Ultrasound-Guided Blocks for Spine Surgery: Part 1—Cervix
Abstract
:1. Introduction
2. Methodology
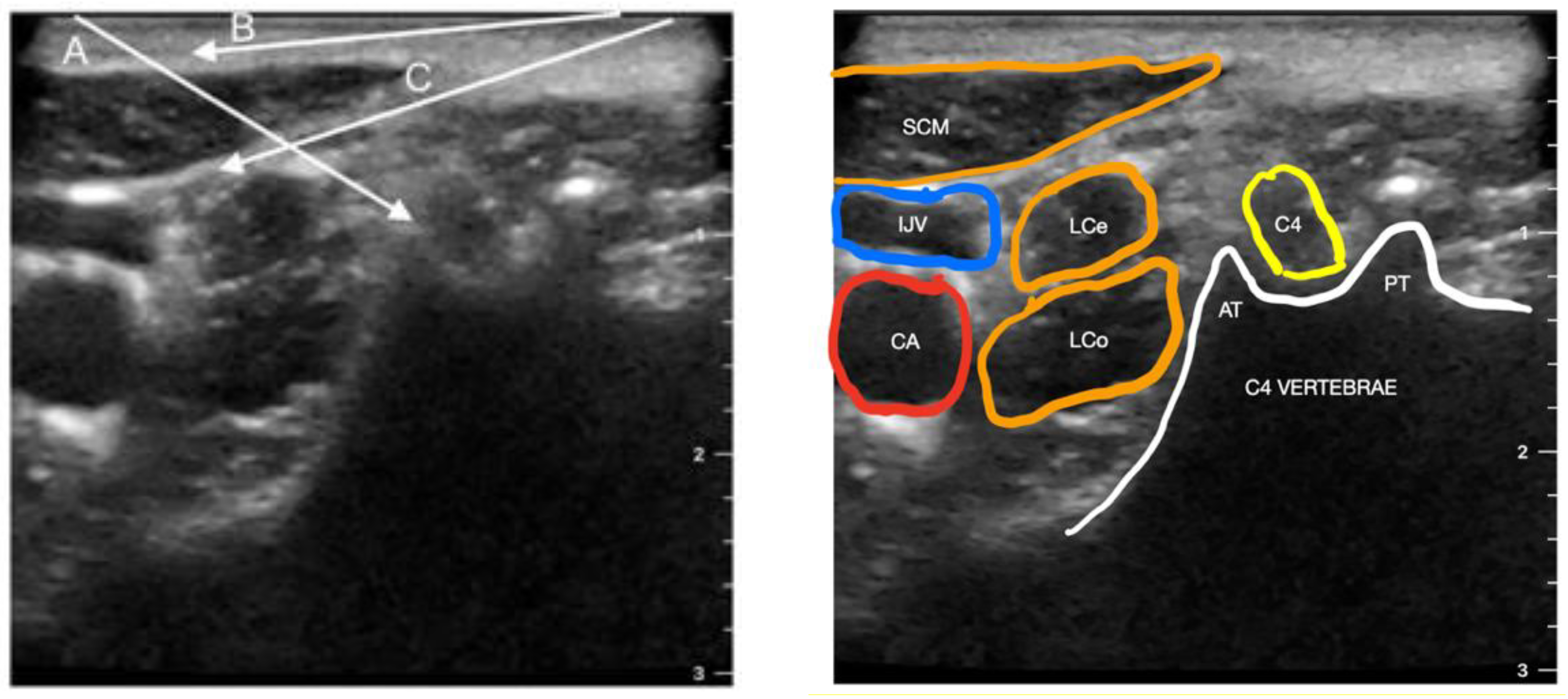
3. Cervical Paravertebral Block (CPVB)
4. Cervical Plexus Block
5. Paraspinal Interfascial Plane Blocks (PIP)
5.1. Multifidus Cervicis Plane Block (MCP)
5.2. Retrolaminar Cervical Block
5.3. Inter-Semispinal Plane Block (ISP)
6. Cervical Erector Spinae Plane Block
7. Stellate Ganglion Block
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lamperti, M.; Tufegdzic, B.; Avitsian, R. Management of Complex Spine Surgery. Curr. Opin. Anaesthesiol. 2017, 30, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Devin, C.J.; McGirt, M.J. Best Evidence in Multimodal Pain Management in Spine Surgery and Means of Assessing Postoperative Pain and Functional Outcomes. J. Clin. Neurosci. 2015, 22, 930–938. [Google Scholar] [CrossRef] [PubMed]
- Bernard, J.M.; Surbled, M.; Lagarde, D.; Trennec, A. Analgesia after surgery of the spine in adults and adolescents. Cah. Anesthesiol. 1995, 43, 557–564. [Google Scholar] [PubMed]
- Bono, C.M.; Ghiselli, G.; Gilbert, T.J.; Kreiner, D.S.; Reitman, C.; Summers, J.T.; Baisden, J.L.; Easa, J.; Fernand, R.; Lamer, T.; et al. An Evidence-Based Clinical Guideline for the Diagnosis and Treatment of Cervical Radiculopathy from Degenerative Disorders. Spine J. 2011, 11, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Kjaer, P.; Kongsted, A.; Hartvigsen, J.; Isenberg-Jørgensen, A.; Schiøttz-Christensen, B.; Søborg, B.; Krog, C.; Møller, C.M.; Halling, C.M.B.; Lauridsen, H.H.; et al. National Clinical Guidelines for Non-Surgical Treatment of Patients with Recent Onset Neck Pain or Cervical Radiculopathy. Eur. Spine J. 2017, 26, 2242–2257. [Google Scholar] [CrossRef]
- Hoy, D.G.; Protani, M.; De, R.; Buchbinder, R. The Epidemiology of Neck Pain. Best Pract. Res. Clin. Rheumatol. 2010, 24, 783–792. [Google Scholar] [CrossRef]
- Woods, B.I.; Hilibrand, A.S. Cervical Radiculopathy: Epidemiology, Etiology, Diagnosis, and Treatment. J. Spinal Disord Tech. 2015, 28, E251–E259. [Google Scholar] [CrossRef] [PubMed]
- Basques, B.A.; Bohl, D.D.; Golinvaux, N.S.; Gruskay, J.A.; Grauer, J.N. Preoperative Factors Affecting Length of Stay after Elective ACDF with and without Corpectomy: A Multivariate Analysis of an Academic Center Cohort. Spine 2014, 39, 939. [Google Scholar] [CrossRef] [Green Version]
- Adamson, T.; Godil, S.S.; Mehrlich, M.; Mendenhall, S.; Asher, A.L.; McGirt, M.J. Anterior Cervical Discectomy and Fusion in the Outpatient Ambulatory Surgery Setting Compared with the Inpatient Hospital Setting: Analysis of 1000 Consecutive Cases. J. Neurosurg. Spine 2016, 24, 878–884. [Google Scholar] [CrossRef] [Green Version]
