HTA and Gastric Cancer: Evaluating Alternatives in Third- and Fourth-Line Patients
Abstract
1. Introduction
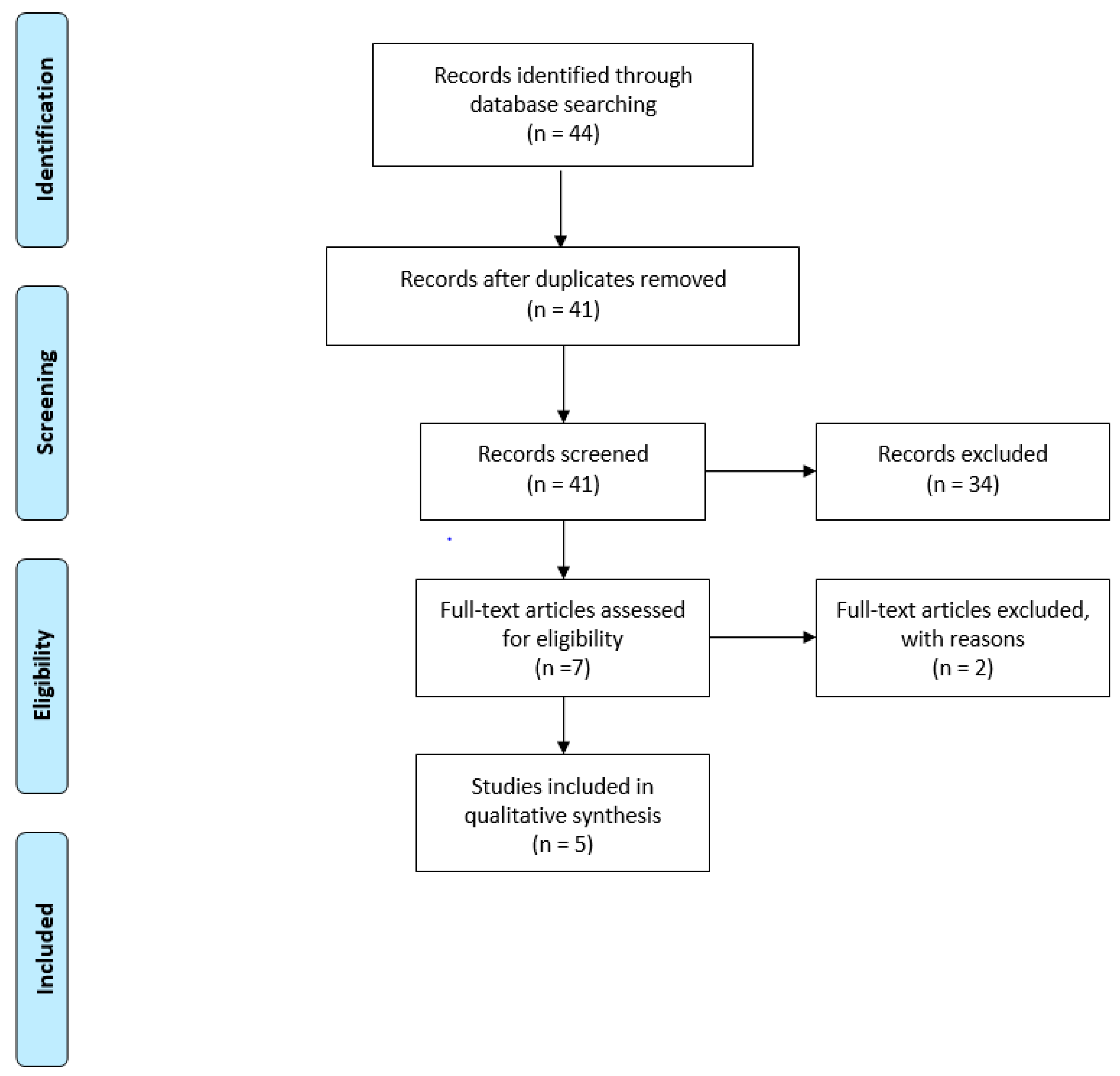
2. Methods
- (i)
- general relevance of the disease;
- (ii)
- technical relevance of the technologies under investigation;
- (iii)
- safety;
- (iv)
- efficacy;
- (v)
- economic-financial impact;
- (vi)
- social and ethical impact;
- (vii)
- equity impact;
- (viii)
- legal impact;
- (ix)
- organizational impact.
