Groundwater Quality, Health Risk Assessment, and Source Distribution of Heavy Metals Contamination around Chromite Mines: Application of GIS, Sustainable Groundwater Management, Geostatistics, PCAMLR, and PMF Receptor Model
Abstract
:1. Introduction
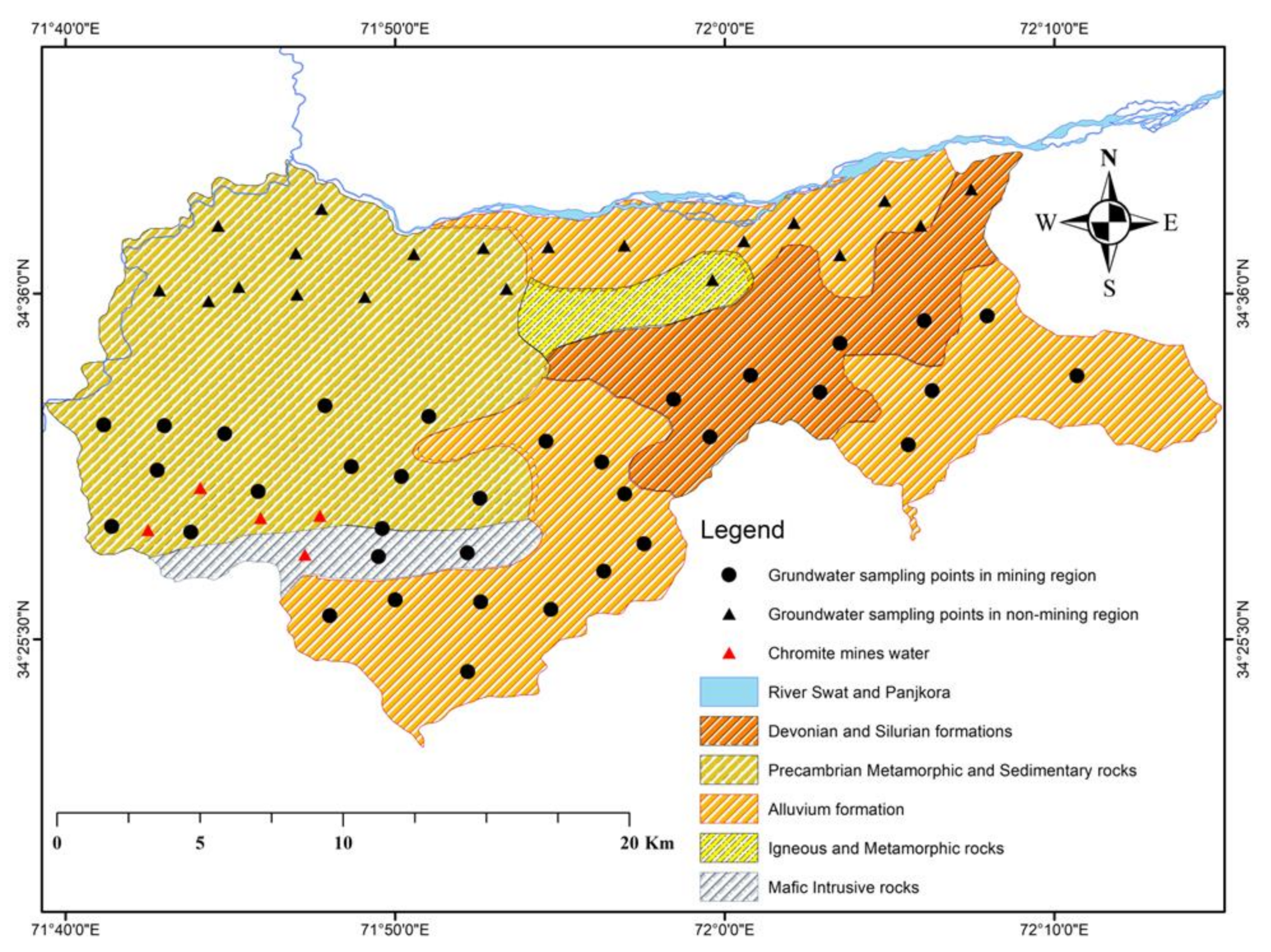
2. Study Area
2.1. Description of the Study Area
2.2. Climate and Hydrology
2.3. Local Geological Setting of the Area
3. Methodology
3.1. Groundwater Sampling
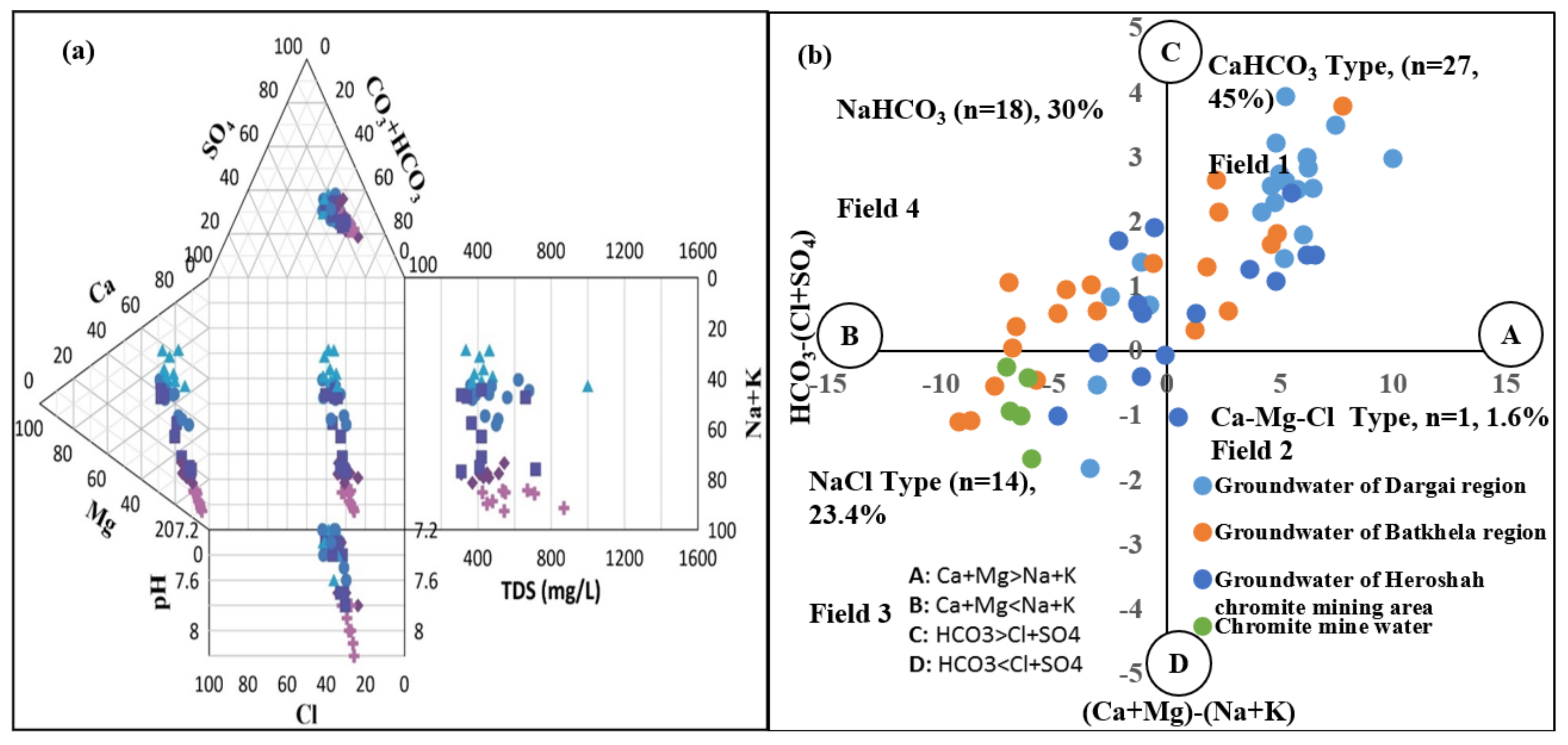
3.2. Gibbs and Chadha Plotting
3.3. Nemarow’s Pollution Index
3.4. Human Health Risk Assessment (HHRA) Model
3.5. Statistical Analysis
3.6. Groundwater Mapping, Geochemical Speciation, and Mineral Phases
3.7. Positive Matrix Factorization (PMF) Receptor Model
3.8. Precision and Accuracy of Groundwater Data
4. Results and Discussion
4.1. Groundwater Composition
4.2. Gibbs Plotting
4.3. Chadha and Durov Diagrams
4.4. Nemarow’s Pollution Indexing
4.5. Health Risk of Exposure to HMs
4.6. Geospatial Distribution and Representation of Pollution Hotspot
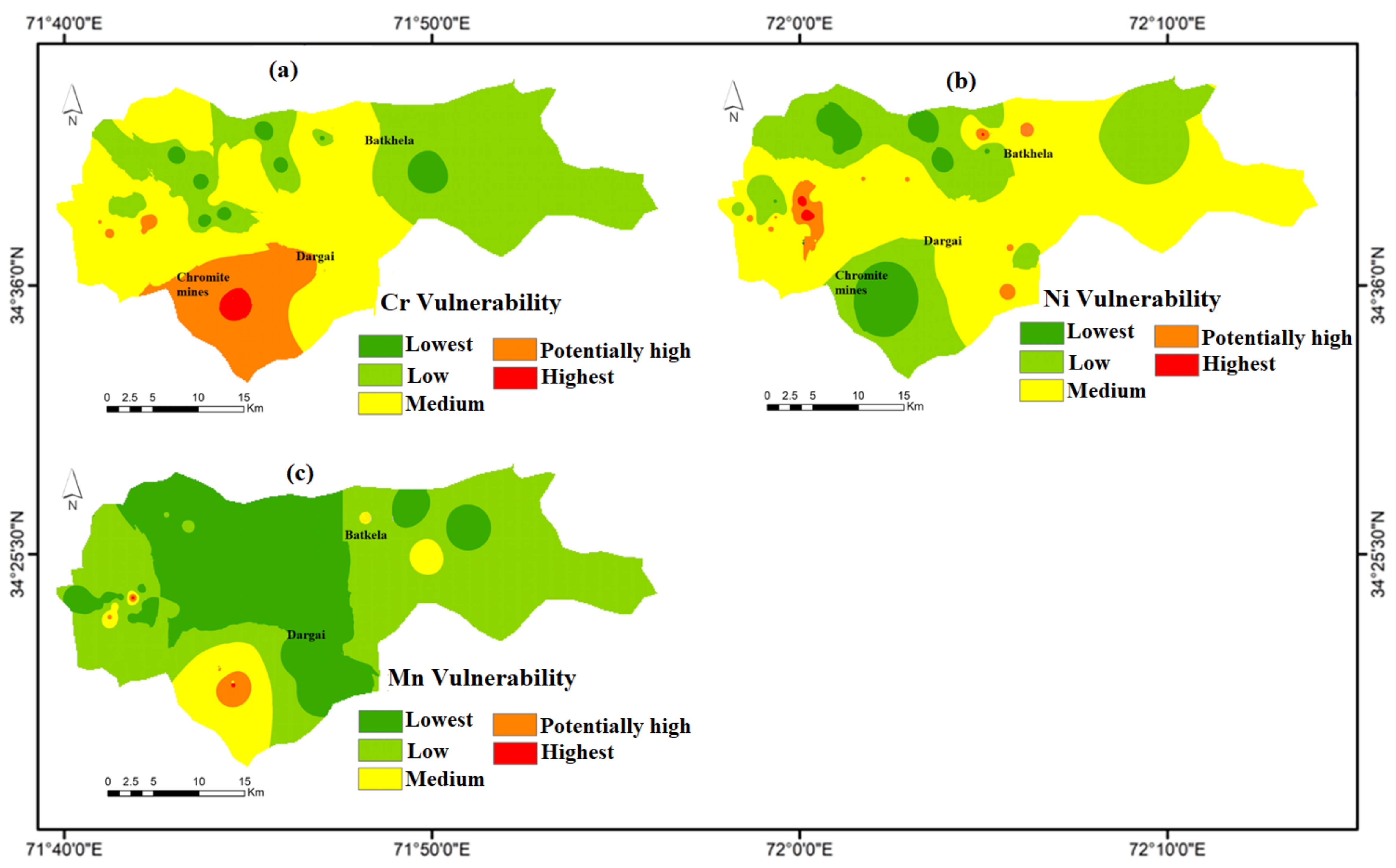
4.7. Groundwater Vulnerability Mapping of HMs
4.8. Geochemical Speciation of HMs and Groundwater Variables
4.9. Mineral Phases of Groundwater Variables
4.10. Hierarchical Agglomerative Cluster Analysis
4.11. Pollution Identification
4.11.1. Principal Component Analysis Multilinear Regression Technique (PCAMLR)
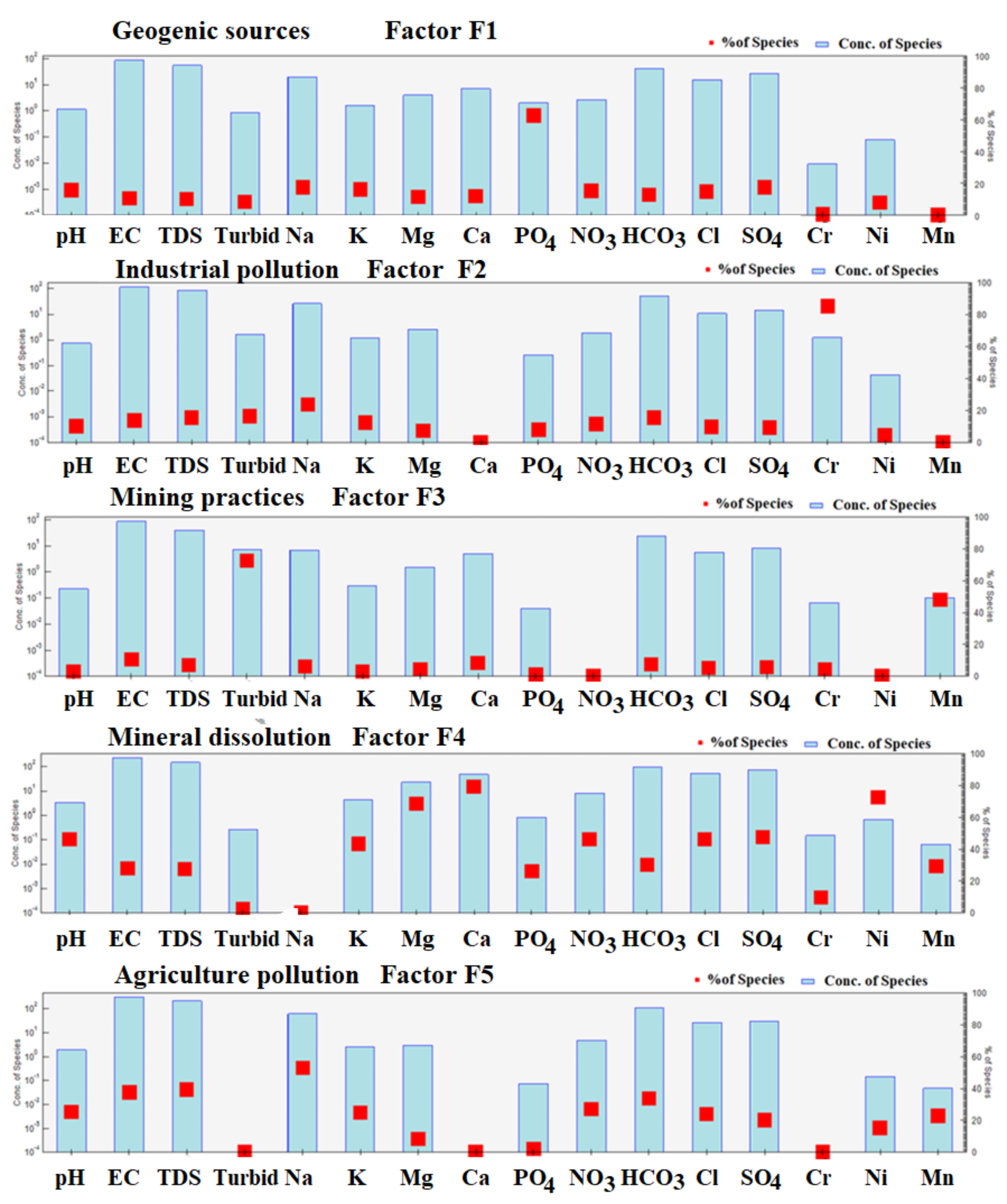
4.11.2. Source Apportionment Using PMF
4.12. Sustainable Groundwater Management
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Ethics Approval and Consent to Participate
References
- Shams, M.; Tavakkoli Nezhad, N.; Dehghan, A.; Alidadi, H.; Paydar, M.; Mohammadi, A.A.; Zarei, A. Heavy metals exposure, carcinogenic and non-carcinogenic human health risks assessment of groundwater around mines in Joghatai, Iran. Int. J. Environ. Anal. Chem. 2022, 102, 1884–1899. [Google Scholar] [CrossRef]
- Sankhla, M.S.; Kumar, R. Contaminant of heavy metals in groundwater & its toxic effects on human health & environment. Int. J. Environ. Sci. Nat. Res. 2019, 18, 555996. [Google Scholar]
- Rashid, A.; Ayub, M.; Javed, A.; Khan, S.; Gao, X.; Li, C.; Ullah, Z.; Sardar, T.; Muhammad, J.; Nazneen, S. Potentially harmful metals, and health risk evaluation in groundwater of Mardan, Pakistan: Application of geostatistical approach and geographic information system. Geosci. Front. 2021, 12, 101128. [Google Scholar] [CrossRef]
- Testa, S.M.; Guertin, J.; Jacobs, J.; Avakian, C. Sources of Chromium Contamination in Soil and Groundwater; CRC Press: Boca Raton, FL, USA, 2004; pp. 143–164. [Google Scholar]
- Sharma, A.; Kapoor, D.; Wang, J.; Shahzad, B.; Kumar, V.; Bali, A.S.; Jasrotia, S.; Zheng, B.; Yuan, H.; Yan, D. Chromium bioaccumulation and its impacts on plants: An overview. Plants 2020, 9, 100. [Google Scholar] [CrossRef] [Green Version]
- Tripathi, S.M.; Chaurasia, S. Detection of Chromium in surface and groundwater and its bio-absorption using bio-wastes and vermiculite. Eng. Sci. Technol. Int. J. 2020, 23, 1153–1161. [Google Scholar] [CrossRef]
- Aithani, D.; Jyethi, D.S.; Siddiqui, Z.; Yadav, A.K.; Khillare, P. Source apportionment, pollution assessment, and ecological and human health risk assessment due to trace metals contaminated groundwater along urban river floodplain. Groundw. Sustain. Dev. 2020, 11, 100445. [Google Scholar] [CrossRef]
- Ertani, A.; Mietto, A.; Borin, M.; Nardi, S. Chromium in agricultural soils and crops: A review. Water Air Soil Pollut. 2017, 228, 190. [Google Scholar] [CrossRef]
- Benítez, S.V.B. Understanding of (Bio) Geochemical Processes Which Control Chromium Release, Speciation and Isotopic Fractionation in Ultramafic Environments Impacted by Mining Activitites. Ph.D. Thesis, Université Paris-Est; Università degli studi, Cassino, Italy, 2018. [Google Scholar]
- Liu, M.; Xu, Y.; Nawab, J.; Rahman, Z.; Khan, S.; Idress, M.; Ali, A.; Ahmad, R.; Khan, S.A.; Khan, A. Contamination features, geo-accumulation, enrichments and human health risks of toxic heavy metal (loids) from fish consumption collected along Swat river, Pakistan. Environ. Technol. Innov. 2020, 17, 100554. [Google Scholar] [CrossRef]
- Kumar, A.; Maiti, S.K. Assessment of potentially toxic heavy metal contamination in agricultural fields, sediment, and water from an abandoned chromite-asbestos mine waste of Roro hill, Chaibasa, India. Environ. Earth Sci. 2015, 74, 2617–2633. [Google Scholar] [CrossRef]
- Fida, M.; Li, P.; Wang, Y.; Alam, S.; Nsabimana, A. Water contamination and human health risks in Pakistan: A review. Expo. Health 2022, 1–21. [Google Scholar] [CrossRef]
- United Nations Environment Programme; International Resource Panel; United Nations Environment Programme; Sustainable Consumption; Production Branch. Decoupling Natural Resource Use and Environmental Impacts from Economic Growth; UNEP: Paris, France; Earthprint: Stevenage, UK, 2011. [Google Scholar]
- Brichieri-Colombi, J. Food security, irrigation and water stress: Logical chain or environmental myth? Occas. Pap. 2003, 57. [Google Scholar]
- Khan, M.; Ellahi, A.; Niaz, R.; Ghoneim, M.E.; Tag-eldin, E.; Rashid, A. Water quality assessment of alpine glacial blue water lakes and glacial-fed rivers. Geomat. Nat. Hazards Risk 2022, 13, 2597–2617. [Google Scholar] [CrossRef]
- Saxena, G.; Purchase, D.; Mulla, S.I.; Saratale, G.D.; Bharagava, R.N. Phytoremediation of heavy metal-contaminated sites: Eco-environmental concerns, field studies, sustainability issues, and future prospects. Rev. Environ. Contam. Toxicol. 2019, 249, 71–131. [Google Scholar]
- Sikdar, A.; Hossain, M.S.; Feng, S. Heavy Metal Pollution of Environment by Mine Tailings and the Potential Reclamation Techniques: A Review. J. Biol. Agric. Health 2020, 10, 33–37. [Google Scholar]
- Ge, X.; Ma, Y.; Song, X.; Wang, G.; Zhang, H.; Zhang, Y.; Zhao, H. β-FeOOH nanorods/carbon foam-based hierarchically porous monolith for highly effective arsenic removal. ACS Appl. Mater. Interfaces 2017, 9, 13480–13490. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Shao, P.; Ding, L.; Xi, Y.; Zhang, K.; Yang, L.; Shi, H.; Luo, X. Protonation of rhodanine polymers for enhancing the capture and recovery of Ag+ from highly acidic wastewater. Environ. Sci. Nano 2019, 6, 3307–3315. [Google Scholar] [CrossRef]
- Zou, J.; Wang, M.; Jiang, W.; Liu, D. Chromium accumulation and its effects on other mineral elements in Amaranthus viridis L. Acta Biol. Crac. Ser. Bot. 2006, 48, 7–12. [Google Scholar]