- Gruskay, J.A.; Fu, M.; Basques, B.A.; Bohl, D.D.; Buerba, R.A.; Webb, M.L.; Grauer, J.N. Factors Affecting Length of Stay and Complications After Elective Anterior Cervical Discectomy and Fusion: A Study of 2164 Patients From The American College of Surgeons National Surgical Quality Improvement Project Database (ACS NSQIP). Clin. Spine Surg. 2016, 29, E34–E42. [Google Scholar] [CrossRef]
- Fu, M.C.; Gruskay, J.A.; Samuel, A.M.; Sheha, E.D.; Derman, P.B.; Iyer, S.; Grauer, J.N.; Albert, T.J. Outpatient Anterior Cervical Discectomy and Fusion Is Associated with Fewer Short-Term Complications in One- A Nd Two-Level Cases. Spine 2017, 42, 1044–1049. [Google Scholar] [CrossRef]
- Mohandas, A.; Summa, C.; Worthington, W.B.; Lerner, J.; Foley, K.T.; Bohinski, R.J.; Lanford, G.B.; Holden, C.; Wohns, R.N.W. Best Practices for Outpatient Anterior Cervical Surgery. Spine 2017, 42, E648–E659. [Google Scholar] [CrossRef]
- Singh, K.; Bohl, D.D.; Ahn, J.; Massel, D.H.; Mayo, B.C.; Narain, A.S.; Hijji, F.Y.; Louie, P.K.; Long, W.W.; Modi, K.D.; et al. Multimodal Analgesia Versus Intravenous Patient-Controlled Analgesia for Minimally Invasive Transforaminal Lumbar Interbody Fusion Procedures. Spine 2017, 42, 1145–1150. [Google Scholar] [CrossRef]
- Mathiesen, O.; Dahl, B.; Thomsen, B.A.; Kitter, B.; Sonne, N.; Dahl, J.B.; Kehlet, H. A Comprehensive Multimodal Pain Treatment Reduces Opioid Consumption after Multilevel Spine Surgery. Eur. Spine J. 2013, 22, 2089–2096. [Google Scholar] [CrossRef] [Green Version]
- Apfelbaum, J.L.; Chen, C.; Mehta, S.S.; Gan, T.J. Postoperative Pain Experience: Results from a National Survey Suggest Postoperative Pain Continues to Be Undermanaged. Anesth. Analg. 2003, 97, 534–540. [Google Scholar] [CrossRef] [Green Version]
- Fleege, C.; Arabmotlagh, M.; Almajali, A.; Rauschmann, M. Pre- and Postoperative Fast-Track Treatment Concepts in Spinal Surgery: Patient Information and Patient Cooperation. Orthopade 2014, 43, 1062–1069. [Google Scholar] [CrossRef]
- Maheshwari, K.; Avitsian, R.; Sessler, D.I.; Makarova, N.; Tanios, M.; Raza, S.; Traul, D.; Rajan, S.; Manlapaz, M.; MacHado, S.; et al. Multimodal Analgesic Regimen for Spine SurgeryA Randomized Placebo-Controlled Trial. Anesthesiology 2020, 132, 992–1002. [Google Scholar] [CrossRef]
- Bautmans, I.; Njemini, R.; De Backer, J.; De Waele, E.; Mets, T. Surgery-Induced Inflammation in Relation to Age, Muscle Endurance, and Self-Perceived Fatigue. J. Gerontol. A Biol. Sci. Med. Sci. 2010, 65, 266–273. [Google Scholar] [CrossRef] [Green Version]
- Cummins, T.R.; Sheets, P.L.; Waxman, S.G. The Roles of Sodium Channels in Nociception: Implications for Mechanisms of Pain. Pain 2007, 131, 243–257. [Google Scholar] [CrossRef] [Green Version]
- Miralda, I.; Uriarte, S.M.; McLeish, K.R. Multiple Phenotypic Changes Define Neutrophil Priming. Front. Cell. Infect. Microbiol. 2017, 7, 217. [Google Scholar] [CrossRef] [Green Version]