- (1)
- scientific evidence derived from a narrative literature review, for the definition of comparative safety and efficacy indicators, as well as for the definition of the potentially eligible population to FTD/TPI treatment;
- (2)
- health economics tools for the economic evaluation of the patient’s clinical pathway, assuming the investigated drugs, and for budget impact analysis;
- (3)
- qualitative approaches, by means of the development of a specific online qualitative questionnaire through the LimeSurvey platform, which was sent to a mailing list composed of 25 healthcare professionals directly involved in the proper mGC patients care and treatment, and was filled in by 2 oncologists, 3 pharmacists, and 3 nurses.
- P = patient (s) or population = adult patients with metastatic gastric cancer, on third- or fourth-line treatment.
- I = “Intervention,” = trifluridine/tipiracil (FTD/TPI).
- C = “Comparators,” i.e., traditional treatment = traditional treatment used in the clinical practice, consisting of best supportive care (BSC), FOLFIRI (5-FU, irinotecan, and leucovorin), docetaxel, and irinotecan.
- O = outcomes of interest declined as “overall survival—OS”, “progression-free survival—PFS”, “12-month OS rate”, “12-month PFS rate”, “3-month OS rate”, “3-month PFS rate”, and “Adverse events occurrence rate”.
- The therapy choice phase, considering all the procedures and activities performed to choose the best therapy for the mCG patients, based on their clinical conditions.
- The drug administration phase, consisting of the costs related to the administration of the therapy based on the drug duration cycle, assuming a 3-month time period.
- The treatment monitoring phase, regarding the panel of examinations, visits, and diagnostic procedures aimed at monitoring the therapeutical success, allowing timely intervention of any adverse events or treatment-related complications that occur.
- The pathology monitoring phase, regarding the panel of examinations, visits, and diagnostic procedures aimed at monitoring gastric cancer progression.
- The management and resolution of drug-related adverse events phase.
3. Results
3.1. Results from the Literature Evidence
3.2. Results from the Economic Analysis
3.3. Results from the Qualitative Assessment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Chivu-Economescu, M.; Matei, L.; Necula, L.G.; Dragu, D.L.; Bleotu, C.; Diaconu, C.C. New therapeutic options opened by the molecular classification of gastric cancer. World J. Gastroenterol. 2018, 24, 1942–1961. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhu, X.; Ren, X. Third-line chemotherapy in advanced gastric cancer. Medicine 2017, 96, e6884. [Google Scholar] [CrossRef] [PubMed]
- Smyth, E.C.; Verheij, M.; Allum, W.; Cunningham, D.; Cervantes, A.; Arnold, D.; Committee, E.G. Gastric cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2016, 27 (Suppl. 5), 27. [Google Scholar] [CrossRef]
- Fanotto, V.; Uccello, M.; Pecora, I.; Rimassa, L.; Leone, F.; Rosati, G.; Santini, D.; Giampieri, R.; Di Donato, S.; Tomasello, G.; et al. Outcomes of Advanced Gastric Cancer Patients Treated with at Least Three Lines of Systemic Chemotherapy. Oncologist 2017, 22, 1463–1469. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.M.; Park, S.H. Chemotherapy beyond second-line in advanced gastric cancer. World J. Gastroenterol. 2015, 21, 8811–8816. [Google Scholar] [CrossRef]
- Smyth, E.C.; Moehler, M. Late-line treatment in metastatic gastric cancer: Today and tomorrow. Ther. Adv. Med. Oncol. 2019, 11, 1758835919867522. [Google Scholar] [CrossRef]
- Shitara, K.; Doi, T.; Dvorkin, M.; Mansoor, W.; Arkenau, H.-T.; Prokharau, A.; Alsina, M.; Ghidini, M.; Faustino, C.; Gorbunova, V.; et al. Trifluridine/tipiracil versus placebo in patients with heavily pretreated metastatic gastric cancer (TAGS): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2018, 19, 1437–1448. [Google Scholar] [CrossRef]
- Pasquini, G.; Vasile, E.; Caparello, C.; Vivaldi, C.; Musettini, G.; Lencioni, M.; Petrini, I.; Fornaro, L.; Falcone, A. Third-Line Chemotherapy with Irinotecan plus 5-Fluorouracil in Caucasian Metastatic Gastric Cancer Patients. Oncology 2016, 91, 311–316. [Google Scholar] [CrossRef] [PubMed]
- EUNetHTA. The HTA Core Model, Version 3.0. April 2016. Available online: https://www.eunethta.eu/wp-content/uploads/2018/03/HTACoreModel3.0-1.pdf (accessed on 15 November 2022).