- Gallios, G.P.; Vaclavikova, M. Removal of chromium (VI) from water streams: A thermodynamic study. Environ. Chem. Lett. 2008, 6, 235–240. [Google Scholar] [CrossRef]
- Oh, Y.J.; Song, H.; Shin, W.S.; Choi, S.J.; Kim, Y.-H. Effect of amorphous silica and silica sand on removal of chromium (VI) by zero-valent iron. Chemosphere 2007, 66, 858–865. [Google Scholar] [CrossRef]
- Zink, S.; Schoenberg, R.; Staubwasser, M. Isotopic fractionation and reaction kinetics between Cr (III) and Cr (VI) in aqueous media. Geochim. Cosmochim. Acta 2010, 74, 5729–5745. [Google Scholar] [CrossRef]
- Liang, J.; Huang, X.; Yan, J.; Li, Y.; Zhao, Z.; Liu, Y.; Ye, J.; Wei, Y. A review of the formation of Cr (VI) via Cr (III) oxidation in soils and groundwater. Sci. Total Environ. 2021, 774, 145762. [Google Scholar] [CrossRef]
- Wolf, R.E.; Morrison, J.M.; Goldhaber, M.B. Simultaneous determination of Cr (III) and Cr (VI) using reversed-phased ion-pairing liquid chromatography with dynamic reaction cell inductively coupled plasma mass spectrometry. J. Anal. At. Spectrom. 2007, 22, 1051–1060. [Google Scholar] [CrossRef]
- Mpouras, T.; Polydera, A.; Dermatas, D.; Verdone, N.; Vilardi, G. Multi wall carbon nanotubes application for treatment of Cr (VI)-contaminated groundwater; Modeling of batch & column experiments. Chemosphere 2021, 269, 128749. [Google Scholar]
- Bagchi, D.; Stohs, S.J.; Downs, B.W.; Bagchi, M.; Preuss, H.G. Cytotoxicity and oxidative mechanisms of different forms of chromium. Toxicology 2002, 180, 5–22. [Google Scholar] [CrossRef] [PubMed]
- Eastmond, D.A.; MacGregor, J.T.; Slesinski, R.S. Trivalent chromium: Assessing the genotoxic risk of an essential trace element and widely used human and animal nutritional supplement. Crit. Rev. Toxicol. 2008, 38, 173–190. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J.B. Recent advances in the nutritional biochemistry of trivalent chromium. Proc. Nutr. Soc. 2004, 63, 41–47. [Google Scholar] [CrossRef]
- Costa, M. Potential hazards of hexavalent chromate in our drinking water. Toxicol. Appl. Pharmacol. 2003, 188, 1–5. [Google Scholar] [CrossRef]
- Mishra, S.; Bharagava, R.N. Toxic and genotoxic effects of hexavalent chromium in environment and its bioremediation strategies. J. Environ. Sci. Health Part C 2016, 34, 1–32. [Google Scholar] [CrossRef]
- Boutroy, E.; Dare, S.A.; Beaudoin, G.; Barnes, S.-J.; Lightfoot, P.C. Magnetite composition in Ni-Cu-PGE deposits worldwide: Application to mineral exploration. J. Geochem. Explor. 2014, 145, 64–81. [Google Scholar] [CrossRef]
- Duggal, V.; Rani, A.; Mehra, R.; Balaram, V. Risk assessment of metals from groundwater in northeast Rajasthan. J. Geol. Soc. India 2017, 90, 77–84. [Google Scholar] [CrossRef]
- Okogbue, C.O.; Ukpai, S.N. Evaluation of trace element contents in groundwater in Abakaliki metropolis and around the abandoned mine sites in the southern part, Southeastern Nigeria. Environ. Earth Sci. 2013, 70, 3351–3362. [Google Scholar] [CrossRef]
- Kaur, S.; Mehra, R.; Kumar, M. Quantification of health risks and spatial distribution of heavy metals in groundwater of Lower Himalayas, India. Int. J. Environ. Sci. Technol. 2021, 19, 3201–3218. [Google Scholar] [CrossRef]
- Perraki, M.; Vasileiou, E.; Bartzas, G. Tracing the origin of chromium in groundwater: Current and new perspectives. Curr. Opin. Environ. Sci. Health 2021, 22, 100267. [Google Scholar] [CrossRef]
- Mishra, S.; Bharagava, R.N.; More, N.; Yadav, A.; Zainith, S.; Mani, S.; Chowdhary, P. Heavy metal contamination: An alarming threat to environment and human health. In Environmental Biotechnology: For Sustainable Future; Springer: Berlin/Heidelberg, Germany, 2019; pp. 103–125. [Google Scholar]
- Antin, D. The South African mining sector: An industry at a crossroads. In Economy Report South Africa; Hanns Seidel Foundation: Johannesburg, South Africa, 2013. [Google Scholar]
- Hattula, A.; Rekola, T. Exploration geophysics at the Pyhäsalmi mine and grade control work of the Outokumpu Group. Geophysics 2000, 65, 1961–1969. [Google Scholar] [CrossRef]
- Naz, A.; Mishra, B.K.; Gupta, S.K. Human health risk assessment of chromium in drinking water: A case study of Sukinda chromite mine, Odisha, India. Expo. Health 2016, 8, 253–264. [Google Scholar] [CrossRef]
- Hove, G.; Rathaha, T.; Mugiya, P. The Impact of Human Activities on the Environment, Case of Mhondongori in Zvishavane, Zimbabwe. J. Geosci. Environ. Prot. 2020, 8, 330–349. [Google Scholar] [CrossRef]
- Bacani, M.R.B.; Farin, A.N. Assessment of Heavy Metals in Agricultural Crops Near Mining Areas in Zambales, Philippines. Nat. Environ. Pollut. Technol. 2018, 17, 869–876. [Google Scholar]
- Kudabayeva, K.; Baspakova, A.; Bazargaliyev, E.; Sakhanova, S.; Nuftieva, A. The significance of the imbalance of chromium and other trace elements in the development of goiter in school Aktobe region of the Republic of Kazakhstan. Curr. Sci. 2017, 112, 1107. [Google Scholar]
- Zhang, Z.; Guo, G.; Zhao, H.; Wu, D. Partitioning, leachability, and speciation of chromium in the size-fractions of soil contaminated by chromate production. Chemosphere 2021, 263, 128308. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, H.; He, L.; Lu, K.; Sarmah, A.; Li, J.; Bolan, N.S.; Pei, J.; Huang, H. Using biochar for remediation of soils contaminated with heavy metals and organic pollutants. Environ. Sci. Pollut. Res. 2013, 20, 8472–8483. [Google Scholar] [CrossRef]
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, mechanism and health effects of some heavy metals. Interdiscip. Toxicol. 2014, 7, 60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bianconi, E.; Piovesan, A.; Facchin, F.; Beraudi, A.; Casadei, R.; Frabetti, F.; Vitale, L.; Pelleri, M.C.; Tassani, S.; Piva, F. An estimation of the number of cells in the human body. Ann. Hum. Biol. 2013, 40, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Rashid, A.; Khan, S.; Ayub, M.; Sardar, T.; Jehan, S.; Zahir, S.; Khan, M.S.; Muhammad, J.; Khan, R.; Ali, A.; et al. Mapping human health risk from exposure to potential toxic metal contamination in groundwater of Lower Dir, Pakistan: Application of multivariate and geographical information system. Chemosphere 2019, 225, 785–795. [Google Scholar] [CrossRef] [PubMed]
- Zayed, A.M.; Terry, N. Chromium in the environment: Factors affecting biological remediation. Plant Soil 2003, 249, 139–156. [Google Scholar] [CrossRef]