- Pobereskin, L.H.; Sneyd, J.R. Wound Infiltration with Bupivacaine after Surgery to the Cervical Spine Using a Posterior Approach. Br. J. Anaesth. 2000, 84, 87–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sperber, G.H. Clinically Oriented Anatomy. J. Anat. 2006, 208, 393. [Google Scholar] [CrossRef]
- Kumar Batra, D.; Krishnan, K.; Resident, S.; Agarwal, A.; Kumar Batra, R. Paravertebral Block. J. Anaesthesiol. Clin. Pharmacol. 2011, 27, 5. [Google Scholar] [CrossRef]
- Kappis, M. Other Experiences with Sympathectomy. Klin. Wochenschr. 1923, 2, 1441. [Google Scholar] [CrossRef]
- EASON, M.J.; WYATT, R. Paravertebral Thoracic Block-a Reappraisal. Anaesthesia 1979, 34, 638–642. [Google Scholar] [CrossRef]
- Boezaart, A.P.; Koorn, R.; Rosenquist, R.W. Paravertebral Approach to the Brachial Plexus: An Anatomic Improvement in Technique. Reg. Anesth. Pain Med. 2003, 28, 241–244. [Google Scholar] [CrossRef]
- Mariano, E.R.; Loland, V.J.; Ilfeld, B.M. Interscalene Perineural Catheter Placement Using an Ultrasound-Guided Posterior Approach. Reg. Anesth. Pain Med. 2009, 34, 60–63. [Google Scholar] [CrossRef] [Green Version]
- Antonakakis, J.G.; Sites, B.D.; Shiffrin, J. Ultrasound-Guided Posterior Approach for the Placement of a Continuous Interscalene Catheter. Reg. Anesth. Pain Med. 2009, 34, 64–68. [Google Scholar] [CrossRef]
- Butterworth, J.F.; Mackey, D.C.; Wasnick, J.D. Morgan & Mikhail’s Clinical Anesthesiology, 7th ed.; McGraw-Hill Education: New York, NY, USA, 2022; ISBN 1260473791. [Google Scholar]
- Boezaart, A.P. Please Don’t Blame the Block. Anesth. Analg. 2007, 104, 211–212. [Google Scholar] [CrossRef]
- Perisanidis, C.; Saranteas, T.; Kostopanagiotou, G. Ultrasound-Guided Combined Intermediate and Deep Cervical Plexus Nerve Block for Regional Anaesthesia in Oral and Maxillofacial Surgery. Dentomaxillofac. Radiol. 2013, 42, 29945724. [Google Scholar] [CrossRef] [Green Version]
- Saranteas, T.; Kostopanagiotou, G.G.; Anagnostopoulou, S.; Mourouzis, K.; Sidiropoulou, T. A Simple Method for Blocking the Deep Cervical Nerve Plexus Using an Ultrasound-Guided Technique. Anaesth. Intensive Care 2011, 39, 971–972. [Google Scholar] [PubMed]
- Wan, Q.; Yang, H.; Li, X.; Lin, C.; Ke, S.; Wu, S.; Ma, C. Ultrasound-Guided versus Fluoroscopy-Guided Deep Cervical Plexus Block for the Treatment of Cervicogenic Headache. Biomed. Res. Int. 2017, 2017, 4654803. [Google Scholar] [CrossRef] [PubMed]
- Sandeman, D.J.; Griffiths, M.J.; Lennox, A.F. Ultrasound Guided Deep Cervical Plexus Block. Anaesth. Intensive. Care 2006, 34, 240–244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naja, Z.; Lönnqvist, P.A. Somatic Paravertebral Nerve Blockade. Incidence of Failed Block and Complications. Anaesthesia 2001, 56, 1184–1188. [Google Scholar] [CrossRef]