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; The PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Jadad, A.R.; Moore, R.A.; Carroll, D.; Jenkinson, C.; Reynolds, D.J.M.; Gavaghan, D.J.; McQuay, H.J. Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Control. Clin. Trials. 1996, 17, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle–Ottawa Scale (NOS) for Assessing the Quality of Non-Randomized Studies in Meta-Analysis. 2000. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 15 November 2022).
- Patton, M.Q. Qualitative Research & Evaluation Methods, 3rd ed.; Sage Publications: Thousand Oaks, CA, USA, 2002. [Google Scholar]
- Foglia, E.; Lettieri, E.; Ferrario, L.; Porazzi, E.; Garagiola, E.; Pagani, R.; Bonfanti, M.; Lazzarotti, V.; Manzini, R.; Masella, C.; et al. Technology assessment in hospitals: Lessons learned from an empirical experiment. Int. J. Technol. Assess. Health Care 2017, 33, 288–296. [Google Scholar] [CrossRef] [PubMed]
- Mitton, C.; Dionne, F.; Damji, R.; Campbell, D.; Bryan, S. Difficult decisions in times of constraint: Criteria based Resource Allocation in the Vancouver Coastal Health Authority. BMC Health Serv. Res. 2011, 11, 169. [Google Scholar] [CrossRef]
- Jansen, H. The Logic of Qualitative Survey Research and its Position in the Field of Social Research Methods. Forum Qual. Soc. Res. 2010, 11, 11. [Google Scholar]
- Tabernero, J.; Alsina, M.; Shitara, K.; Doi, T.; Dvorkin, M.; Mansoor, W.; Arkenau, H.-T.; Prokharau, A.; Ghidini, M.; Faustino, C.; et al. Health-related quality of life associated with trifluridine/tipiracil in heavily pretreated metastatic gastric cancer: Results from TAGS. Gastric Cancer 2020, 23, 689–698. [Google Scholar] [CrossRef]
- Tabernero, J.; Shitara, K.; Zaanan, A.; Doi, T.; Lorenzen, S.; van Cutsem, E.; Fornaro, L.; Catenacci, D.; Fougeray, R.; Moreno, S.; et al. Trifluridine/tipiracil versus placebo for third or later lines of treatment in metastatic gastric cancer: An exploratory subgroup analysis from the TAGS study. ESMO Open 2021, 6, 100200. [Google Scholar] [CrossRef]
- Kang, E.J.; Im, S.A.; Oh, D.Y.; Han, S.W.; Kim, J.S.; Choi, I.S.; Kim, J.W.; Kim, Y.J.; Kim, J.H.; Kim, T.Y.; et al. Irinotecan combined with 5-fluorouracil and leucovorin third-line chemotherapy after failure of fluoropyrimidine, platinum, and taxane in gastric cancer: Treatment outcomes and a prognostic model to predict survival. Gastric Cancer 2013, 16, 581–589. [Google Scholar] [CrossRef]
- Nishimura, T.; Iwasa, S.; Nagashima, K.; Okita, N.; Takashima, A.; Honma, Y.; Kato, K.; Hamaguchi, T.; Yamada, Y.; Shimada, Y.; et al. Irinotecan monotherapy as third-line treatment for advanced gastric cancer refractory to fluoropyrimidines, platinum, and taxanes. Gastric Cancer 2017, 20, 655–662. [Google Scholar] [CrossRef]
- Lee, M.J.; Hwang, I.G.; Jang, J.S.; Choi, J.H.; Park, B.B.; Chang, M.H.; Kim, S.T.; Park, S.H.; Kang, M.H.; Kang, J.H. Outcomes of third-line docetaxel-based chemotherapy in advanced gastric cancer who failed previous oxaliplatin-based and irinotecan-based chemotherapies. Cancer Res. Treat. 2012, 44, 235–241. [Google Scholar] [CrossRef]