- Qian, A.; Liao, P.; Yuan, S.; Luo, M. Efficient reduction of Cr (VI) in groundwater by a hybrid electro-Pd process. Water Res. 2014, 48, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.R.; Riyazuddin, P. Seasonal variation of redox species and redox potentials in shallow groundwater: A comparison of measured and calculated redox potentials. J. Hydrol. 2012, 444, 187–198. [Google Scholar] [CrossRef]
- Kumar, A.R.; Riyazuddin, P. Chromium speciation in a contaminated groundwater: Redox processes and temporal variability. Environ. Monit. Assess. 2011, 176, 647–662. [Google Scholar] [CrossRef]
- Mills, C.T.; Morrison, J.M.; Goldhaber, M.B.; Ellefsen, K.J. Chromium (VI) generation in vadose zone soils and alluvial sediments of the southwestern Sacramento Valley, California: A potential source of geogenic Cr (VI) to groundwater. Appl. Geochem. 2011, 26, 1488–1501. [Google Scholar] [CrossRef]
- Zhang, L.; Qin, X.; Tang, J.; Liu, W.; Yang, H. Review of arsenic geochemical characteristics and its significance on arsenic pollution studies in karst groundwater, Southwest China. Appl. Geochem. 2017, 77, 80–88. [Google Scholar] [CrossRef]
- Nikolenko, O.; Jurado, A.; Borges, A.V.; Knöller, K.; Brouyère, S. Isotopic composition of nitrogen species in groundwater under agricultural areas: A review. Sci. Total Environ. 2018, 621, 1415–1432. [Google Scholar] [CrossRef] [PubMed]
- Ogundiran, M.; Osibanjo, O. Mobility and speciation of heavy metals in soils impacted by hazardous waste. Chem. Speciat. Bioavailab. 2009, 21, 59–69. [Google Scholar] [CrossRef]
- Thakare, M.; Sarma, H.; Datar, S.; Roy, A.; Pawar, P.; Gupta, K.; Pandit, S.; Prasad, R. Understanding the holistic approach to plant-microbe remediation technologies for removing heavy metals and radionuclides from soil. Curr. Res. Biotechnol. 2021, 3, 84–98. [Google Scholar] [CrossRef]
- Tauqeer, H.M.; Fatima, M.; Rashid, A.; Shahbaz, A.K.; Ramzani, P.M.A.; Farhad, M.; Basharat, Z.; Turan, V.; Iqbal, M. The current scenario and prospects of immobilization remediation technique for the management of heavy metals contaminated soils. In Approaches to the Remediation of Inorganic Pollutants; Springer: Berlin/Heidelberg, Germany, 2021; pp. 155–185. [Google Scholar]
- Kumar, M.; Kushwaha, A.; Goswami, L.; Singh, A.K.; Sikandar, M. A review on advances and mechanism for the phycoremediation of cadmium contaminated wastewater. Clean. Eng. Technol. 2021, 5, 100288. [Google Scholar] [CrossRef]
- Rashid, A.; Ayub, M.; Ullah, Z.; Ali, A.; Khattak, S.A.; Ali, L.; Gao, X.; Li, C.; Khan, S.; El-Serehy, H.A. Geochemical Modeling Source Provenance, Public Health Exposure, and Evaluating Potentially Harmful Elements in Groundwater: Statistical and Human Health Risk Assessment (HHRA). Int. J. Environ. Res. Public Health 2022, 19, 6472. [Google Scholar] [CrossRef]
- Foster, S.; Chilton, J.; Nijsten, G.-J.; Richts, A. Groundwater—A global focus on the ‘local resource’. Curr. Opin. Environ. Sustain. 2013, 5, 685–695. [Google Scholar] [CrossRef]
- Gleeson, T.; Richter, B. How much groundwater can we pump and protect environmental flows through time? Presumptive standards for conjunctive management of aquifers and rivers. River Res. Appl. 2018, 34, 83–92. [Google Scholar] [CrossRef]
- Khalid, S. An assessment of groundwater quality for irrigation and drinking purposes around brick kilns in three districts of Balochistan province, Pakistan, through water quality index and multivariate statistical approaches. J. Geochem. Explor. 2019, 197, 14–26. [Google Scholar]
- Qureshi, A.S. Groundwater governance in Pakistan: From colossal development to neglected management. Water 2020, 12, 3017. [Google Scholar] [CrossRef]
- Ngene, B.U.; Nwafor, C.O.; Bamigboye, G.O.; Ogbiye, A.S.; Ogundare, J.O.; Akpan, V.E. Assessment of water resources development and exploitation in Nigeria: A review of integrated water resources management approach. Heliyon 2021, 7, e05955. [Google Scholar] [CrossRef]
- Basharat, M. Water management in the Indus Basin in Pakistan: Challenges and opportunities. In Indus River Basin; Elsevier: Amsterdam, The Netherlands, 2019; pp. 375–388. [Google Scholar]
- Mohan, S.; Kuipally, N. Groundwater and Conjunctive Use Management. In Handbook of Water Resources Management: Discourses, Concepts and Examples; Springer: Berlin/Heidelberg, Germany, 2021; pp. 735–775. [Google Scholar]
- Iyyapazham, S. Managing Water Resources in Agriculture and Watersheds: Modeling Using GIS and Dynamic Simulation. Ph.D. Thesis, University of Massachusetts Amherst, Amherst, MA, USA, 2007. [Google Scholar]
- Grönwall, J.T.; Mulenga, M.; McGranahan, G. Groundwater, Self-Supply and Poor Urban Dwellers: A Review with Case Studies of Bangalore and Lusaka; IIED: Amsterdam, The Netherlands, 2010. [Google Scholar]
- Talpur, S.A.; Noonari, T.M.; Rashid, A.; Ahmed, A.; Jat Baloch, M.Y.; Talpur, H.A.; Soomro, M.H. Hydrogeochemical signatures and suitability assessment of groundwater with elevated fluoride in unconfined aquifers Badin district, Sindh, Pakistan. SN Appl. Sci. 2020, 2, 1038. [Google Scholar] [CrossRef]
- Iqbal, J.; Su, C.; Rashid, A.; Yang, N.; Baloch, M.Y.J.; Talpur, S.A.; Ullah, Z.; Rahman, G.; Rahman, N.U.; Sajjad, M.M. Hydrogeochemical assessment of groundwater and suitability analysis for domestic and agricultural utility in Southern Punjab, Pakistan. Water 2021, 13, 3589. [Google Scholar] [CrossRef]
- Gorelick, S.M.; Zheng, C. Global change and the groundwater management challenge. Water Resour. Res. 2015, 51, 3031–3051. [Google Scholar] [CrossRef]
- Habib, Z. Water Availability, Use and Challenges in Pakistan-Water Sector Challenges in the Indus Basin and Impact of Climate Change; Food & Agriculture Organization: Rome, Italy, 2021. [Google Scholar]
- Noor, S.; Rashid, A.; Javed, A.; Khattak, J.A.; Farooqi, A. Hydrogeological properties, sources provenance, and health risk exposure of fluoride in the groundwater of Batkhela, Pakistan. Environ. Technol. Innov. 2022, 25, 102239. [Google Scholar] [CrossRef]
- Pérez Hoyos, I.C.; Krakauer, N.Y.; Khanbilvardi, R.; Armstrong, R.A. A review of advances in the identification and characterization of groundwater dependent ecosystems using geospatial technologies. Geosciences 2016, 6, 17. [Google Scholar] [CrossRef] [Green Version]
- Tofiloski, S. Geospatial Analysis of Water-Associated Infectious Diseases: Case of Myanmar. Master’s Thesis, Central European University, Budapest, Hungary, 2018. [Google Scholar]