- Kleopa, K.A.; Rosenberg, H.; Heiman-Patterson, T. Permanent Loss of Cervical Spinal Cord Function Associated with Interscalene Block Performed under General Anesthesia. Anesthesiology 2000, 93, 1541–1544. [Google Scholar] [CrossRef]
- Vosylius, S.; Uvarovas, V.; Svediene, S.; Satkauskas, I. Resuscitation Following a Bupivacaine Injection for a Cervical Paravertebral Block. Open Med. 2020, 14, 953–958. [Google Scholar] [CrossRef]
- Elder, J.B.; Hoh, D.J.; Liu, C.Y.; Wang, M.Y. Postoperative Continuous Paravertebral Anesthetic Infusion for Pain Control in Posterior Cervical Spine Surgery: A Case-Control Study. Neurosurgery 2010, 66, ons99–ons107. [Google Scholar] [CrossRef]
- Boezaart, A.P.; Nosovitch, M.A. Carotid Endarterectomy Using Single Injection Posterior Cervical Paravertebral Block. Anesth. Analg. 2005, 101, 1885–1886. [Google Scholar] [CrossRef]
- Merle, J.C.; Mazoit, J.X.; Desgranges, P.; Abhay, K.; Rezaiguia, S.; Dhonneur, G.; Duvaldestin, P. A Comparison of Two Techniques for Cervical Plexus Blockade: Evaluation of Efficacy and Systemic Toxicity. Anesth. Analg. 1999, 89, 1366–1370. [Google Scholar] [CrossRef]
- Wang, H.; Ma, L.; Yang, D.; Wang, T.; Wang, Q.; Zhang, L.; Ding, W. Cervical Plexus Anesthesia versus General Anesthesia for Anterior Cervical Discectomy and Fusion Surgery: A Randomized Clinical Trial. Medicine 2017, 96, e6119. [Google Scholar] [CrossRef]
- Mariappan, R.; Mehta, J.; Massicotte, E.; Nagappa, M.; Manninen, P.; Venkatraghavan, L. L’effet d’un Bloc Du Plexus Cervical Superficiel Sur La Qualité de La Récupération Postopératoire Après Une Discectomie Cervicale Antérieure et Fusion: Une Étude Randomisée Contrôlée. Can. J. Anesth. 2015, 62, 883–890. [Google Scholar] [CrossRef] [Green Version]
- Hand, W.R.; Taylor, J.M.; Harvey, N.R.; Epperson, T.I.; Gunselman, R.J.; Bolin, E.D.; Whiteley, J. Thoracolumbar Interfascial Plane (TLIP) Block: A Pilot Study in Volunteers. Can. J. Anaesth. 2015, 62, 1196–1200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ueshima, H.; Sakai, R.; Otake, H. Clinical Experiences of Ultrasound-Guided Thoracolumbar Interfascial Plane Block: A Clinical Experience. J. Clin. Anesth. 2016, 33, 499. [Google Scholar] [CrossRef]
- Ueshima, H.; Otake, H. RETRACTED: Clinical Efficacy of Modified Thoracolumbar Interfascial Plane Block. J. Clin. Anesth. 2016, 30, 74–75. [Google Scholar] [CrossRef]
- Ueshima, H.; Otake, H. RETRACTED: Blocking of Multiple Posterior Branches of Cervical Nerves Using a Cervical Interfascial Plane Block. J. Clin. Anesth. 2017, 38, 5. [Google Scholar] [CrossRef] [PubMed]
- Ohgoshi, Y.; Izawa, H.; Kori, S.; Matsukawa, M. Multifidus Cervicis Plane Block Is Effective for Cervical Spine Surgery. Can. J. Anesth. 2017, 64, 329–330. [Google Scholar] [CrossRef]
