Impact of Plasticizer on the Intestinal Epithelial Integrity and Tissue-Repairing Ability within Cells in the Proximity of the Human Gut Microbiome
Abstract
:1. Introduction
2. Materials and Methods
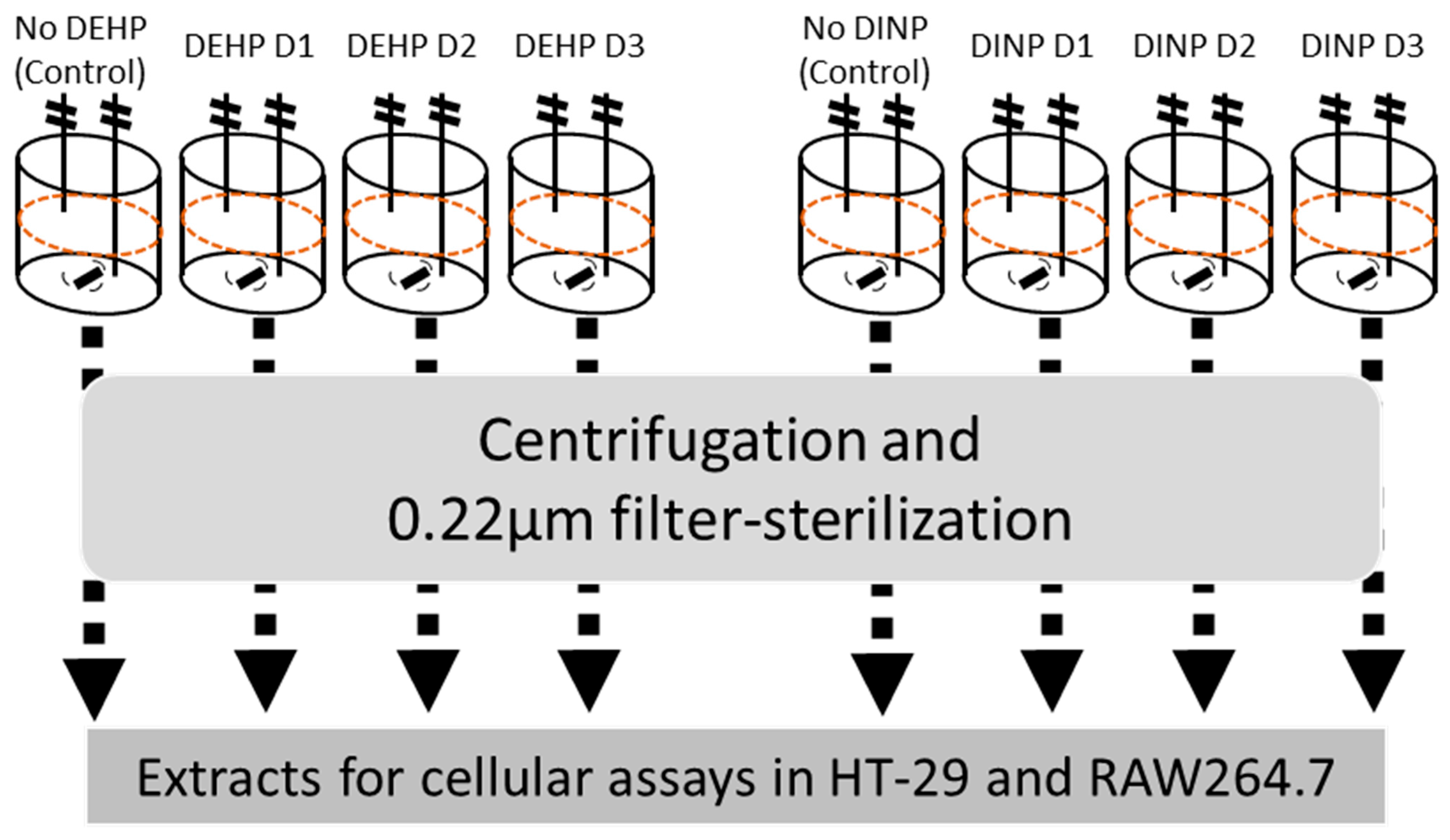
2.1. Batch Culture and Preparation of Luminal Extracts
2.2. DNA Extraction, 16s rDNA Sequencing and Microbial Composition Analysis
2.3. Cell Culture and Viability Assay
2.4. RNA Extraction and Reverse Transcription Quantitative PCR
2.5. Western Blot
2.6. Immunofluorescence
2.7. Statistical Analysis
3. Results
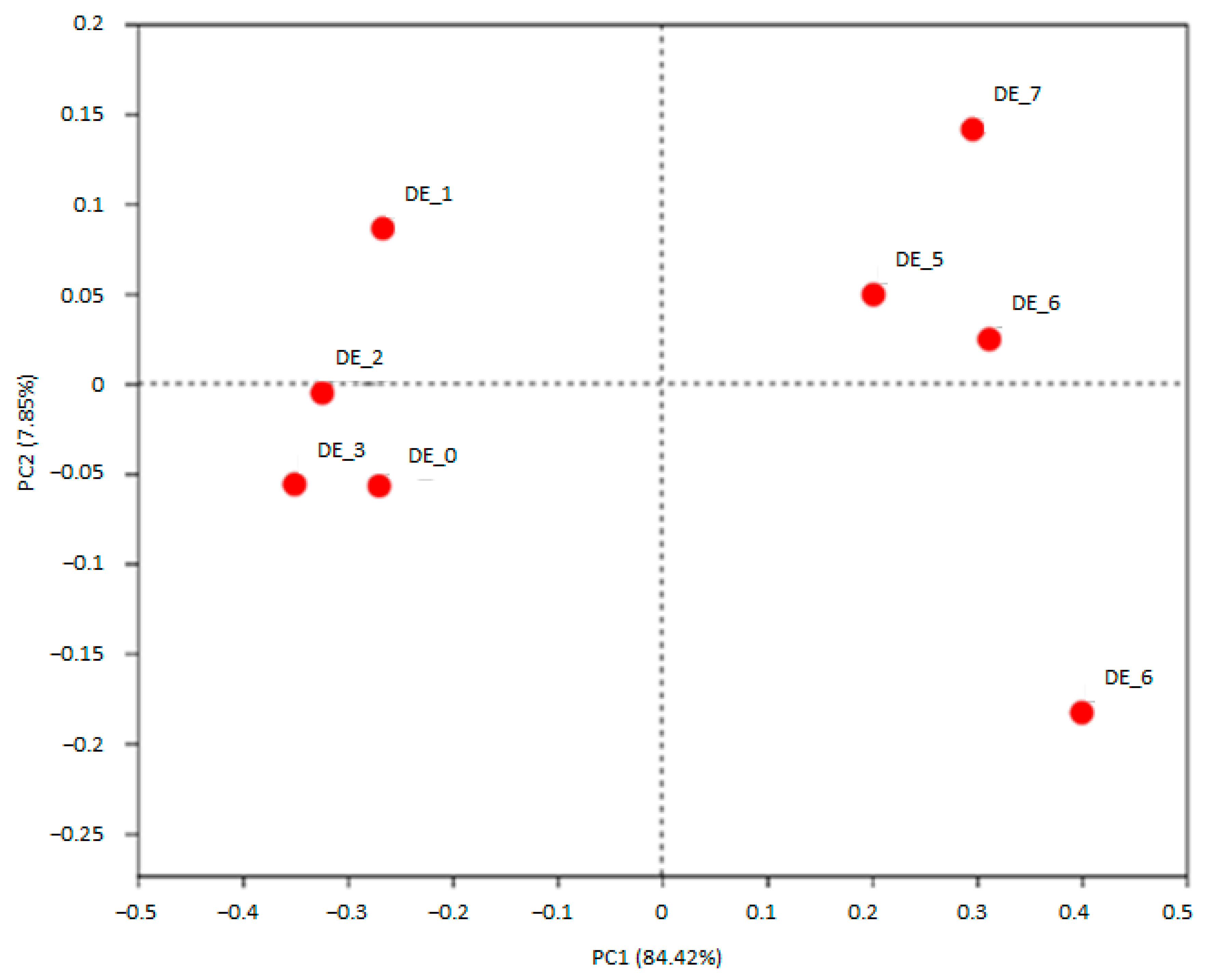
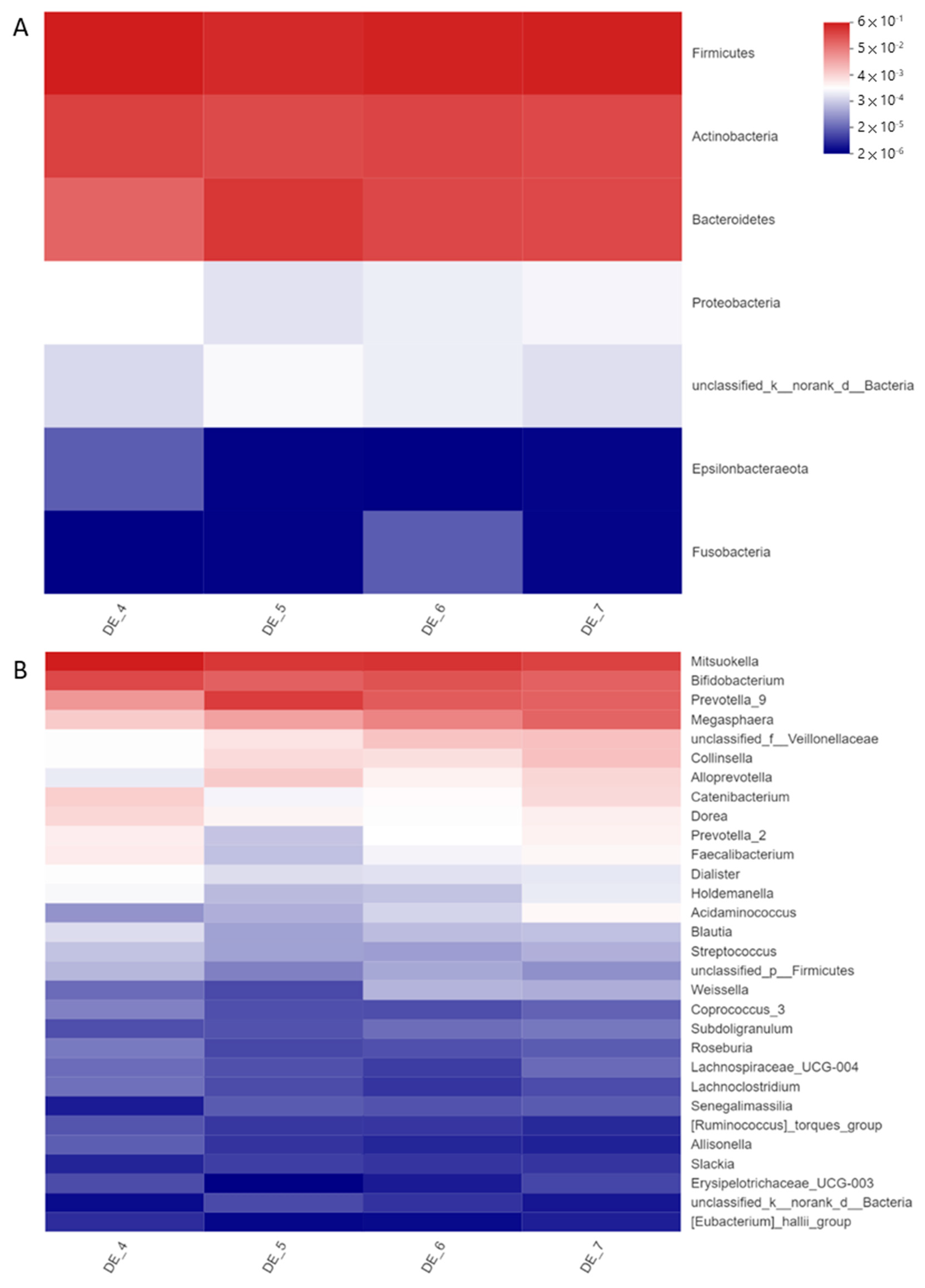
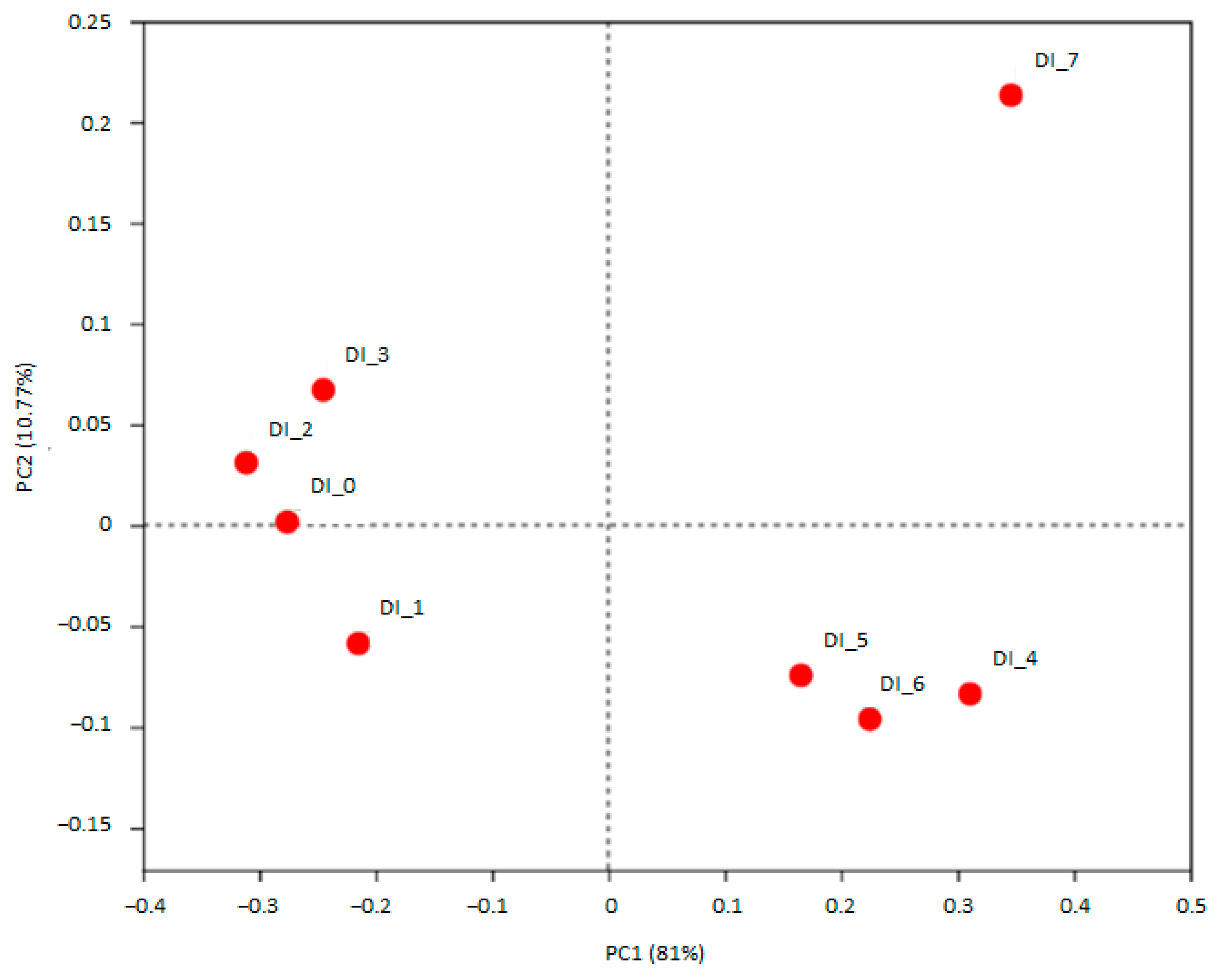
3.1. DEHP and DINP Altered the Compositions of Gut Microbiota
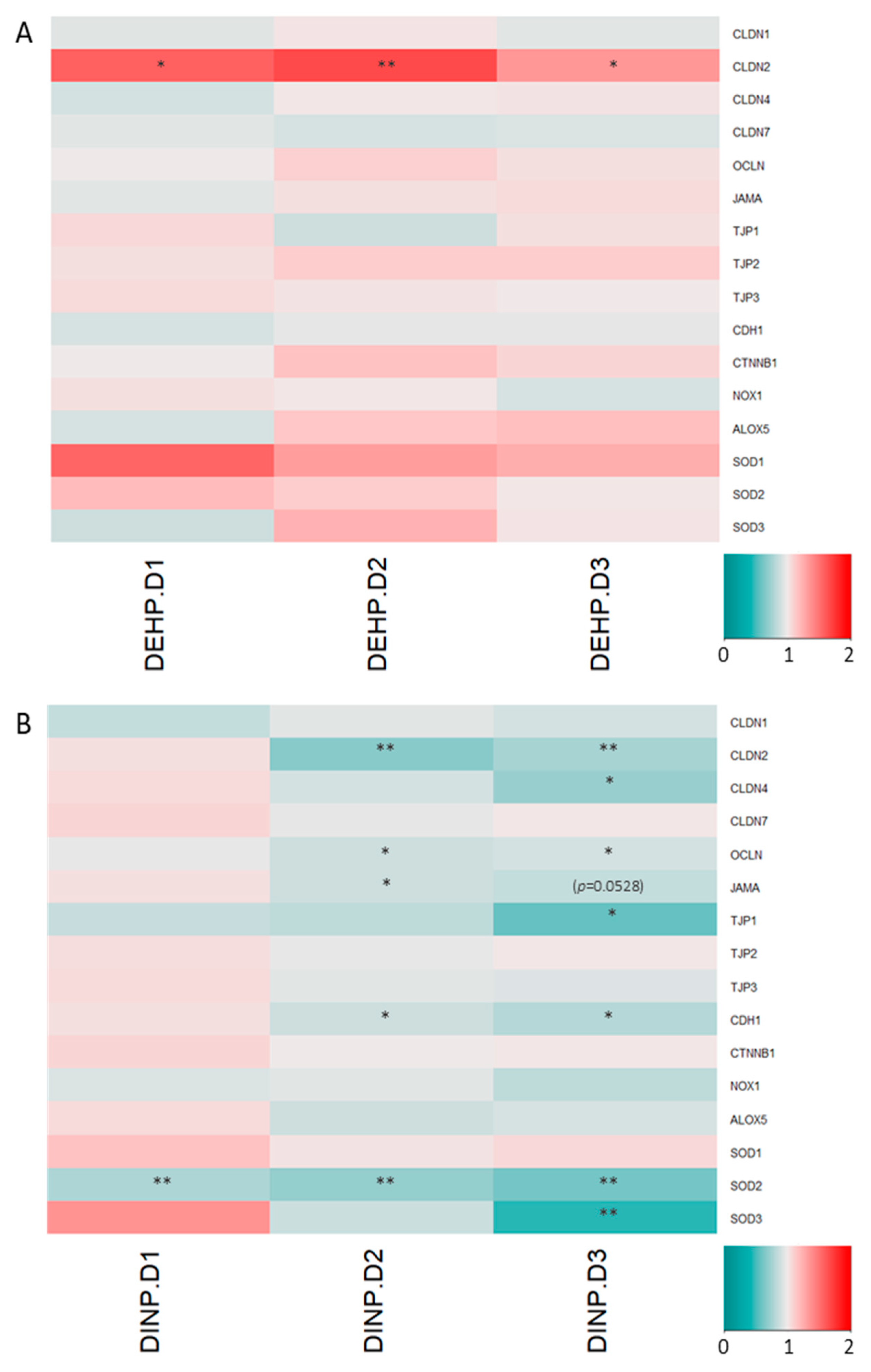