- Kang, J.H.; Lee, S.I.; Lim, D.H.; Park, K.W.; Oh, S.Y.; Kwon, H.C.; Hwang, I.G.; Lee, S.C.; Nam, E.; Shin, D.B.; et al. Salvage chemotherapy for pretreated gastric cancer: A randomized phase III trial comparing chemotherapy plus best supportive care with best supportive care alone. J. Clin. Oncol. 2012, 30, 1513–1518. [Google Scholar] [CrossRef]
- Mayer, R.J.; Van Cutsem, E.; Falcone, A.; Yoshino, T.; Garcia-Carbonero, R.; Mizunuma, N.; Yamazaki, K.; Shimada, Y.; Tabernero, J.; Komatsu, Y.; et al. Randomized Trial of TAS-102 for Refractory Metastatic Colorectal Cancer. N. Engl. J. Med. 2015, 372, 1909–1919. [Google Scholar] [CrossRef] [PubMed]
- Gourzoulidis, G.; Koulentaki, M.; Koumarianou, A.; Samadas, E.; Androulakis, N.; Xynogalos, S.; Papakotoulas, P.; Boukovinas, I.; Karamouzis, M.; Souglakos, J.; et al. Cost-effectiveness of trifluridine/tipiracil as a third-line treatment of metastatic gastric cancer, including adenocarcinoma of the gastrohesophageal junction, among patients previously treated in Greece. Expert. Rev. Pharm. Outcomes Res. 2022, 22, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Takushima, Y.; Igarashi, A.; Yoshihara, H.; Shitara, K.; Doi, T. Cost-effectiveness of trifluridine/tipiracil against nivolumab for heavily pretreated metastatic gastric cancer in Japan. Jpn. J. Clin. Oncol. 2021, 51, 1383–1390. [Google Scholar] [CrossRef] [PubMed]
- Loh, A.Z.; Tan, J.S.; Tan, J.; Lyn, T.Y.; Krishna, L.K.; Goh, C.R. Place of care at end of life: What factors are associated with patients’ and their family members’ preferences? Am. J. Hosp. Palliat. Med. 2016, 33, 669–677. [Google Scholar] [CrossRef] [PubMed]
- Waller, A.; Sanson-Fisher, R.; Zdenkowski, N.; Douglas, C.; Hall, A.; Walsh, J. The right place at the right time: Medical oncology outpatients’ perceptions of location of end-of-life care. J. Natl. Compr. Cancer Netw. 2018, 16, 35–41. [Google Scholar] [CrossRef] [PubMed]
| Baseline Scenario | Innovative Scenario #1 | Innovative Scenario #2 | |
|---|---|---|---|
| FTD/TPI | 10% | 41% | 50% |
| BSC | 45% | 29.5% | 25% |
| FOLFIRI | 45% | 29.5% | 25% |
| FTD/TPI [8] | BSC [8] | FOLFIRI [9] | |
|---|---|---|---|
| Median OS (months) | 5.7 | 3.6 | 7.5 |
| 12-month OS (% of patients) | 21% | 13% | 16% |
| Mortality rate considering a median follow-up equal to 10.7 months (% of patients) | 72% | 82% | n.a. |
| Median PFS (months) | 2.0 | 1.8 | 3.3 |
| FTD/TPI [8] | BSC [8] | FOLFIRI [9,19] | |
|---|---|---|---|
| Nausea | 3% | 3% | 2.1% (based on the summary of product characteristics) |
| Anemia | 20% | 8% | 6.30% |
| Decreased appetite | 9% | 6% | not applicable |
| Vomiting | 4% | 2% | 3.03% |
| Diarrhea | 3% | 2% | 9.09% |
| Fatigue | 7% | 6% | 1.30% |
| Neutropenia | 34% | 0% | 36.8% |
| Asthenia | 5% | 7% | 3.03% |
| Thrombocytopenia | 3% | 0% | 1.90% |
| Leucopenia | 9% | 0% | not applicable |
| Abdominal pain | 4% | 9% | not applicable |
| Constipation | 2% | 2% | 1.90% |
| Back pain | 1% | 2% | not applicable |
| Increased blood alkaline phosphatase concentrations | 3% | 3% | not applicable |
| Dyspnea | 2% | 3% | not applicable |
| Dysphagia | 3% | 2% | not applicable |
| Ascites | 4% | 6% | not applicable |