- Ohgoshi, Y.; Nishizakura, R.; Takahashi, Y.; Takeda, K.; Nakayama, H.; Kawamata, M.; Kurahashi, K. Novel Ultrasound-Guided Inter-Semispinal Plane Block: A Comparative Pilot Study in Healthy Volunteers. J. Anesth. 2018, 32, 143–146. [Google Scholar] [CrossRef]
- Hochberg, U.; de Santiago Moraga, J. Ultrasound-Guided Retrolaminar Cervical Block. Reg. Anesth. Pain Med. 2022, 47, 73–74. [Google Scholar] [CrossRef]
- Mohamed, Z.E.; Zarad, C.A.; Flifel, M.E.; Abou Elmaaty, A.A. The Efficacy of Ultrasound-Guided Multifidus Cervicis Plane Block versus Greater Occipital Nerve Block for Cervicogenic Headache. Egypt. J. Neurol. Psychiatry Neurosurg. 2021, 57, 11. [Google Scholar] [CrossRef]
- Fredriksen, T.A.; Antonaci, F.; Sjaastad, O. Cervicogenic Headache: Too Important to Be Left Un-Diagnosed. J. Headache Pain 2015, 16, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Onishi, E.; Toda, N.; Kameyama, Y.; Yamauchi, M.; Ueshima, H. Comparison of Clinical Efficacy and Anatomical Investigation between Retrolaminar Block and Erector Spinae Plane Block. Biomed. Res. Int. 2019, 2019, 2578396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, H.M.; Choi, Y.J.; Kwon, H.J.; O, J.; Cho, T.H.; Kim, S.H. Comparison of Injectate Spread and Nerve Involvement between Retrolaminar and Erector Spinae Plane Blocks in the Thoracic Region: A Cadaveric Study. Anaesthesia 2018, 73, 1244–1250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hochberg, U.; Perez, M.F.; Brill, S.; Khashan, M.; de Santiago, J. A New Solution to an Old Problem: Ultrasound-Guided Cervical Retrolaminar Injection for Acute Cervical Radicular Pain: Prospective Clinical Pilot Study and Cadaveric Study. Spine 2021, 46, 1370–1377. [Google Scholar] [CrossRef] [PubMed]
- Khashan, M.; de Santiago, J.; Pardo, I.; Regev, G.; Ophir, D.; Salame, K.; Lidar, Z.; Brill, S.; Hochberg, U. Ultrasound-Guided Cervical Retro-Laminar Block for Cervical Radicular Pain: A Comparative Analysis. Spine 2022, 47, 1351–1356. [Google Scholar] [CrossRef]
- Mostafa, S.F.; Abu Elyazed, M.M.; Eid, G.M.; Belal, A.M. Inter-Semispinal Plane (ISP) Block for Postoperative Analgesia Following Cervical Spine Surgery: A Prospective Randomized Controlled Trial. J. Clin. Anesth. 2022, 83, 110974. [Google Scholar] [CrossRef]
- Chin, K.J.; Adhikary, S.D.; Forero, M. Erector Spinae Plane (ESP) Block: A New Paradigm in Regional Anesthesia and Analgesia. Curr. Anesthesiol. Rep. 2019, 9, 271–280. [Google Scholar] [CrossRef]
- Forero, M.; Adhikary, S.D.; Lopez, H.; Tsui, C.; Chin, K.J. The Erector Spinae Plane Block: A Novel Analgesic Technique in Thoracic Neuropathic Pain. Reg. Anesth. Pain Med. 2016, 41, 621–627. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.L.; Tseng, V. Proposal to Standardize the Nomenclature for Paraspinal Interfascial Plane Blocks. Reg. Anesth. Pain Med. 2019, 44, 1106–1107. [Google Scholar] [CrossRef] [Green Version]