- Ahmad, I.; Dar, M.A.; Fenta, A.; Halefom, A.; Nega, H.; Andualem, T.G.; Teshome, A. Spatial configuration of groundwater potential zones using OLS regression method. J. Afr. Earth Sci. 2021, 177, 104147. [Google Scholar] [CrossRef]
- Medici, G.; Langman, J.B. Pathways and Estimate of Aquifer Recharge in a Flood Basalt Terrain; A Review from the South Fork Palouse River Basin (Columbia River Plateau, USA). Sustainability 2022, 14, 11349. [Google Scholar] [CrossRef]
- Ducci, D.; Sellerino, M. Vulnerability mapping of groundwater contamination based on 3D lithostratigraphical models of porous aquifers. Sci. Total Environ. 2013, 447, 315–322. [Google Scholar] [CrossRef]
- Gordon, F.D. Freshwater Resources and Interstate Cooperation: Strategies to Mitigate an Environmental Risk; SUNY Press: Albany, NY, USA, 2008. [Google Scholar]
- Rashid, A.; Guan, D.-X.; Farooqi, A.; Khan, S.; Zahir, S.; Jehan, S.; Khattak, S.A.; Khan, M.S.; Khan, R. Fluoride prevalence in groundwater around a fluorite mining area in the flood plain of the River Swat, Pakistan. Sci. Total Environ. 2018, 635, 203–215. [Google Scholar] [CrossRef] [Green Version]
- Arun, P.V. A comparative analysis of different DEM interpolation methods. Egypt. J. Remote Sens. Space Sci. 2013, 16, 133–139. [Google Scholar]
- Shukla, K.; Kumar, P.; Mann, G.S.; Khare, M. Mapping spatial distribution of particulate matter using Kriging and Inverse Distance Weighting at supersites of megacity Delhi. Sustain. Cities Soc. 2020, 54, 101997. [Google Scholar] [CrossRef]
- Linnik, V.G.; Bauer, T.V.; Minkina, T.M.; Mandzhieva, S.S.; Mazarji, M. Spatial distribution of heavy metals in soils of the flood plain of the Seversky Donets River (Russia) based on geostatistical methods. Environ. Geochem. Health 2020, 44, 319–333. [Google Scholar] [CrossRef] [PubMed]
- Ali, L.; Rashid, A.; Khattak, S.A.; Gao, X.; Jehan, S.; Javed, A. Geochemical modeling, fate distribution, and risk exposure of potentially toxic metals in the surface sediment of the Shyok suture zone, northern Pakistan. Int. J. Sediment Res. 2021, 36, 656–667. [Google Scholar] [CrossRef]
- Khan, I.; Choudhry, Z.I.; Ali, A. Impact of Adult Literacy Centers on Women Social Lives in District Malakand Khyber Pakhtunkhwa, Pakistan: A Case Study of NCHD Adult Literacy Program. Global Social Sciences Review 2018, 3, 130–141. [Google Scholar] [CrossRef]
- Rashid, A.; Ayub, M.; Khan, S.; Ullah, Z.; Ali, L.; Gao, X.; Li, C.; El-Serehy, H.A.; Kaushik, P.; Rasool, A. Hydrogeochemical assessment of carcinogenic and non-carcinogenic health risks of potentially toxic elements in aquifers of the Hindukush ranges, Pakistan: Insights from groundwater pollution indexing, GIS-based, and multivariate statistical approaches. Environ. Sci. Pollut. Res. 2022, 29, 75744–75768. [Google Scholar] [CrossRef] [PubMed]
- Rashid, A.; Farooqi, A.; Gao, X.; Zahir, S.; Noor, S.; Khattak, J.A. Geochemical modeling, source apportionment, health risk exposure and control of higher fluoride in groundwater of sub-district Dargai, Pakistan. Chemosphere 2020, 243, 125409. [Google Scholar] [CrossRef] [PubMed]
- Singh, U.K.; Ramanathan, A.; Subramanian, V. Groundwater chemistry and human health risk assessment in the mining region of East Singhbhum, Jharkhand, India. Chemosphere 2018, 204, 501–513. [Google Scholar] [CrossRef]
- Epa, U. Maximum contaminant level goals and national primary drinking water regulations for lead and copper; final rule. Fed. Regist. 1991, 56, 26460. [Google Scholar]
- EPA USA. A Review of the Reference Dose and Reference Concentration Processes; EPA/630/P-02; U.S. Environmental Protection Agency, Risk Assessment Forum: Washington, DC, USA, 2002. [Google Scholar]
- World Health Organization. Guidelines for Drinking-water Quality: Surveillance and control of community supplies; World Health Organization: Geneva, Switzerland, 1997; Volume 3. [Google Scholar]
- Avigliano, E.; Schenone, N.F. Human health risk assessment and environmental distribution of trace elements, glyphosate, fecal coliform and total coliform in Atlantic Rainforest mountain rivers (South America). Microchem. J. 2015, 122, 149–158. [Google Scholar] [CrossRef]
- Saha, N.; Rahman, M.S.; Ahmed, M.B.; Zhou, J.L.; Ngo, H.H.; Guo, W. Industrial metal pollution in water and probabilistic assessment of human health risk. J. Environ. Manag. 2017, 185, 70–78. [Google Scholar] [CrossRef]
- Parkhurst, D.L. User’s Guide to PHREEQC: A Computer Program for Speciation, Reaction-Path, Advective-Transport, and Inverse Geochemical Calculations; US Department of the Interior, US Geological Survey: Reston, VA, USA, 1995; Volume 95. [Google Scholar]
- Kinniburgh, D.; Cooper, D. PhreePlot: Creating graphical Output with PHREEQC; UK Center for Ecology & Hydrology: Lancaster, UK, 2011. [Google Scholar]
- Zhang, Y.; Chen, H.; Liu, C.; Chen, R.; Wang, Y.; Teng, Y. Developing an integrated framework for source apportionment and source-specific health risk assessment of PAHs in soils: Application to a typical cold region in China. J. Hazard. Mater. 2021, 415, 125730. [Google Scholar] [CrossRef]
- Xiong, Y.; Bari, M.A.; Xing, Z.; Du, K. Ambient volatile organic compounds (VOCs) in two coastal cities in western Canada: Spatiotemporal variation, source apportionment, and health risk assessment. Sci. Total Environ. 2020, 706, 135970. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Teng, Y.; Chen, H.; Hu, B.; Yue, W. Groundwater pollution and risk assessment based on source apportionment in a typical cold agricultural region in Northeastern China. Sci. Total Environ. 2019, 696, 133972. [Google Scholar] [CrossRef]
- Chacón-Mateos, M. Application of Source Apportionment Methods to Identify Emission Sources with the Help of Ambient Air Quality Measurements in Six European Cities. Master’s Thesis, University of Stuttgart, Stuttgart, Germany, 2019. [Google Scholar]
- Shim, C. Constraining Global Biogenic Emissions and Exploring Source Contributions to Tropospheric Ozone: Modeling Applications. Ph.D. Thesis, Georgia Institute of Technology, Atlanta, GA, USA, 2006. [Google Scholar]
- Sowlat, M.H.; Hasheminassab, S.; Sioutas, C. Source apportionment of ambient particle number concentrations in central Los Angeles using positive matrix factorization (PMF). Atmos. Chem. Phys. 2016, 16, 4849–4866. [Google Scholar] [CrossRef] [Green Version]
- WHO. Guidelines for Drinking-Water Quality; World Health Organization: Geneva, Switzerland, 2011; pp. 303–304. [Google Scholar]