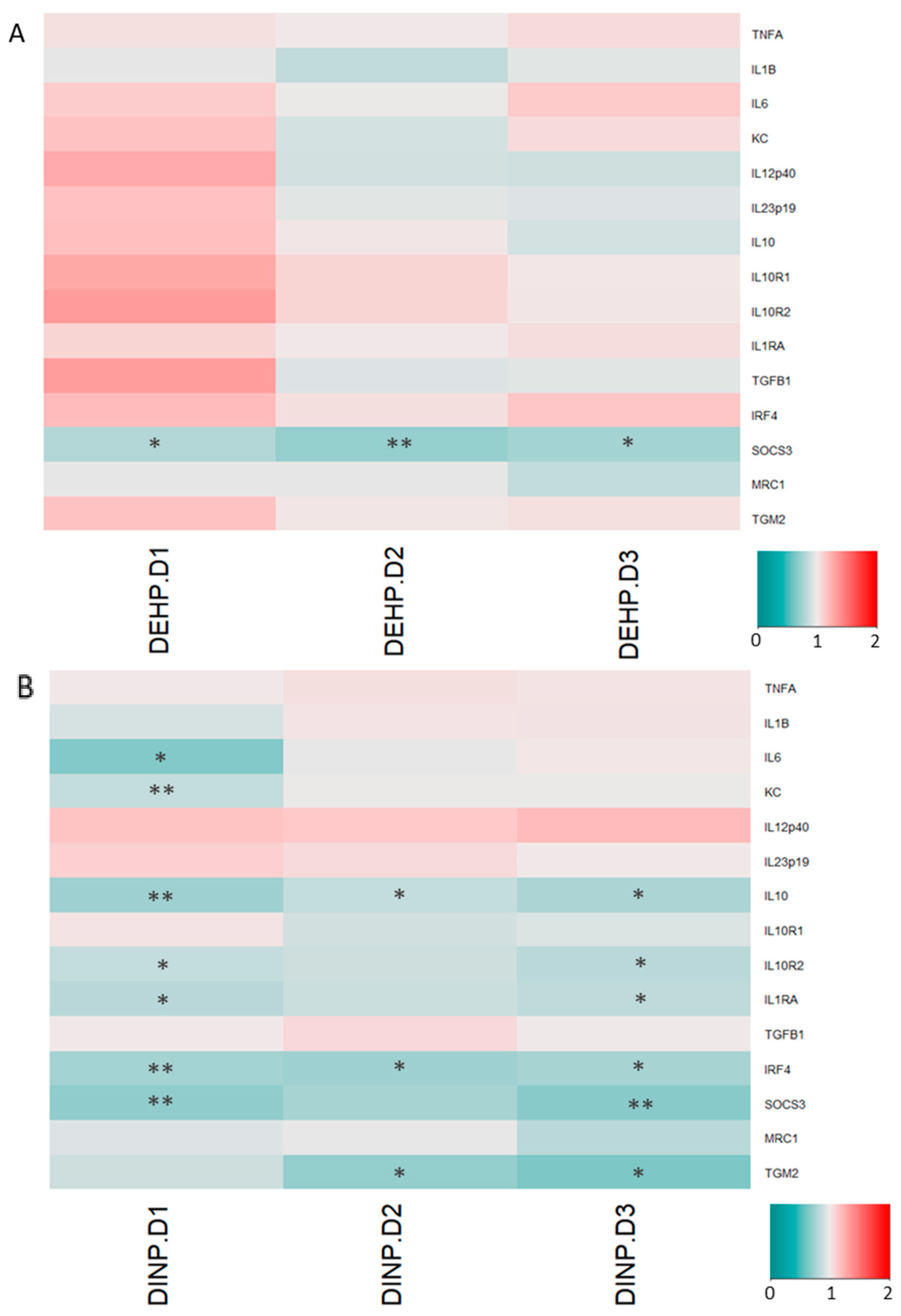
3.2. Luminal Extracts from Plasticizer-Treated Microbiota Altered Gene Expressions in HT-29 and RAW264.7 Cells
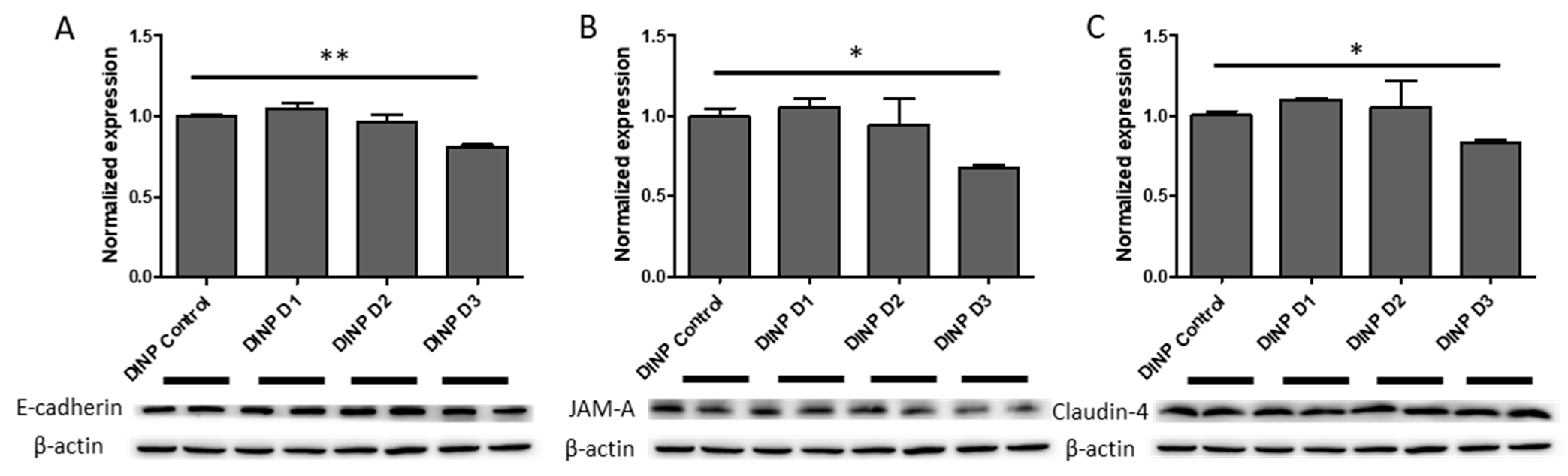
3.3. Microbiota Metabolites from Fermenter Exposed to High-Dose DINP Reduced Protein Expressions of E-Cadherin, JAM-A and Claudin-4 in HT-29
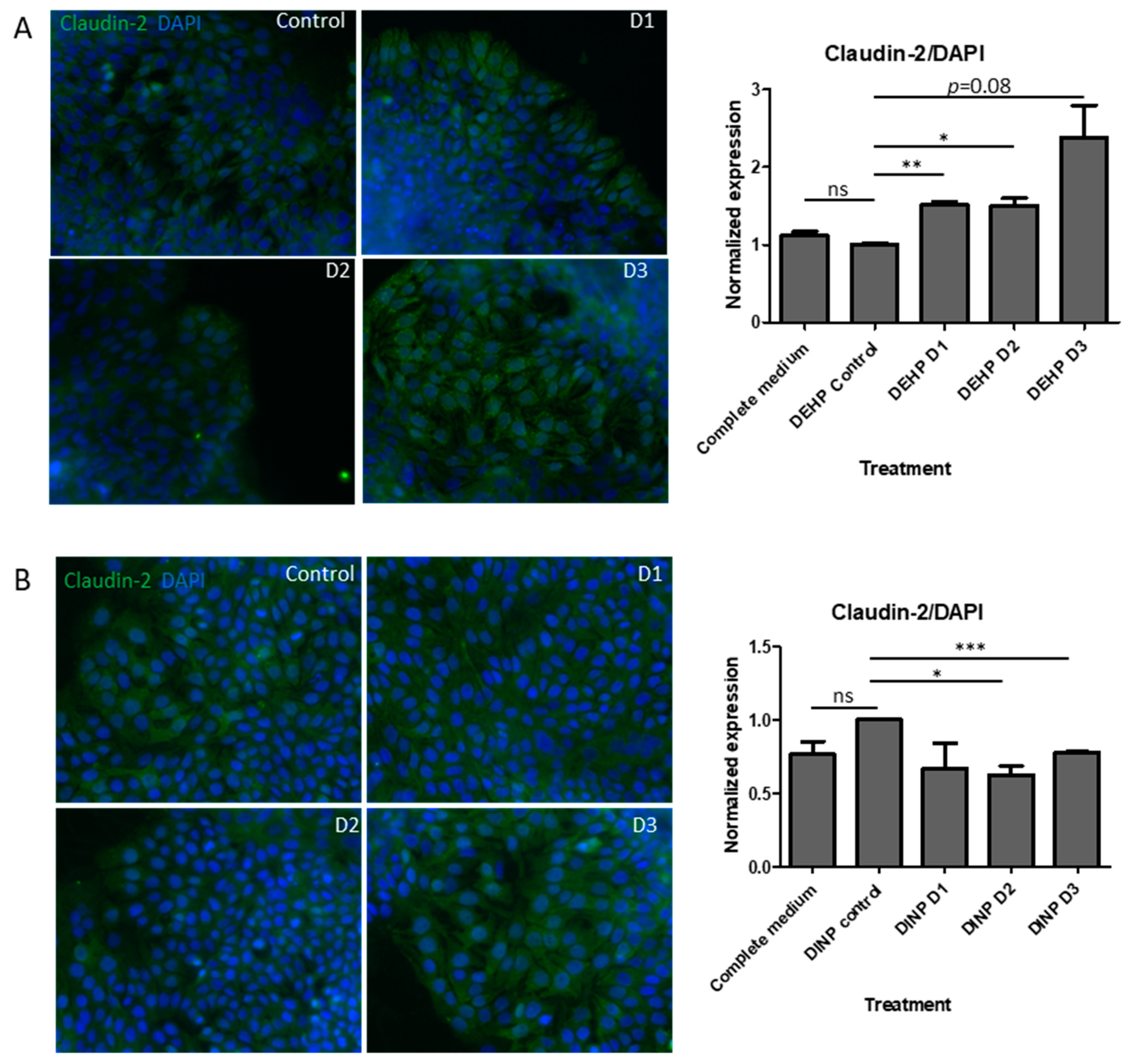
3.4. DEHP Fermenter Luminal Extracts Promoted Claudin-2 Expression in HT-29, whereas Reduction of this Protein Was Observed after Treatment of DINP Fermenter Extracts
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hogberg, J.; Hanberg, A.; Berglund, M.; Skerfving, S.; Remberger, M.; Calafat, A.M.; Filipsson, A.F.; Jansson, B.; Johansson, N.; Appelgren, M.; et