- Jain, K.; Jaiswal, V.; Puri, A. Erector Spinae Plane Block: Relatively New Block on Horizon with a Wide Spectrum of Application—A Case Series. Indian J. Anaesth. 2018, 62, 809–813. [Google Scholar] [CrossRef]
- Tsui, B.C.H.; Fonseca, A.; Munshey, F.; McFadyen, G.; Caruso, T.J. The Erector Spinae Plane (ESP) Block: A Pooled Review of 242 Cases. J. Clin. Anesth. 2019, 53, 29–34. [Google Scholar] [CrossRef]
- Goyal, A.; Kamath, S.; Kalgudi, P.; Krishnakumar, M. Perioperative Analgesia with Erector Spinae Plane Block for Cervical Spine Instrumentation Surgery. Saudi. J. Anaesth. 2020, 14, 263. [Google Scholar] [CrossRef] [PubMed]
- Goyal, A.; Kalgudi, P.; Sriganesh, K. Ultrasound-Guided Erector Spinae Plane Block for Perioperative Analgesia in Cervical and Thoracic Spine Surgeries—A Case Series. Neurol. India 2021, 69, 487. [Google Scholar] [CrossRef] [PubMed]
- Kwon, A.H.; Xu, J.L. Cervical Erector Spinae Plane Block: Is It Feasible for Cervical Spine Surgeries? Reg. Anesth. Pain Med. 2021, 46, 552–553. [Google Scholar] [CrossRef] [PubMed]
- Ohgoshi, Y. Semispinalis Capitis Muscle Is a Key Factor in Defining Cervical Nerve Block Effect. Reg. Anesth. Pain Med. 2021, 46, 457–458. [Google Scholar] [CrossRef] [PubMed]
- Tsui, B.C.H.; Elsharkawy, H. Novel Cervical Erector Spinae Plane Block: Interfascial Layers Remain Important in the Spread of Anesthetics. Reg. Anesth. Pain Med. 2021, 46, 458–459. [Google Scholar] [CrossRef] [PubMed]
- Möller, T.B.; Reif, E. Pocket Atlas of Sectional Anatomy: Computed Tomography and Magnetic Resonance Imaging; Thieme Medical Publishers: New York, NY, USA, 2007. [Google Scholar]
- Civelek, E.; Karasu, A.; Cansever, T.; Hepgul, K.; Kiris, T.; SabancI, A.; Canbolat, A. Surgical Anatomy of the Cervical Sympathetic Trunk during Anterolateral Approach to Cervical Spine. Eur. Spine J. 2008, 17, 991–995. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Sinha, C.; Kumar, A.; Sinha, A.K. Ultrasound-Guided Stellate Ganglion Block for Resistant Ventricular Tachycardia. Saudi. J. Anaesth. 2017, 11, 372. [Google Scholar] [CrossRef]
- Cai, C.; Dai, M.Y.; Tian, Y.; Zhang, P.; Wittwer, E.D.; Rho, R.H.; Kapa, S.; McLeod, C.J.; Mulpuru, S.K.; Lee, H.C.; et al. Electrophysiologic Effects and Outcomes of Sympatholysis in Patients with Recurrent Ventricular Arrhythmia and Structural Heart Disease. J. Cardiovasc. Electrophysiol. 2019, 30, 1499–1507. [Google Scholar] [CrossRef]
- Yoo, Y.; Lee, C.S.; Kim, Y.C.; Moon, J.Y.; Finlayson, R.J. A Randomized Comparison between 4, 6 and 8 ML of Local Anesthetic for Ultrasound-Guided Stellate Ganglion Block. J. Clin. Med. 2019, 8, 1314. [Google Scholar] [CrossRef] [Green Version]