- Changsheng, H.; Akram, W.; Rashid, A.; Ullah, Z.; Shah, M.; Alrefaei, A.F.; Kamel, M.; Aleya, L.; Abdel-Daim, M.M. Quality Assessment of Groundwater Based on Geochemical Modelling and Water Quality Index (WQI). Water 2022, 14, 3888. [Google Scholar]
- Gao, Y.; Qian, H.; Ren, W.; Wang, H.; Liu, F.; Yang, F. Hydrogeochemical characterization and quality assessment of groundwater based on integrated-weight water quality index in a concentrated urban area. J. Clean. Prod. 2020, 260, 121006. [Google Scholar] [CrossRef]
- Ghalib, H.B. Groundwater chemistry evaluation for drinking and irrigation utilities in east Wasit province, Central Iraq. Appl. Water Sci. 2017, 7, 3447–3467. [Google Scholar] [CrossRef] [Green Version]
- Swathi, S.; Umesh, S. Nemerow’s pollution index: For ground water quality assessment. J. Environ. Sci. Pollut. Res. 2015, 1, 23–31. [Google Scholar]
- Khan, K.; Lu, Y.; Khan, H.; Zakir, S.; Khan, S.; Khan, A.A.; Wei, L.; Wang, T. Health risks associated with heavy metals in the drinking water of Swat, northern Pakistan. J. Environ. Sci. 2013, 25, 2003–2013. [Google Scholar]
- Bhuiyan, M.A.H.; Bodrud-Doza, M.; Islam, A.T.; Rakib, M.; Rahman, M.S.; Ramanathan, A. Assessment of groundwater quality of Lakshimpur district of Bangladesh using water quality indices, geostatistical methods, and multivariate analysis. Environ. Earth Sci. 2016, 75, 1020. [Google Scholar] [CrossRef]
- Javed, S.; Ali, A.; Ullah, S. Spatial assessment of water quality parameters in Jhelum city (Pakistan). Environ. Monit. Assess. 2017, 189, 119. [Google Scholar] [CrossRef]
- McKnight, U.S.; Finkel, M. A system dynamics model for the screening-level long-term assessment of human health risks at contaminated sites. Environ. Model. Softw. 2013, 40, 35–50. [Google Scholar] [CrossRef]
- Subba Rao, N.; Sunitha, B.; Adimalla, N.; Chaudhary, M. Quality criteria for groundwater use from a rural part of Wanaparthy District, Telangana State, India, through ionic spatial distribution (ISD), entropy water quality index (EWQI) and principal component analysis (PCA). Environ. Geochem. Health 2020, 42, 579–599. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Asthana, H.; Rena, V.; Kumar, P.; Kushawaha, J.; Mukherjee, S. Hydrogeochemical processes controlling fluoride enrichment within alluvial and hard rock aquifers in a part of a semi-arid region of Northern India. Environ. Earth Sci. 2018, 77, 475. [Google Scholar] [CrossRef]
- Hussien, B.M.; Faiyad, A.S. Modeling the hydrogeochemical processes and source of ions in the groundwater of aquifers within Kasra-Nukhaib region (West Iraq). Int. J. Geosci. 2016, 7, 1156–1181. [Google Scholar] [CrossRef]
- Dinka, M.O.; Loiskandl, W.; Ndambuki, J.M. Hydrochemical characterization of various surface water and groundwater resources available in Matahara areas, Fantalle Woreda of Oromiya region. J. Hydrol. Reg. Stud. 2015, 3, 444–456. [Google Scholar] [CrossRef] [Green Version]
- Khatri, N.; Tyagi, S. Influences of natural and anthropogenic factors on surface and groundwater quality in rural and urban areas. Front. Life Sci. 2015, 8, 23–39. [Google Scholar] [CrossRef]
- Delina, R.E.; Arcilla, C.; Otake, T.; Garcia, J.J.; Tan, M.; Ito, A. Chromium occurrence in a nickel laterite profile and its implications to surrounding surface waters. Chem. Geol. 2020, 558, 119863. [Google Scholar] [CrossRef]
- Paulukat, C.; Døssing, L.N.; Mondal, S.K.; Voegelin, A.R.; Frei, R. Oxidative release of chromium from Archean ultramafic rocks, its transport and environmental impact–A Cr isotope perspective on the Sukinda valley ore district (Orissa, India). Appl. Geochem. 2015, 59, 125–138. [Google Scholar] [CrossRef]
- Marieni, C.; Matter, J.M.; Teagle, D.A. Experimental study on mafic rock dissolution rates within CO2-seawater-rock systems. Geochim. Cosmochim. Acta 2020, 272, 259–275. [Google Scholar] [CrossRef]
- Deng, J.; Zhang, J.; Yin, H.; Hu, W.; Zhu, J.; Wang, X. Ecological risk assessment and source apportionment of metals in the surface sediments of river systems in Lake Taihu Basin, China. Environ. Sci. Pollut. Res. 2020, 27, 25943–25955. [Google Scholar] [CrossRef]
- Rashid, A.; Khattak, S.A.; Ali, L.; Zaib, M.; Jehan, S.; Ayub, M.; Ullah, S. Geochemical profile and source identification of surface and groundwater pollution of District Chitral, Northern Pakistan. Microchem. J. 2019, 145, 1058–1065. [Google Scholar] [CrossRef]
- Wang, X.; Tang, Z. The first large-scale bioavailable Sr isotope map of China and its implication for provenance studies. Earth-Sci. Rev. 2020, 210, 103353. [Google Scholar] [CrossRef]
- Balderacchi, M.; Benoit, P.; Cambier, P.; Eklo, O.M.; Gargini, A.; Gemitzi, A.; Gurel, M.; Kløve, B.; Nakic, Z.; Predaa, E. Groundwater pollution and quality monitoring approaches at the European level. Crit. Rev. Environ. Sci. Technol. 2013, 43, 323–408. [Google Scholar] [CrossRef]
- Chai, L.; Wang, Y.; Wang, X.; Ma, L.; Cheng, Z.; Su, L. Pollution characteristics, spatial distributions, and source apportionment of heavy metals in cultivated soil in Lanzhou, China. Ecol. Indic. 2021, 125, 107507. [Google Scholar] [CrossRef]
- Alexandrov, D.; Eisner, L.; Waheed, U.B.; Kaka, S.I.; Greenhalgh, S.A. Detection, location, and source mechanism determination with large noise variations in surface microseismic monitoring. Geophysics 2020, 85, KS197–KS206. [Google Scholar] [CrossRef]
- Olmedo, P.; Hernández, A.; Pla, A.; Femia, P.; Navas-Acien, A.; Gil, F. Determination of essential elements (copper, manganese, selenium and zinc) in fish and shellfish samples. Risk and nutritional assessment and mercury–selenium balance. Food Chem. Toxicol. 2013, 62, 299–307. [Google Scholar] [CrossRef]
- Arvaniti, F.; Panagiotakos, D.B. Healthy indexes in public health practice and research: A review. Crit. Rev. Food Sci. Nutr. 2008, 48, 317–327. [Google Scholar] [CrossRef]
- Waring, R.H.; Harris, R.; Mitchell, S. Plastic contamination of the food chain: A threat to human health? Maturitas 2018, 115, 64–68. [Google Scholar] [CrossRef]
- Ali, H.; Khan, E.; Ilahi, I. Environmental chemistry and ecotoxicology of hazardous heavy metals: Environmental persistence, toxicity, and bioaccumulation. J. Chem. 2019, 2019. [Google Scholar] [CrossRef]






| Water Sources | Water in Mine Region (n = 35) | Water Control Area (n = 20) | Chromite Mines Water (n = 5) | ||||
|---|---|---|---|---|---|---|---|
| Statistics | Range | Mean ± SD | Range | Mean ± SD | Range | Mean ± SD | WHO Limit |
| pH | 7.2–8.0 | 7.4 ± 0.2 | 7.3–8.2 | 7.71 ± 0.26 | 6.5–7.2 | 6.8 ± 0.25 | 6.5–9.2 |
| Depth m | 30–135 | 74.0 ± 25.5 | 27.0–95.0 | 64.6 ± 18.84 | 15.0–25.0 | 20.0 ± 3.81 | - |