al. Phthalate diesters and their metabolites in human breast milk, blood or serum, and urine as biomarkers of exposure in vulnerable populations. Environ. Health Perspect. 2008, 116, 334–339. [Google Scholar] [CrossRef] [Green Version]
- Rusyn, I.; Corton, J.C. Mechanistic considerations for human relevance of cancer hazard of di(2-ethylhexyl) phthalate. Mutat. Res. 2012, 750, 141–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rowdhwal, S.S.S.; Chen, J. Toxic Effects of Di-2-ethylhexyl Phthalate: An Overview. Biomed. Res. Int. 2018, 2018, 1750368. [Google Scholar] [CrossRef] [Green Version]
- Hao, C.; Cheng, X.; Xia, H.; Ma, X. The endocrine disruptor mono-(2-ethylhexyl) phthalate promotes adipocyte differentiation and induces obesity in mice. Biosci. Rep. 2012, 32, 619–629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vessa, B.; Perlman, B.; McGovern, P.G.; Morelli, S.S. Endocrine disruptors and female fertility: A review of pesticide and plasticizer effects. F. S. Rep. 2022, 3, 86–90. [Google Scholar] [CrossRef]
- Zhang, J.N.; Zhang, R.Q.; Liu, J.C.; Li, L.; Shen, W.; Sun, X.F. Di (2-ethylhexyl) Phthalate Exposure Impairs the microRNAs Expression Profile During Primordial Follicle Assembly. Front. Endocrinol. 2019, 10, 877. [Google Scholar] [CrossRef] [Green Version]
- Hines, C.J.; Hopf, N.B.; Deddens, J.A.; Silva, M.J.; Calafat, A.M. Estimated daily intake of phthalates in occupationally exposed groups. J. Expo. Sci. Environ. Epidemiol. 2011, 21, 133–141. [Google Scholar] [CrossRef] [Green Version]