- Makharita, M.Y.; Amr, Y.M.; El-Bayoumy, Y. Effect of Early Stellate Ganglion Blockade for Facial Pain from Acute Herpes Zoster and Incidence of Postherpetic Neuralgia. Pain Physician 2012, 15, 467–474. [Google Scholar] [CrossRef]
- Lee, W.; Jae Hyeon, P.; Seong Min, C.; Yu-Sun, M.; Kwan Sik, S. Effects of Stellate Ganglion Block on Breast Cancer-Related Lymphedema: Comparison of Various Injectates. Eur. J. Cancer 2015, 51, S302. [Google Scholar] [CrossRef]
- Bryce-Smith, r. Stellate ganglion block. Anaesthesia 1952, 7, 154–156. [Google Scholar] [CrossRef]
- Smith, D.W. Stellate Ganglion Block: The Tissue Displacement Method. Am. J. Surg. 1951, 82, 344–348. [Google Scholar] [CrossRef] [PubMed]
- Ugur, F.; Gulcu, N.; Gunes, I.; Özcan, N. Skin Marking for Stellate Block With Fluoroscopic Guidance. Reg. Anesth. Pain Med. 2006, 31, 386–387. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Yao, P.; Li, H.; Han, Z.; Wang, S.; Hong, T.; Zhao, G. CT-Guided Stellate Ganglion Pulsed Radiofrequency Stimulation for Facial and Upper Limb Postherpetic Neuralgia. Front. Neurosci. 2019, 13, 170. [Google Scholar] [CrossRef] [Green Version]
- Erickson, S.J.; Hogan, Q.H. CT-Guided Injection of the Stellate Ganglion: Description of Technique and Efficacy of Sympathetic Blockade. Radiology 1993, 188, 707–709. [Google Scholar] [CrossRef]
- Gofeld, M.; Bhatia, A.; Abbas, S.; Ganapathy, S.; Johnson, M. Development and Validation of a New Technique for Ultrasound-Guided Stellate Ganglion Block. Reg. Anesth. Pain Med. 2009, 34, 475–479. [Google Scholar] [CrossRef]
- Narouze, S. Ultrasound-Guided Stellate Ganglion Block: Safety and Efficacy. Curr. Pain Headache Rep. 2014, 18, 1–5. [Google Scholar] [CrossRef]
- Kapral, S.; Krafft, P.; Gosch, M.; Weinstabl, C.; Fleischmann, D. Ultrasound Imaging for Stellate Ganglion Block: Direct Visualization of Puncture Site and Local Anesthetic Spread: A Pilot Study. Reg. Anesth. J. Neural Blockade Obstet. Surg. Pain Control. 1995, 20, 323–328. [Google Scholar] [CrossRef]
- Ghai, A.; Kaushik, T.; Wadhera, R.; Wadhera, S. Stellate Ganglion Blockade-Techniques and Modalities. Acta Anaesthesiol. Belg. 2016, 67, 1–5. [Google Scholar]
- Ghai, A.; Kaushik, T.; Kundu, Z.S.; Wadhera, S.; Wadhera, R. Evaluation of New Approach to Ultrasound Guided Stellate Ganglion Block. Saudi. J. Anaesth. 2016, 10, 161. [Google Scholar] [CrossRef]
- Shan, H.H.; Chen, H.F.; Ni, Y.; Yang, J.X.; Zhou, X.L. Effects of Stellate Ganglion Block Through Different Approaches Under Guidance of Ultrasound. Front. Surg. 2022, 8, 740. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, S.; Tripathi, S. Cardiac Arrest Following Stellate Ganglion Block Performed under Ultrasound Guidance. Anaesthesia 2010, 65, 1042. [Google Scholar] [CrossRef] [PubMed]