| Elevation m | 290–495 | 376 ± 57.8 | 310–500 | 347 ± 23.92 | 430–470 | 452.0 ± 16.43 | - |
| EC µS/cm | 480–1500 | 782 ± 249.1 | 480–1400 | 760 ± 233.31 | 1200–1500 | 1370 ± 139.6 | 400 |
| Temperature °C | 27.5–31.2 | 30 ± 1.0 | 27.4–30.2 | 28.6 ± 0.76 | 27.5–31.2 | 29.2 ± 1.50 | - |
| Turbidity NTU | 2.4–14 | 6.0 ± 2.7 | 3.2–12.0 | 6.7 ± 2.83 | 33.0–70.0 | 47.6 ± 16.47 | 5.0 |
| ORP mV | 140–280 | 196 ± 36.5 | 160–225 | 191 ± 19 | 170–260 | 212.0 ± 38.34 | - |
| TDS mg/L | 310–980 | 503 ± 164.3 | 310.0–1000 | 512.5 ± 174.8 | 750.0–980.0 | 848.0 ± 84.38 | 1000 |
| Na mg/L | 25–210 | 86 ± 53.1 | 30.0–245 | 152.3 ± 71.05 | 180.0–255.0 | 213.0 ± 27.75 | 200 |
| K mg/L | 6.0–12.0 | 10 ± 1.8 | 6.0–12.0 | 9.6 ± 2.01 | 8.0–12.0 | 10.6 ± 1.95 | 12 |
| Mg mg/L | 25–60 | 40 ± 8.5 | 12.0–45.0 | 27.7 ± 9.90 | 21.0–30.0 | 25.2 ± 3.19 | 50 |
| Ca mg/L | 25–125 | 66 ± 25.5 | 15.0–110 | 49.8 ± 27.65 | 25.0–50.0 | 39.0 ± 9.62 | 100 |
| PO4 mg/L | 1.33–8.5 | 3.0 ± 1.9 | 1.5–10.0 | 3.5 ± 1.93 | 1.5–6.5 | 3.7 ± 1.92 | 0.1 |
| NO3 mg/L | 10–28 | 18 ± 4.7 | 10–27 | 17 ± 4 | 10.0–15.0 | 12.8 ± 2.17 | 50 |
| HCO3 mg/L | 200–650 | 293 ± 70.1 | 215–580 | 350 ± 74 | 400–630 | 451.0 ± 30.90 | 500 |
| Cl mg/L | 75–145 | 110 ± 14.1 | 95–145 | 115 ± 13 | 100–125 | 115.0 ± 9.35 | 250 |
| SO4 mg/L | 100–210 | 153 ± 27.2 | 140–190 | 161 ± 15 | 140–180 | 159.0 ± 14.75 | 500 |
| Cr mg/L | 0.02–4.5 | 1.3 ± 1.2 | 0.02–2.3 | 0.7 ± 0.81 | 5.8–6.0 | 5.9 ± 0.11 | 0.05 |
| Ni mg/L | 0.4–3.8 | 1.4 ± 0.28 | 0.05–3.6 | 1.2 ± 0.23 | 3.2–5.8 | 4.2 ± 0.52 | 3.0 |
| Mn mg/L | 0.05–0.8 | 0.4 ± 0.12 | 0.04–0.6 | 0.3 ± 0.10 | 0.6–1.2 | 0.8 ± 0.21 | 0.5 |
| Groundwater Variables | Groundwater in the Mining Region (n = 35) | Groundwater in the Non-Mining Region (n = 20) | Chromite Mines Water (n = 5) | |||
|---|---|---|---|---|---|---|
| Statistic | Range | Mean ± SD | Range | Mean ± SD | Range | Mean ± SD |
| pH | 0.92–1.02 | 0.95 ± 0.03 | 0.93–1.04 | 0.98 ± 0.03 | 0.83–0.92 | 0.87 ± 0.03 |
| EC µS/cm | 1.20–3.75 | 1.96 ± 0.62 | 1.20–3.50 | 1.90 ± 0.58 | 3.00–3.75 | 3.43 ± 0.35 |
| Turbidity NTU | 0.48–2.80 | 1.29 ± 0.55 | 0.64–2.40 | 1.33 ± 0.57 | 6.60–14.00 | 9.52 ± 3.29 |
| TDS mg/L | 0.31–0.98 | 0.50 ± 0.16 | 0.31–1.00 | 0.51 ± 0.17 | 0.75–0.98 | 0.85 ± 0.08 |
| Na mg/L | 0.13–1.05 | 0.43 ± 0.27 | 0.15–1.23 | 0.76 ± 0.36 | 0.90–1.28 | 1.07 ± 0.14 |
| K mg/L | 0.50–1.00 | 0.83 ± 0.15 | 0.50–1.00 | 0.80 ± 0.17 | 0.67–1.00 | 0.88 ± 0.16 |
| Mg mg/L | 0.50–1.20 | 0.80 ± 0.17 | 0.24–0.90 | 0.55 ± 0.20 | 0.42–0.60 | 0.50 ± 0.06 |
| Ca mg/L | 0.25–1.25 | 0.66 ± 0.25 | 0.15–1.10 | 0.50 ± 0.28 | 0.25–0.50 | 0.39 ± 0.10 |
| PO4 mg/L | 13.3–85.0 | 30.0 ± 19.3 | 15.0–100.0 | 35.0 ± 19.3 | 15.0–65.0 | 37.0 ± 19.2 |
| NO3 mg/L | 0.20–0.56 | 0.37 ± 0.09 | 0.20–0.55 | 0.35 ± 0.08 | 0.20–0.30 | 0.26 ± 0.04 |
| HCO3 mg/L | 0.40–1.30 | 0.62 ± 0.20 | 0.43–1.10 | 0.71 ± 0.17 | 1.02–1.26 | 1.11 ± 0.09 |
| Cl mg/L | 0.30–0.58 | 0.44 ± 0.06 | 0.38–0.58 | 0.46 ± 0.05 | 0.40–0.50 | 0.46 ± 0.04 |
| SO4 mg/L | 0.20–0.42 | 0.31 ± 0.05 | 0.28–0.38 | 0.32 ± 0.03 | 0.28–0.36 | 0.32 ± 0.03 |
| Cr mg/L | 0.4–90.0 | 26.9 ± 20.8 | 0.2–45.0 | 13.3 ± 10.3 | 115.0–120.0 | 116.0 ± 2.2 |
| Ni mg/L | 0.13–0.73 | 0.39 ± 0.13 | 0.01–0.60 | 0.25 ± 0.18 | 0.73–0.75 | 0.74 ± 0.11 |
| Mn mg/L | 0.02–1.60 | 0.36 ± 0.31 | 0.22–1.00 | 0.36 ± 0.20 | 0.56–2.06 | 1.14 ± 0.74 |
| CDI Children | CDI Males | CDI Females | ||||
|---|---|---|---|---|---|---|
| Groundwater in the mining region | ||||||
| Range | Mean ± SD | Range | Mean ± SD | Range | Mean ± SD | |
| Cr | 0.002–0.500 | 0.150 ± 0.130 | 0.001–0.173 | 0.052 ± 0.045 | 0.001–0.218 | 0.065 ± 0.057 |
| Ni | 0.044–0.244 | 0.130 ± 0.043 | 0.015–0.085 | 0.045 ± 0.015 | 0.019–0.106 | 0.057 ± 0.019 |
| Mn | 0.001–0.089 | 0.020 ± 0.017 | 0.0004–0.031 | 0.007 ± 0.006 | 0.0005–0.039 | 0.009 ± 0.007 |
| Groundwater in non-mining region | ||||||
| Cr | 0.001–0.250 | 0.074 ± 0.088 | 0.001–0.087 | 0.026 ± 0.031 | 0.001–0.218 | 0.065 ± 0.057 |
| Ni | 0.002–0.200 | 0.084 ± 0.057 | 0.001–0.069 | 0.029 ± 0.020 | 0.015–0.101 | 0.057 ± 0.019 |
| Mn | 0.012–0.056 | 0.020 ± 0.011 | 0.004–0.019 | 0.007 ± 0.004 | 0.0001–0.039 | 0.009 ± 0.007 |
| Chromite mines water | ||||||
| Cr | 0.639–0.667 | 0.644 ± 0.011 | 0.221–0.231 | 0.223 ± 0.004 | 0.278–0.290 | 0.281 ± 0.005 |
| Ni | 0.246–0.250 | 0.248 ± 0.002 | 0.085–0.087 | 0.086 ± 0.001 | 0.107–0.109 | 0.108 ± 0.001 |
| Mn | 0.031–0.114 | 0.063 ± 0.037 | 0.011–0.040 | 0.022 ± 0.013 | 0.014–0.050 | 0.027 ± 0.016 |
| HQ Children | HQ Males | HQ Females | ||||
| Groundwater in the mining region | ||||||
| Cr | 0.001–0.333 | 0.100 ± 0.087 | 0.001–0.115 | 0.035 ± 0.030 | 0.001–0.145 | 0.043 ± 0.038 |
| Ni | 2.222–12.222 | 6.502 ± 2.151 | 0.769–4.231 | 2.251 ± 0.744 | 0.968–5.323 | 2.832 ± 0.937 |
| Mn | 0.008–0.635 | 0.142 ± 0.123 | 0.003–0.220 | 0.049 ± 0.042 | 0.003–0.276 | 0.062 ± 0.053 |
| Groundwater in non-mining region | ||||||
| Cr | 0.001–0.167 | 0.049 ± 0.059 | 0.0001–0.058 | 0.017 ± 0.020 | 0.0001–0.073 | 0.022 ± 0.026 |
| Ni | 0.111–10.000 | 4.181 ± 2.852 | 0.038–3.462 | 1.447 ± 0.987 | 0.048–4.355 | 1.821 ± 1.242 |
| Mn | 0.087–0.397 | 0.143 ± 0.077 | 0.030–0.137 | 0.050 ± 0.027 | 0.038–0.173 | 0.062 ± 0.033 |
| Chromite mines water | ||||||
| Cr | 0.426–0.444 | 0.430 ± 0.007 | 0.147–0.154 | 0.149 ± 0.003 | 0.185–0.194 | 0.187 ± 0.003 |
| Ni | 12.278–12.500 | 12.422 ± 0.097 | 4.250–4.327 | 4.300 ± 0.034 | 5.347–5.444 | 5.410 ± 0.042 |
| Mn | 0.222–0.817 | 0.451 ± 0.262 | 0.077–0.283 | 0.156 ± 0.091 | 0.097–0.356 | 0.196 ± 0.114 |
| THI | 12.926–13.761 | 13.303 ± 0.366 | 4.474–4.764 | 4.605 ± 0.128 | 13.629–15.994 | 14.793 ± 0.159 |
| Carcinogenic risk | ||||||
| Groundwater in the mining region | ||||||
| Cr | 0.001–0.250 | 0.075 ± 0.065 | 0.0001–0.087 | 0.026 ± 0.023 | 0.000–0.109 | 0.033 ± 0.028 |
| Ni | 0.040–0.222 | 0.118 ± 0.039 | 0.014–0.077 | 0.041 ± 0.014 | 0.018–0.097 | 0.052 ± 0.017 |
| Groundwater in non-mining region | ||||||
| Cr | 0.001–0.125 | 0.037 ± 0.044 | 0.0001–0.043 | 0.013 ± 0.015 | 0.0001–0.054 | 0.016 ± 0.019 |
| Ni | 0.002–0.182 | 0.076 ± 0.052 | 0.001–0.063 | 0.026 ± 0.018 | 0.001–0.079 | 0.033 ± 0.023 |
| Chromite mines water | ||||||
| Cr | 0.319–0.333 | 0.322 ± 0.006 | 0.111–0.115 | 0.112 ± 0.002 | 0.139–0.145 | 0.140 ± 0.002 |
| Ni | 0.223–0.228 | 0.226 ± 0.002 | 0.077–0.079 | 0.078 ± 0.001 | 0.097–0.099 | 0.098 ± 0.001 |
| THI | 0.542–0.561 | 0.548 ± 0.008 | 0.188–0.194 | 0.190 ± 0.003 | 0.436–0.444 | 0.432 ± 0.003 |
