [CrossRef] [PubMed]
- Reichelt, M.; Zerboni, L.; Arvin, A.M. Mechanisms of Varicella-Zoster Virus Neuropathogenesis in Human Dorsal Root Ganglia. J. Virol. 2008, 82, 3971–3983. [Google Scholar] [CrossRef] [PubMed]
- Dopico, X.C.; Evangelou, M.; Ferreira, R.C.; Guo, H.; Pekalski, M.L.; Smyth, D.J.; Cooper, N.; Burren, O.S.; Fulford, A.J.; Hennig, B.J.; et al. Widespread Seasonal Gene Expression Reveals Annual Differences in Human Immunity and Physiology. Nat. Commun. 2015, 6, 7000. [Google Scholar] [CrossRef]
- Moyal, D.D.; Fourtanier, A.M. Broad-Spectrum Sunscreens Provide Better Protection from Solar Ultraviolet-Simulated Radiation and Natural Sunlight-Induced Immunosuppression in Human Beings. J. Am. Acad. Dermatol. 2008, 58 (Suppl. S2), S149–S154. [Google Scholar] [CrossRef]
- Fatahzadeh, M.; Schwartz, R.A. Human Herpes Simplex Virus Infections: Epidemiology, Pathogenesis, Symptomatology, Diagnosis, and Management. J. Am. Acad. Dermatol. 2007, 57, 737–763. [Google Scholar] [CrossRef]
- Freeman, R.G.; Knox, J.M. Influence of Temperature on Ultraviolet Injury. Arch. Dermatol. 1964, 89, 858–864. [Google Scholar] [CrossRef]
- MacGillivray, D.M.; Kollmann, T.R. The Role of Environmental Factors in Modulating Immune Responses in Early Life. Front. Immunol. 2014, 5, 434. [Google Scholar] [CrossRef]
- Denda, M.; Sato, J.; Masuda, Y.; Tsuchiya, T.; Koyama, J.; Kuramoto, M.; Elias, P.M.; Feingold, K.R. Exposure to a Dry Environment Enhances Epidermal Permeability Barrier Function. J. Investig. Dermatol. 1998, 111, 858–863. [Google Scholar] [CrossRef]
- Fleming, D.M.; Cross, K.W.; Cobb, W.A.; Chapman, R.S. Gender Difference in the Incidence of Shingles. Epidemiol. Infect. 2004, 132, 1–5. [Google Scholar] [CrossRef]
- Opstelten, W.; Van Essen, G.A.; Schellevis, F.; Verheij, T.J.M.; Moons, K.G.M. Gender as an Independent Risk Factor for Herpes Zoster: A Population-Based Prospective Study. Ann. Epidemiol. 2006, 16, 692–695. [Google Scholar] [CrossRef]
- Ho, T.-Y.; Chung, C.-H.; Shen, Y.-P.; Chen, L.-C.; Chien, W.-C.; Wu, Y.-T. Herpes Zoster Increased Risk of Neuralgic Amyotrophy: A Retrospective, Population-Based Matched Cohort Study. J. Neurovirol. 2019, 25, 91–100. [Google Scholar] [CrossRef]


| Variables | Sum | Mean (SD) | Minimum | P25 | P50 | P75 | Maximum |
|---|---|---|---|---|---|---|---|
| Outpatient visits | 43,547 | 23.85 (6.79) | 0.00 | 19.00 | 24.00 | 28.00 | 50.00 |
| Male | 19,846 | 10.87 (3.91) | 0.00 | 8.00 | 11.00 | 13.00 | 27.00 |
| Female | 23,701 | 12.89 (4.54) | 0.00 | 10.00 | 13.00 | 16.00 | 31.00 |