| Hyponatremia | 1% | 4% | not applicable |
| Increased γ-glutamyl transferase concentrations | 2% | 3% | not applicable |
| Economic resources devoted to the management of drug-related adverse events | EUR 281.23 | EUR 157.02 | EUR 106.47 |
| FTD/TPI * | BSC * | FOLFIRI * | |
|---|---|---|---|
| Therapy choice phase * | EUR 77.47 | EUR 77.47 | EUR 77.47 |
| Drug administration phase * | EUR 5956.53 | EUR 1443.29 | EUR 5583.82 |
| Treatment monitoring phase * | EUR 202.23 | - | EUR 210.00 |
| Pathology monitoring phase * | EUR 357.40 | EUR 357.40 | EUR 357.40 |
| Management and resolution of drug-related adverse events phase * | EUR 281.23 | EUR 157.02 | EUR 106.47 |
| Total Costs per treatment | EUR 6874.86 | EUR 2035.17 | EUR 6335.16 |
| Baseline Scenario | Innovative Scenario #1 | Difference (EUR) | Difference (%) | |
| FTD/TPI | EUR 1,036,729.25 | EUR 4,250,589.92 | EUR 3,213,860.67 | 310% |
| BSC | EUR 1,381,069.35 | EUR 905,367.68 | EUR −475,701.66 | −34% |
| FOLFIRI | EUR 4,299,038.19 | EUR 2,818,258.37 | EUR −1,480,779,82 | −34% |
| Total | EUR 6,716,836.78 | EUR 7,974,215.97 | EUR 1,257,379.19 | 19% |
| Baseline Scenario | Innovative Scenario #2 | Difference (EUR) | Difference (%) | |
| FTD/TPI | EUR 1,036,729.25 | EUR 5,183,646.25 | EUR 4,146,917.00 | 400% |
| BSC | EUR 1,381,069.35 | EUR 767,260.75 | EUR −613,808.60 | −44% |
| FOLFIRI | EUR 4,299,038.19 | EUR 2,388,354.55 | EUR −1,910,683.64 | −44% |
| Total | EUR 6,716,836.78 | EUR 8,339,261.55 | EUR 1,622,424.76 | 24% |
| Equity Impact | FOLFIRI | BSC | FTD/TPI | p-Value |
| Access to care on the local level | 1.88 | 1.88 | 1.00 | 0.048 |
| Access to care for a person of a legally protected status | 2.00 | 2.00 | 2.00 | Not applicable |
| Impact on the hospital waiting list | −1.38 | 0.00 | 1.75 | 0.004 |
| Generation of health migrations | 0.00 | 0.00 | 0.00 | Not applicable |
| Existence of a factor limiting the use of the therapy for specific groups of patients | 0.00 | 0.00 | 0.00 | Not applicable |
| Iniquity | 0.00 | 0.00 | 0.00 | Not applicable |
| Average value for equity aspects | 0.42 | 0.65 | 0.79 | 0.590 |
| Social and ethical impact | FOLFIRI | BSC | FTD/TPI | p-value |
| Ability of the therapy to protect patients’ autonomy | 1.88 | 1.88 | 2.38 | 0.112 |
| Ability of the therapy to protect patients’ dignity | 2.38 | 2.48 | 2.38 | 0.459 |
| Ability of the therapy to protect patients’ religion | 1.63 | 1.63 | 1.63 | Not applicable |
| Impact of the therapy on social costs | 0.00 | 0.00 | 0.00 | Not applicable |
| Patients and citizens can have a good level of understanding of technology | 1.00 | 1.00 | 1.00 | Not applicable |
| Impact of the therapy on patient satisfaction | 1.25 | 1.00 | 1.75 | 0.041 |
| Impact of the therapy on patients’ quality of life | 1.50 | 1.00 | 2.00 | 0.028 |
| Impact of the therapy on caregivers’ quality of life | 1.25 | 1.00 | 1.50 | 0.011 |
| Average value for social and ethical aspects | 1.36 | 1.25 | 1.58 | 0.045 |
| Legal impact | FOLFIRI | BSC | FTD/TPI | p-value |
| Permission level of the drugs | 0.00 | 0.00 | 0.00 | Not applicable |
| Need for inclusion of the drugs’ registry | 0.00 | 0.00 | 0.00 | Not applicable |