| Groundwater of Mining Region (n = 35) | Groundwater of Non-Mining Region (n = 20) | Chromite Mines Water (n = 5) | ||||
|---|---|---|---|---|---|---|
| Statistic | Range | Mean ± SD | Range | Mean ± SD | Range | Mean ± SD |
| OH− | 2.0 × 10−4–2.0 × 10−3 | 9.0 × 10−4 ± 1.0 × 10−3 | 3.0 × 10−4–3.0 × 10−3 | 1.0 × 10−3 ± 2.0 × 10−3 | 5.0 × 10−5–3.0 × 10−4 | 2.0 × 10−4 ± 1.0 × 10−4 |
| H+ | 1.0 × 10−5–9.0 × 10−5 | 5.0 × 10−5 ± 6.0 × 10−5 | 8.0 × 10−6–7.0 × 10−5 | 4.0 × 10−5 ± 5.0 × 10−5 | 8.0 × 10−5–4.0 × 10−4 | 3.0 × 10−4 ± 2.0 × 10−4 |
| HCO3− | 0.184–0.354 | 0.269 ± 0.1207 | 0.205–0.315 | 0.26 ± 0.078 | 0.372–0.419 | 0.396 ± 0.034 |
| CO3−2 | 3.0 × 10−1–0.004 | 0.002 ± 0.0023 | 5.0 × 10−1–0.005 | 0.003 ± 0.001 | 1.0 × 10−1–7.0 × 10−1 | 4.0 × 10−1 ± 4.0 × 10−1 |
| Ca+2 | 0.007–0.021 | 0.014 ± 0.0094 | 0.003–0.019 | 0.011 ± 0.008 | 0.005–0.008 | 0.007 ± 0.002 |
| Cl− | 0.075–0.145 | 0.11 ± 0.0495 | 0.095–0.145 | 0.12 ± 0.035 | 0.1–0.125 | 0.113 ± 0.018 |
| K+ | 0.005–0.01 | 0.007 ± 0.0031 | 0.005–0.01 | 0.007 ± 0.003 | 0.007–0.009 | 0.008 ± 0.002 |
| Mg+2 | 0.009–0.013 | 0.011 ± 0.003 | 0.003–0.01 | 0.007 ± 0.005 | 0.006–0.007 | 0.006 ± 8.0 × 10−1 |
| NO3− | 0.01–0.027 | 0.018 ± 0.012 | 0.01–0.026 | 0.018 ± 0.012 | 0.01–0.015 | 0.012 ± 0.003 |
| Na+ | 0.021–0.159 | 0.09 ± 0.0971 | 0.024–0.187 | 0.106 ± 0.116 | 0.142–0.192 | 0.167 ± 0.036 |
| PO4−3 | 2.0 × 10−5–3.0 × 10−4 | 3.0 × 10−4 ± 4.0 × 10−4 | 3.0 × 10−5–7.0 × 10−4 | 3.0 × 10−4 ± 4.0 × 10−4 | 2.0 × 10−6–5.0 × 10−5 | 3.0 × 10−5 ± 4.0 × 10−5 |
| SO4−2 | 0.076–0.104 | 0.09 ± 0.0196 | 0.097–0.12 | 0.109 ± 0.016 | 0.096–0.107 | 0.101 ± 0.007 |
| Cr+2 | 4.0× 10−17–6.0 × 10−16 | 3.0 × 10−16 ± 4.0 × 10−16 | 1.0× 10−17–1.0 × 10−16 | 6.0 × 10−17 ± 7.0 × 10−17 | 2.0× 10−14–1.0 × 10−13 | 7.0 × 10−14 ± 7.0 × 10−14 |
| Cr+3 | 5.0 × 10−5–3.0 × 10−3 | 2.0 × 10−3 ± 3.0 × 10−3 | 3.0 × 10−5–5.0 × 10−7 | 3.0 × 10−4 ± 4.0 × 10−4 | 9.0 × 10−2–4.0 × 10−1 | 2.0 × 10−1 ± 2.0 × 10−1 |
| Cr+6 | 2.0 × 10−14–2.0 × 10−7 | 1.0 × 10−7 ± 2.0 × 10−7 | 3.0 × 10−14–2.0 × 10−6 | 1.0 × 10−6 ± 2.0 × 10−6 | 1.0× 10−16–4.0 × 10−12 | 2.0 × 10−12 ± 2.0 × 10−12 |
| Ni+2 | 3.0 × 10−2–5.0 × 10−2 | 4.0 × 10−2 ± 5.0 × 10−2 | 1.0 × 10−3–4.0 × 10−2 | 2.0 × 10−2 ± 3.0 × 10−2 | 7.0 × 10−3–8.0 × 10−3 | 8.0 × 10−3 ± 7.0 × 10−4 |
| Mn+2 | 7.0 × 10−3–6.0 × 10−1 | 3.0 × 10−1 ± 0.0004 | 7.0 × 10−2–4.0 × 10−1 | 2.0 × 10−1 ± 3.0 × 10−1 | 2.0 × 10−1–8.0 × 10−1 | 5.0 × 10−1 ± 4.0 × 10−1 |
| Mn+3 | 3.0× 10−24–8.0 × 10−23 | 4.0 × 10−23 ± 5.0 × 10−23 | 3.0×10−23–5.0×10−23 | 4.0 × 10−23 ± 2.0 × 10−23 | 4.0× 10−23–1.0 × 10−22 | 7.0 × 10−23 ± 4.0 × 10−23 |
| Locations | Groundwater of Mining Region (n = 35) | Groundwater of Non-Mining Region (n = 20) | Chromite Mines Water (n = 5) | |||||
|---|---|---|---|---|---|---|---|---|
| Statistic | Range | Mean ± SD | Range | Mean ± SD | Rang | Mean ± SD | Formula | |
| Anhydrite | 0.03–0.79 | 0.41 ± 0.21 | 0.27–0.74 | 0.25 ± 0.28 | 0.05–0.32 | 0.17 ± 0.12 | CaSO4 | |
| Aragonite | 1.78–2.80 | 2.23 ± 0.25 | 2.11–2.58 | 2.38 ± 0.13 | 1.07–1.94 | 1.53 ± 0.31 | CaCO3 | |
| Ca3(PO4)2(beta) | 2.63–6.20 | 4.55 ± 0.84 | 3.76–5.80 | 4.78 ± 0.61 | 0.40–3.94 | 2.48 ± 1.32 | Ca3(PO4)2 | |
| Ca4H(PO4)3:3H2O | 1.74–6.45 | 4.29 ± 1.15 | 2.91–5.90 | 4.44 ± 0.94 | −0.78–3.70 | 1.93 ± 1.70 | Ca4H(PO4)3:3H2O | |
| CaCrO4 | −16.86–−10.92 | −14.68 ± 1.54 | −16.52–−10.28 | −13.62 ± 1.61 | −20.55–−15.46 | −18.26 ± 1.83 | CaCrO4 | |
| CaHPO4 | 0.24–1.49 | 0.86 ± 0.35 | 0.29–1.24 | 0.79 ± 0.34 | −0.04–0.89 | 0.59 ± 0.39 | CaHPO4 | |
| CaHPO4:2H2O | −0.05–1.21 | 0.57 ± 0.35 | 0.02–0.95 | 0.50 ± 0.34 | −0.34–0.60 | 0.30 ± 0.39 | CaHPO4:2H2O | |
| Calcite | 1.96–2.98 | 2.41 ± 0.25 | 2.29–2.76 | 2.56 ± 0.13 | 1.25–2.12 | 1.71 ± 0.31 | CaCO3 | |
| Gypsum | 0.27–1.03 | 0.64 ± 0.21 | −0.03–0.98 | 0.48 ± 0.28 | 0.23–0.56 | 0.40 ± 0.12 | CaSO4:2H2O | |
| Huntite | 4.17–7.78 | 5.43 ± 0.91 | 4.46–6.82 | 6.00 ± 0.67 | 1.19–4.28 | 2.73 ± 1.10 | CaMg3(CO3)4 | |
| Hydroxylapatite | 10.77–16.80 | 14.01 ± 1.38 | 12.98–16.12 | 14.52 ± 0.92 | 6.61–12.76 | 10.13 ± 2.26 | Ca5(PO4)3OH | |
| Lime | −20.93–−19.31 | −20.25 ± 0.41 | −20.42–−19.46 | −19.89 ± 0.26 | −22.59–−20.92 | −21.79 ± 0.60 | CaO | |
| Portlandite | −11.04–−9.43 | −10.36 ± 0.41 | −10.53–−9.57 | −10.00 ± 0.26 | −12.70–−11.03 | −11.90 ± 0.60 | Ca(OH)2 | |
| Dolomite | 4.35–5.93 | 4.86 ± 0.46 | 4.46–5.52 | 5.16 ± 0.30 | 2.67–4.29 | 3.50 ± 0.57 | CaMg(CO3)2 | |
| Artinite | −4.30–−1.69 | −3.41 ± 0.61 | −4.04–−2.17 | −2.92 ± 0.53 | −6.6–−4.37 | −5.60 ± 0.81 | MgCO3:Mg(OH)2:3H2O | |
| Brucite | −4.98–−3.41 | −4.48 ± 0.39 | −4.82–−3.57 | −4.13 ± 0.35 | −6.69–−5.16 | −6.00 ± 0.55 | Mg(OH)2 | |
| Epsomite | −2.26–−1.71 | −1.95 ± 0.13 | −2.50–−1.81 | −2.13 ± 0.18 | −2.25–−2.11 | −2.18 ± 0.05 | MgSO4:7H2O | |
| Hydromagnesite | −5.62–0.15 | −3.51 ± 1.31 | −5.00–−1.29 | −2.61 ± 1.10 | −9.85–−5.34 | −7.71 ± 1.60 | Mg5(CO3)4(OH)2:4H2O | |
| Magnesite | 0.90–1.96 | 1.30 ± 0.23 | 1.02–1.67 | 1.44 ± 0.19 | 0.28–1.02 | 0.64 ± 0.26 | MgCO3 | |
| Mg3(PO4)2 | −2.32–−0.05 | −1.34 ± 0.64 | −2.03–0.13 | −1.15 ± 0.52 | −5.09–−1.95 | −3.33 ± 1.16 | Mg3(PO4)2 | |
| MgCr2O4 | 0.06–6.70 | 3.60 ± 1.69 | 0.33–7.08 | 3.62 ± 1.87 | −0.12–4.05 | 1.76 ± 1.50 | MgCr2O4 | |
| MgCrO4 | −24.66–−18.60 | −22.41 ± 1.55 | −24.41–−17.88 | −21.36 ± 1.71 | −28.15–−23.19 | −25.9 ± 1.79 | MgCrO4 | |
| MgHPO4:3H2O | −0.85–0.22 | −0.34 ± 0.29 | −0.79–0.11 | −0.42 ± 0.27 | −1.12–−0.28 | −0.59 ± 0.34 | MgHPO4:3H2O | |
| Nesquehonite | −1.90–−0.85 | −1.50 ± 0.23 | −1.79–−1.14 | −1.36 ± 0.19 | −2.54–−1.79 | −2.18 ± 0.26 | MgCO3:3H2O | |
| Periclase | −9.71–−8.15 | −9.21 ± 0.39 | −9.56–−8.30 | −8.87 ± 0.35 | −11.42–−9.89 | −10.73 ± 0.55 | MgO | |
| Mg(OH)2 | −6.93–−5.36 | −6.43 ± 0.39 | −6.77–−5.52 | −6.08 ± 0.35 | −8.64–−7.11 | −7.95 ± 0.55 | Mn(OH)2 | |
| Halite | −4.48–−3.57 | −4.07 ± 0.29 | −4.41–−3.46 | −3.80 ± 0.26 | −3.72–−3.48 | −3.59 ± 0.09 | NaCl | |
| Mirabilite | −4.09–−2.31 | −3.25 ± 0.54 | −3.94–−2.17 | −2.70 ± 0.53 | −2.46–−2.15 | −2.32 ± 0.12 | Na2SO4:10H2O | |
| Na2Cr2O7 | −41.51–−29.11 | −36.81 ± 3.09 | −40.22–−27.03 | −34.33 ± 3.66 | −44.66–−36.59 | −41.22 ± 2.95 | Na2Cr2O7 | |
| Na2CrO4 | −22.90–−15.37 | −20.24 ± 1.91 | −21.99–−14.07 | −18.46 ± 2.28 | −24.55–−19.91 | −22.61 ± 1.71 | Na2CrO4 | |
| Natron | −6.28–−3.51 | −5.17 ± 0.75 | −6.18–−3.21 | −4.31 ± 0.83 | −4.91–−4.36 | −4.69 ± 0.21 | Na2CO3:10H2O | |
| Thenardite | −5.47–−3.68 | −4.64 ± 0.54 | −5.33–−3.53 | −4.07 ± 0.54 | −3.82–−3.49 | −3.67 ± 0.13 | Na2SO4 | |
| Thermonatrite | −8.18–−5.39 | −7.08 ± 0.76 | −8.08–−5.10 | −6.20 ± 0.84 | −6.79–−6.25 | −6.57 ± 0.20 | Na2CO3:H2O | |