| 40 years | 11,078 | 6.07 (2.69) | 0.00 | 4.00 | 6.00 | 8.00 | 17.00 |
| ≥40 years | 32,469 | 17.78 (5.83) | 0.00 | 14.00 | 18.00 | 21.00 | 42.00 |
| Daily mean Temperature (°C) | 16.85 (9.21) | −6.20 | 8.60 | 17.60 | 24.50 | 34.80 | |
| DTR (°C) | 8.39 (3.77) | 0.80 | 5.50 | 8.30 | 11.10 | 19.80 | |
| Relative humidity (%) | 76.51 (11.98) | 39.20 | 68.70 | 77.00 | 85.70 | 98.30 |
| lag0 | lag1 | lag2 | lag3 | lag4 | lag5 | lag6 | lag7 | |
|---|---|---|---|---|---|---|---|---|
| MT | ||||||||
| Female | 1.023 (0.990,1.057) | 1.020 (0.993,1.046) | 1.016 (0.995,1.037) | 1.012 (0.995,1.030) | 1.009 (0.992,1.026) | 1.005 (0.985,1.026) | 1.002 (0.976,1.028) | 0.998 (0.966,1.031) |
| Male | 1.032 (0.997,1.068) | 1.025 (0.997,1.054) | 1.018 (0.996,1.041) | 1.012 (0.993,1.031) | 1.005 (0.987,1.024) | 0.998 (0.977,1.021) | 0.992 (0.965,1.020) | 0.985 (0.952,1.020) |
| 0–40 years | 1.032 (0.986,1.081) | 1.019 (0.982,1.058) | 1.007 (0.978,1.037) | 0.994 (0.970,1.019) | 0.982 (0.958,1.006) | 0.969 (0.942,0.998) * | 0.957 (0.923,0.993) * | 0.945 (0.903,0.990) * |
| ≥40 years | 1.026 (0.997,1.055) | 1.023 (1.001,1.047) * | 1.021 (1.003,1.039) * | 1.019 (1.004,1.034) * | 1.016 (1.001,1.032) * | 1.014 (0.996,1.032) | 1.012 (0.989,1.035) | 1.009 (0.981,1.038) |
| RH | ||||||||
| Female | 1.008 (0.998,1.018) | 1.007 (0.999,1.015) | 1.006 (1.000,1.012) | 1.005 (1.000,1.009) * | 1.004 (1.000,1.008) | 1.003 (0.998,1.007) | 1.002 (0.995,1.008) | 1.000 (0.992,1.009) |
| Male | 1.008 (0.997,1.019) | 1.007 (0.998,1.015) | 1.005 (0.999,1.012) | 1.004 (0.999,1.009) | 1.002 (0.998,1.007) | 1.001 (0.996,1.006) | 1.000 (0.993,1.006) | 0.998 (0.990,1.007) |
| 0–40 years | 0.998 (0.984,1.013) | 1.000 (0.988,1.011) | 1.001 (0.992,1.010) | 1.002 (0.996,1.009) | 1.003 (0.998,1.009) | 1.005 (0.998,1.011) | 1.006 (0.997,1.015) | 1.007 (0.996,1.019) |
| ≥40 years | 1.011 (1.003,1.020) * | 1.009 (1.002,1.016) * | 1.007 (1.002,1.013) * | 1.005 (1.001,1.009) * | 1.003 (0.999,1.006) | 1.001 (0.997,1.005) | 0.999 (0.993,1.004) | 0.997 (0.990,1.004) |
| lag0 | lag1 | lag2 | lag3 | lag4 | lag5 | lag6 | lag7 | |
|---|---|---|---|---|---|---|---|---|
| MT | ||||||||
| Female | 0.975 (0.943,1.009) | 0.984 (0.958,1.011) | 0.994 (0.973,1.015) | 1.003 (0.986,1.020) | 1.012 (0.995,1.030) | 1.022 (1.000,1.044) * | 1.032 (1.004,1.060) * | 1.041 (1.006,1.078) * |
| Male | 0.991 (0.956,1.026) | 0.992 (0.964,1.020) | 0.993 (0.972,1.015) | 0.994 (0.976,1.012) | 0.995 (0.977,1.014) | 0.997 (0.975,1.019) | 0.998 (0.970,1.027) | 0.999 (0.964,1.036) |