| Fulfillment of the safety requirements | 2.00 | 2.00 | 2.00 | Not applicable |
| Infringement of intellectual property rights | 0.00 | 0.00 | 0.00 | Not applicable |
| Need to regulate the acquisition of the drug | 1.13 | 1.00 | −0.75 | 0.084 |
| The legislation covers the regulation of the therapy for all categories of patients | 1.00 | 1.00 | 1.00 | Not applicable |
| Average value for legal aspects | 0.69 | 0.67 | 0.38 | 0.379 |
| Organizational impact | FOLFIRI | BSC | FTD/TPI | p-value |
| Additional staff | 0.00 | 0.00 | 0.00 | Not applicable |
| Training course for all the healthcare professionals involved | −0.75 | −0.75 | −1.00 | 0.423 |
| Education of patients’ and caregivers | −1.50 | −1.38 | −1.63 | 0.629 |
| Hospital meetings required | −0.63 | −0.71 | −0.38 | 0.289 |
| Learning curve | 0.00 | 0.00 | 0.00 | Not applicable |
| Compliance with hospital protocol | 1.25 | 1.00 | 1.88 | 0.165 |
| Additional hospital spaces or furniture | 0.00 | 0.00 | 0.00 | Not applicable |
| Impact of the drug on hospital waiting lists | −2.23 | 0.00 | 1.25 | 0.032 |
| Impact of the drug on the organizational management of adverse events | −0.38 | −1.50 | −0.75 | 0.117 |
| Impact of the drug on the organizational management of the patient, in terms of follow-up monitoring activities | 0.38 | 0.43 | 0.38 | 0.319 |
| Impact of the drug on internal processes | −1.13 | 0.00 | 1.00 | 0.005 |
| Impact of the drug on the hospital connection process | 0.38 | 0.00 | 0.25 | 0.543 |
| Impact of the drug on the hospital purchasing process | 0.38 | 0.00 | 0.50 | 0.678 |
| Impact of the therapy on the patient’s clinical pathway optimization | 0.63 | 0.57 | 2.00 | 0.009 |
| Impact of the drug on the occupancy of chemotherapy chairs | −1.00 | 0.85 | 3.00 | 0.001 |
| Impact of the drug on the monitoring activities | −1.00 | 0.25 | 0.75 | 0.074 |
| Impact of the drug on the treatment duration | 1.00 | 0.00 | 1.25 | 0.059 |
| Average value for organizational aspects | −0.27 | −0.07 | 0.44 | 0.031 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferrario, L.; Asperti, F.; Aprile, G.; Giuliani, J. HTA and Gastric Cancer: Evaluating Alternatives in Third- and Fourth-Line Patients. Int. J. Environ. Res. Public Health 2023, 20, 2107. https://doi.org/10.3390/ijerph20032107
Ferrario L, Asperti F, Aprile G, Giuliani J. HTA and Gastric Cancer: Evaluating Alternatives in Third- and Fourth-Line Patients. International Journal of Environmental Research and Public Health. 2023; 20(3):2107. https://doi.org/10.3390/ijerph20032107
Chicago/Turabian StyleFerrario, Lucrezia, Federica Asperti, Giuseppe Aprile, and Jacopo Giuliani. 2023. "HTA and Gastric Cancer: Evaluating Alternatives in Third- and Fourth-Line Patients" International Journal of Environmental Research and Public Health 20, no. 3: 2107. https://doi.org/10.3390/ijerph20032107
APA StyleFerrario, L., Asperti, F., Aprile, G., & Giuliani, J. (2023). HTA and Gastric Cancer: Evaluating Alternatives in Third- and Fourth-Line Patients. International Journal of Environmental Research and Public Health, 20(3), 2107. https://doi.org/10.3390/ijerph20032107