| K2Cr2O7 | −35.30–−24.19 | −31.16 ± 2.83 | −34.89–−22.24 | −29.25 ± 3.36 | −40.17–−31.65 | −36.44 ± 3.11 | K2Cr2O7 | |
| K2CrO4 | −20.72–−14.35 | −18.49 ± 1.62 | −20.59–−13.18 | −17.28 ± 1.97 | −23.97–−18.87 | −21.74 ± 1.87 | K2CrO4 | |
| Cr(OH)2 | −17.12–−14.80 | −15.58 ± 0.64 | −17.40–−15.01 | −16.03 ± 0.75 | −15.42–−14.80 | −15.12 ± 0.22 | Cr(OH)2 | |
| Cr(OH)3 | 2.80 | 5.56 | 4.46 ± 0.73 | 2.90–5.74 | 4.29 ± 0.84 | 3.70–5.02 | 4.29±0.48 | Cr(OH)3 |
| Cr2O3 | 6.47 | 12.00 | 9.79 ± 1.46 | 6.66–12.36 | 9.46 ± 1.68 | 8.28–10.92 | 9.46±0.95 | Cr2O3 |
| CrCl2 | −37.53 | −34.87 | −35.88 ± 0.67 | −38.72–−35.33 | −36.86 ± 0.81 | −34.60–−33.73 | −34.12±0.31 | CrCl2 |
| CrCl3 | −38.68 | −35.87 | −36.95 ± 0.68 | −39.73–−36.36 | −37.90 ± 0.81 | −35.64–−34.73 | −35.16±0.32 | CrCl3 |
| Cr-metal | −59.56–−57.16 | −58.08 ± 0.66 | −61.05–−57.61 | −59.10 ± 0.82 | −56.85–−56.06 | −56.37 ± 0.29 | Cr | |
| CrO3 | −28.22–−23.36 | −26.19 ± 1.22 | −27.85–−22.58 | −25.49 ± 1.40 | −29.72–−26.30 | −28.23 ± 1.24 | CrO3 | |
| Bunsenite | −3.06–−1.44 | −2.38 ± 0.34 | −3.62–−1.19 | −2.29 ± 0.53 | −5.15–−3.60 | −4.48 ± 0.56 | NiO | |
| Morenosite | −4.76–−3.95 | −4.24 ± 0.22 | −6.10–−4.01 | −4.66 ± 0.56 | −5.11–−4.98 | −5.05 ± 0.05 | NiSO4:7H2O | |
| Ni(OH)2 | −3.42–−1.80 | −2.74 ± 0.34 | −3.98–−1.54 | −2.64 ± 0.53 | −5.50–−3.96 | −4.84 ± 0.56 | Ni(OH)2 | |
| Ni3(PO4)2 | −2.14–1.08 | −0.24 ± 0.80 | −5.29–0.90 | −0.80 ± 1.64 | −5.65–−2.49 | −3.99 ± 1.17 | Ni3(PO4)2 | |
| Ni4(OH)6SO4 | −10.58–−5.49 | −8.17 ± 1.02 | −13.74–−4.86 | −8.31 ± 1.96 | −17.31–−12.62 | −15.27 ± 1.70 | Ni4(OH)6SO4 | |
| NiCO3 | −2.03–−1.01 | −1.59 ± 0.21 | −3.03–−0.98 | −1.70 ± 0.48 | −3.18–−2.42 | −2.85 ± 0.27 | NiCO3 | |
| Retgersite | −4.86–−4.05 | −4.34 ± 0.22 | −6.20–−4.11 | −4.76 ± 0.56 | −5.21–−5.08 | −5.15 ± 0.05 | NiSO4:6H2O | |
| Hausmannite | −14.14–−5.78 | −10.01 ± 2.10 | −10.88–−4.56 | −7.68 ± 1.84 | −15.38–−10.96 | −13.84 ± 1.71 | Mn3O4 | |
| Birnessite | −12.75–−9.03 | −11.21 ± 0.96 | −11.66–−8.36 | −10.05 ± 0.96 | −14.23–−11.82 | −13.31 ± 0.89 | MnO2 | |
| Bixbyite | −11.27–−5.23 | −8.43 ± 1.53 | −9.09–−4.28 | −6.68 ± 1.40 | −12.62–−9.21 | −11.40 ± 1.30 | Mn2O3 | |
| Manganite | −5.95–−2.93 | −4.53 ± 0.76 | −4.86–−2.46 | −3.66 ± 0.70 | −6.63–−4.92 | −6.01 ± 0.65 | MnOOH | |
| Mn2(SO4)3 | −54.79–−51.05 | −52.59 ± 0.70 | −52.81–−51.49 | −52.40 ± 0.37 | −52.14–−50.98 | −51.65 ± 0.56 | Mn2(SO4)3 | |
| Mn3(PO4)2 | −13.11–−7.57 | −9.72 ± 1.14 | −9.98–−6.90 | −8.81 ± 0.71 | −10.39–−8.81 | −9.75 ± 0.67 | Mn3(PO4)2 | |
| MnCl2:4H2O | −11.4–−9.61 | −10.38 ± 0.36 | −10.66–−9.70 | −10.32 ± 0.21 | −10.17–−9.58 | −9.94 ± 0.25 | MnCl2:4H2O | |
| MnHPO4 | 2.88–−4.63 | 3.93 ± 0.37 | 3.70–4.78 | 4.09 ± 0.27 | 4.14–4.48 | 4.34 ± 0.16 | MnHPO4 | |
| MnSO4 | −10.68–−8.82 | −9.60 ± 0.35 | −9.76–−9.05 | −9.53 ± 0.19 | −9.40–−8.84 | −9.16 ± 0.27 | MnSO4 | |
| Nsutite | −12.16–−8.44 | −10.62 ± 0.96 | −11.07–−7.77 | −9.46 ± 0.96 | −13.64–−11.23 | −12.73 ± 0.89 | MnO2 | |
| Pyrochroite | −7.10–−4.65 | −5.81 ± 0.58 | −6.01–−4.51 | −5.22 ± 0.45 | −6.98–−5.97 | −6.67 ± 0.42 | Mn(OH)2 | |
| Pyrolusite | −10.69–−6.97 | −9.15 ± 0.96 | −9.60–−6.30 | −7.99 ± 0.96 | −12.17–−9.76 | −11.25 ± 0.89 | MnO2 | |
| Rhodochrosite | 0.16–2.41 | 1.45 ± 0.49 | 1.22–2.27 | 1.83 ± 0.30 | 1.20–1.67 | 1.43 ± 0.23 | MnCO3 | |
| O2(g) | −38.30–−35.10 | −37.41 ± 0.87 | −37.90–−34.30 | −36.26 ± 1.05 | −41.11–−38.30 | −39.91 ± 1.02 | O2 | |
| CH4(g) | −73.88–−66.72 | −68.85 ± 1.90 | −75.65–−67.81 | −71.36 ± 2.28 | −66.68–−60.28 | −63.02 ± 2.33 | CH4 | |
| CO2(g) | −0.79–−0.03 | −0.38 ± 0.18 | −0.96–−0.32 | −0.59 ± 0.18 | 0.01–0.80 | 0.47 ± 0.29 | CO2 | |
| Ionic strength | 0.34–0.69 | 0.52 ± 0.25 | 0.43–0.66 | 0.55 ± 0.16 | 0.56–0.68 | 0.62 ± 0.08 | ||
| Total carbon | 0.217–0.458 | 0.3373 ± 0.171 | 0.229–0.411 | 0.32 ± 0.13 | 0.515–0.585 | 0.55 ± 0.05 | ||
| Total CO2 | 0.217–0.458 | 0.3373 ± 0.171 | 0.23–0.411 | 0.32 ± 0.13 | 0.515–0.585 | 0.55 ± 0.05 | ||
| Electrical balance | −0.48–−0.35 | −0.41 ± 0.091 | −0.51–−0.44 | −0.48 ± 0.05 | −0.55–−0.5 | −0.53 ± 0.03 | ||
| Percent error | −71.3–−44.5 | −57.87 ± 18.92 | −84.1–−41.9 | −62.98 ± 29.9 | −56.3–−50.3 | −53.3 ± 4.19 | ||
| Iterations | 10.0–12.0 | 11.0 ± 1.414 | 11.0–12.0 | 11.5 ± 0.71 | 11.0–12.0 | 11.5 ± 0.71 | ||
| Total H | 111.2–111.5 | 111.36 ± 0.156 | 111.3–111.4 | 111.3 ± 0.11 | 111.6–111.8 | 111.7 ± 0.15 | ||
| Total O | 56.59–57.85 | 57.22 ± 0.888 | 56.79–57.63 | 57.21 ± 0.59 | 57.85–57.87 | 57.86 ± 0.01 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rashid, A.; Ayub, M.; Ullah, Z.; Ali, A.; Sardar, T.; Iqbal, J.; Gao, X.; Bundschuh, J.; Li, C.; Khattak, S.A.; et al. Groundwater Quality, Health Risk Assessment, and Source Distribution of Heavy Metals Contamination around Chromite Mines: Application of GIS, Sustainable Groundwater Management, Geostatistics, PCAMLR, and PMF Receptor Model. Int. J. Environ. Res. Public Health 2023, 20, 2113. https://doi.org/10.3390/ijerph20032113
Rashid A, Ayub M, Ullah Z, Ali A, Sardar T, Iqbal J, Gao X, Bundschuh J, Li C, Khattak SA, et al. Groundwater Quality, Health Risk Assessment, and Source Distribution of Heavy Metals Contamination around Chromite Mines: Application of GIS, Sustainable Groundwater Management, Geostatistics, PCAMLR, and PMF Receptor Model. International Journal of Environmental Research and Public Health. 2023; 20(3):2113. https://doi.org/10.3390/ijerph20032113
Chicago/Turabian StyleRashid, Abdur, Muhammad Ayub, Zahid Ullah, Asmat Ali, Tariq Sardar, Javed Iqbal, Xubo Gao, Jochen Bundschuh, Chengcheng Li, Seema Anjum Khattak, and et al. 2023. "Groundwater Quality, Health Risk Assessment, and Source Distribution of Heavy Metals Contamination around Chromite Mines: Application of GIS, Sustainable Groundwater Management, Geostatistics, PCAMLR, and PMF Receptor Model" International Journal of Environmental Research and Public Health 20, no. 3: 2113. https://doi.org/10.3390/ijerph20032113
APA StyleRashid, A., Ayub, M., Ullah, Z., Ali, A., Sardar, T., Iqbal, J., Gao, X., Bundschuh, J., Li, C., Khattak, S. A., Ali, L., El-Serehy, H. A., Kaushik, P., & Khan, S. (2023). Groundwater Quality, Health Risk Assessment, and Source Distribution of Heavy Metals Contamination around Chromite Mines: Application of GIS, Sustainable Groundwater Management, Geostatistics, PCAMLR, and PMF Receptor Model. International Journal of Environmental Research and Public Health, 20(3), 2113. https://doi.org/10.3390/ijerph20032113