| 0–40 years | 0.983 (0.937,1.032) | 0.992 (0.954,1.030) | 1.000 (0.971,1.030) | 1.008 (0.984,1.033) | 1.017 (0.992,1.042) | 1.025 (0.995,1.057) | 1.034 (0.994,1.075) | 1.043 (0.993,1.095) |
| ≥40 years | 0.982 (0.954,1.011) | 0.986 (0.964,1.009) | 0.991 (0.973,1.009) | 0.996 (0.981,1.010) | 1.000 (0.985,1.015) | 1.005 (0.987,1.023) | 1.009 (0.986,1.033) | 1.014 (0.984,1.045) |
| RH | ||||||||
| Female | 1.000 (0.993,1.006) | 1.000 (0.995,1.005) | 1.000 (0.996,1.004) | 1.001 (0.998,1.004) | 1.001 (0.998,1.004) | 1.001 (0.998,1.004) | 1.002 (0.998,1.006) | 1.002 (0.997,1.007) |
| Male | 0.997 (0.990,1.004) | 0.997 (0.992,1.003) | 0.998 (0.994,1.002) | 0.999 (0.996,1.002) | 0.999 (0.997,1.002) | 1.000 (0.997,1.003) | 1.001 (0.997,1.005) | 1.002 (0.996,1.007) |
| 0–40 years | 0.997 (0.988,1.006) | 0.998 (0.991,1.005) | 0.999 (0.993,1.004) | 0.999 (0.995,1.004) | 1.000 (0.997,1.004) | 1.001 (0.997,1.005) | 1.002 (0.996,1.007) | 1.003 (0.995,1.010) |
| ≥40 years | 0.999 (0.993,1.004) | 0.999 (0.995,1.003) | 1.000 (0.996,1.003) | 1.000 (0.997,1.002) | 1.000 (0.998,1.003) | 1.001 (0.998,1.003) | 1.001 (0.998,1.005) | 1.001 (0.997,1.006) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, X.; Fang, X.; Qian, T.; Cai, Y.; Gao, P.; Chen, H.; Wu, Q.; Wu, J.; Fan, Y.; Ye, D. Association between Meteorological Factors and Outpatient Visits for Herpes Zoster in Hefei, China: A Time-Series Analysis. Int. J. Environ. Res. Public Health 2023, 20, 2097. https://doi.org/10.3390/ijerph20032097
Lv X, Fang X, Qian T, Cai Y, Gao P, Chen H, Wu Q, Wu J, Fan Y, Ye D. Association between Meteorological Factors and Outpatient Visits for Herpes Zoster in Hefei, China: A Time-Series Analysis. International Journal of Environmental Research and Public Health. 2023; 20(3):2097. https://doi.org/10.3390/ijerph20032097
Chicago/Turabian StyleLv, Xiaojie, Xinyu Fang, Tingting Qian, Yuyu Cai, Peng Gao, Haifeng Chen, Qing Wu, Jun Wu, Yinguang Fan, and Dongqing Ye. 2023. "Association between Meteorological Factors and Outpatient Visits for Herpes Zoster in Hefei, China: A Time-Series Analysis" International Journal of Environmental Research and Public Health 20, no. 3: 2097. https://doi.org/10.3390/ijerph20032097
APA StyleLv, X., Fang, X., Qian, T., Cai, Y., Gao, P., Chen, H., Wu, Q., Wu, J., Fan, Y., & Ye, D. (2023). Association between Meteorological Factors and Outpatient Visits for Herpes Zoster in Hefei, China: A Time-Series Analysis. International Journal of Environmental Research and Public Health, 20(3), 2097. https://doi.org/10.3390/ijerph20032097