- Brock, C.; Olesen, S.S.; Olesen, A.E.; Frøkjaer, J.B.; Andresen, T.; Drewes, A.M. Opioid-Induced Bowel Dysfunction. Drugs 2012, 72, 1847–1865. [Google Scholar] [CrossRef] [PubMed]
- Stienen, M.N.; Smoll, N.R.; Hildebrandt, G.; Schaller, K.; Tessitore, E.; Gautschi, O.P. Constipation after Thoraco-Lumbar Fusion Surgery. Clin. Neurol. Neurosurg. 2014, 126, 137–142. [Google Scholar] [CrossRef]
- Devulder, J.; De Laat, M.; Rolly, G. Stellate Ganglion Block Alleviates Pseudo-Obstruction Symptoms Followed by Episodes of Hypermetropia: Case Report. Reg. Anesth. 1997, 22, 284–286. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.Y.; Yang, G.T.; Sun, N.N.; Kong, Y.; Liu, Y.F. Efficacy and Safety of Stellate Ganglion Block in Chronic Ulcerative Colitis. World J. Gastroenterol. 2017, 23, 533. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Wu, Q.; Wang, H.; Zhang, Y.; Peng, X.; Chen, L. Effects of Ultrasound-Guided Stellate Ganglion Block on Postoperative Quality of Recovery in Patients Undergoing Breast Cancer Surgery: A Randomized Controlled Clinical Trial. J. Healthc. Eng. 2022, 2022, 7628183. [Google Scholar] [CrossRef]
- Zhu, G.; Kang, Z.; Chen, Y.; Zeng, J.; Su, C.; Li, S. Ultrasound-Guided Stellate Ganglion Block Alleviates Stress Responses and Promotes Recovery of Gastrointestinal Function in Patients. Dig. Liver Dis. 2021, 53, 581–586. [Google Scholar] [CrossRef]
- Peng, K.; Zhang, J.; Chen, W.R.; Liu, H.Y.; Ji, F.H. Ultrasound-Guided Stellate Ganglion Block Improves Gastrointestinal Function After Thoracolumbar Spinal Surgery. Clin. Ther. 2017, 39, 2322–2330. [Google Scholar] [CrossRef]
- Choquet, O.; Dadure, C.; Capdevila, X. Ultrasound-Guided Deep or Intermediate Cervical Plexus Block: The Target Should Be the Posterior Cervical Space. Anesth. Analg. 2010, 111, 1563–1564. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adamczyk, K.; Koszela, K.; Zaczyński, A.; Niedźwiecki, M.; Brzozowska-Mańkowska, S.; Gasik, R. Ultrasound-Guided Blocks for Spine Surgery: Part 1—Cervix. Int. J. Environ. Res. Public Health 2023, 20, 2098. https://doi.org/10.3390/ijerph20032098
Adamczyk K, Koszela K, Zaczyński A, Niedźwiecki M, Brzozowska-Mańkowska S, Gasik R. Ultrasound-Guided Blocks for Spine Surgery: Part 1—Cervix. International Journal of Environmental Research and Public Health. 2023; 20(3):2098. https://doi.org/10.3390/ijerph20032098
Chicago/Turabian StyleAdamczyk, Kamil, Kamil Koszela, Artur Zaczyński, Marcin Niedźwiecki, Sybilla Brzozowska-Mańkowska, and Robert Gasik. 2023. "Ultrasound-Guided Blocks for Spine Surgery: Part 1—Cervix" International Journal of Environmental Research and Public Health 20, no. 3: 2098. https://doi.org/10.3390/ijerph20032098