- Adamovsky, O.; Buerger, A.N.; Vespalcova, H.; Sohag, S.R.; Hanlon, A.T.; Ginn, P.E.; Craft, S.L.; Smatana, S.; Budinska, E.; Persico, M.; et al. Evaluation of Microbiome-Host Relationships in the Zebrafish Gastrointestinal System Reveals Adaptive Immunity Is a Target of Bis(2-ethylhexyl) Phthalate (DEHP) Exposure. Environ. Sci. Technol. 2020, 54, 5719–5728. [Google Scholar] [CrossRef]
- Chang-Liao, W.L.; Hou, M.L.; Chang, L.W.; Lee, C.J.; Tsai, Y.M.; Lin, L.C.; Tsai, T.H. Determination and pharmacokinetics of di-(2-ethylhexyl) phthalate in rats by ultra performance liquid chromatography with tandem mass spectrometry. Molecules 2013, 18, 11452–11466. [Google Scholar] [CrossRef] [Green Version]
- Koch, H.M.; Angerer, J. Di-iso-nonylphthalate (DINP) metabolites in human urine after a single oral dose of deuterium-labelled DINP. Int. J. Hyg. Environ. Health 2007, 210, 9–19. [Google Scholar] [CrossRef]
- McKee, R.H.; El-Hawari, M.; Stoltz, M.; Pallas, F.; Lington, A.W. Absorption, disposition and metabolism of di-isononyl phthalate (DINP) in F-344 rats. J. Appl. Toxicol. 2002, 22, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Nugent, S.G.; Kumar, D.; Rampton, D.S. Intestinal luminal pH in inflammatory bowel disease possible determinants and implications for therapy with aminosalicylates and other drugs. Gut 2001, 48, 571–577. [Google Scholar] [CrossRef] [Green Version]
- Tsumura, Y.; Ishimitsu, S.; Saito, I.; Sakai, H.; Tsuchida, Y.; Tonogai, Y. Estimated daily intake of plasticizers in 1-week duplicate diet samples following regulation of DEHP-containing PVC gloves in Japan. Food Addit. Contam. 2003, 20, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.W.; Lee, C.C.; Pan, W.H.; Chou, W.C.; Huang, H.B.; Chiang, H.C.; Huang, P.C. Estimated Daily Intake and Cumulative Risk Assessment of Phthalates in the General Taiwanese after the 2011 DEHP Food Scandal. Sci. Rep. 2017, 7, 45009. [Google Scholar] [CrossRef] [Green Version]
- Centre for Food Safety; Food and Environmental Hygiene Department; The Government of the Hong Kong Special Administrative Region. Risk Assessment Studies Report No. 57 Chemical Hazard Evaluation—Phthalates in Food; Government of the Hong Kong Special Administrative Region: Hong Kong, China, 2018.
- Song, A.X.; Mao, Y.H.; Siu, K.C.; Wu, J.Y. Bifidogenic effects of Cordyceps sinensis fungal exopolysaccharide and konjac glucomannan after ultrasound and acid degradation. Int. J. Biol. Macromol. 2018, 111, 587–594. [Google Scholar] [CrossRef]
- Zhao, G.; Nyman, M.; Jonsson, J.A. Rapid determination of short-chain fatty acids in colonic contents and faeces of humans and rats by acidified water-extraction and direct-injection gas chromatography. Biomed Chromatogr. 2006, 20, 674–682. [Google Scholar] [CrossRef] [PubMed]
- Putt, K.K.; Pei, R.; White, H.M.; Bolling, B.W. Yogurt inhibits intestinal barrier dysfunction in Caco-2 cells by increasing tight junctions. Food Funct. 2017, 8, 406–414. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, T.; Yoshinaga, N.; Tanabe, S. Interleukin-6 (IL-6) regulates claudin-2 expression and tight junction permeability in intestinal epithelium. J. Biol. Chem. 2011, 286, 31263–31271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, J.; Li, R.; Raes, J.; Arumugam, M.; Burgdorf, K.S.; Manichanh, C.; Nielsen, T.; Pons, N.; Levenez, F.; Yamada, T.; et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010, 464, 59–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frank, D.N.; St. Amand, A.L.; Feldman, R.A.; Boedeker, E.C.; Harpaz, N.; Pace, N.R. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. PNAS 2007, 104, 13780–13785. [Google Scholar] [CrossRef] [Green Version]
- Chung, H.C.; Lee, O.O.; Huang, Y.L.; Mok, S.Y.; Kolter, R.; Qian, P.Y. Bacterial community succession and chemical profiles of subtidal biofilms in relation to larval settlement of the polychaete Hydroides elegans. ISME J. 2010, 4, 817–828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gevers, D.; Kugathasan, S.; Denson, L.A.; Vazquez-Baeza, Y.; Van Treuren, W.; Ren, B.; Schwager, E.; Knights, D.; Song, S.J.; Yassour, M.; et al. The treatment-naive microbiome in new-onset Crohn’s disease. Cell Host Microbe 2014, 15, 382–392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, T.; Liu, R.; Lee, A.; Long, Y.; Du, L.; Lai, S.; Chen, X.; Wang, L.; Si, J.; Owyang, C.; et al. Altered Intestinal Microbiota with Increased Abundance of Prevotella Is Associated with High Risk of Diarrhea-Predominant Irritable Bowel Syndrome. Gastroenterol. Res. Pract. 2018, 2018, 6961783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iljazovic, A.; Roy, U.; Galvez, E.J.C.; Lesker, T.R.; Zhao, B.; Gronow, A.; Amend, L.; Will, S.E.; Hofmann, J.D.; Pils, M.C.; et al. Perturbation of the gut microbiome by Prevotella spp. enhances host susceptibility to mucosal inflammation. Mucosal Immunol. 2020, 14, 113–124. [Google Scholar] [CrossRef]
- Gerasimidis, K.; Bertz, M.; Hanske, L.; Junick, J.; Biskou, O.; Aguilera, M.; Garrick, V.; Russell, R.K.; Blaut, M.; McGrogan, P.; et al. Decline in presumptively protective gut bacterial species and metabolites are paradoxically associated with disease improvement in pediatric Crohn’s disease during enteral nutrition. Inflamm. Bowel Dis. 2014, 20, 861–871. [Google Scholar] [CrossRef]
- Alam, M.T.; Amos, G.C.A.; Murphy, A.R.J.; Murch, S.; Wellington, E.M.H.; Arasaradnam, R.P. Microbial imbalance in inflammatory bowel disease patients at different taxonomic levels. Gut Pathog. 2020, 12, 1. [Google Scholar] [CrossRef]
- Pascal, V.; Pozuelo, M.; Borruel, N.; Casellas, F.; Campos, D.; Santiago, A.; Martinez, X.; Varela, E.; Sarrabayrouse, G.; Machiels, K.; et al. A microbial signature for Crohn’s disease. Gut 2017, 66, 813–822. [Google Scholar] [CrossRef] [Green Version]
- Chiodini, R.J.; Dowd, S.E.; Chamberlin, W.M.; Galandiuk, S.; Davis, B.; Glassing, A. Microbial Population Differentials between Mucosal and Submucosal Intestinal Tissues in Advanced Crohn’s Disease of the Ileum. PLoS ONE 2015, 10, e0134382. [Google Scholar] [CrossRef]
- Lo Presti, A.; Zorzi, F.; Del Chierico, F.; Altomare, A.; Cocca, S.; Avola, A.; De Biasio, F.; Russo, A.; Cella, E.; Reddel, S.; et al. Fecal and Mucosal Microbiota Profiling in Irritable Bowel Syndrome and Inflammatory Bowel Disease. Front. Microbiol. 2019, 10, 1655. [Google Scholar] [CrossRef] [Green Version]
- Caplan, M.S.; Miller-Catchpole, R.; Kaup, S.; Russell, T.; Lickerman, M.; Amer, M.; Xiao, Y.; Thomson, A.R., Jr. Bifidobacterial Supplementation Reduces the Incidence of Necrotizing Enterocolitis in a Neonatal Rat Model. Gastroenterology 1999, 117, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Sommer, F.; Ruhlemann, M.C.; Bang, C.; Rehman, A.; Kaleta, C.; Dempfle, A.; Weidinger, S.; Krauss-Etschmann, S.; Schmidt-Arras, D.; Schulte, D.; et al. Microbiomarkers in inflammatory bowel diseases caveats come with caviar. Gut 2017, 66, 1734–1738. [Google Scholar] [CrossRef]
- Sun, M.; Wu, W.; Chen, L.; Yang, W.; Huang, X.; Ma, C.; Chen, F.; Xiao, Y.; Zhao, Y.; Ma, C.; et al. Microbiota-derived short-chain fatty acids promote Th1 cell IL-10 production to maintain intestinal homeostasis. Nat. Commun. 2018, 9, 3555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, L.; Kelly, C.J.; Battista, K.D.; Schaefer, R.; Lanis, J.M.; Alexeev, E.E.; Wang, R.X.; Onyiah, J.C.; Kominsky, D.J.; Colgan, S.P. Microbial-Derived Butyrate Promotes Epithelial Barrier Function through IL-10 Receptor–Dependent Repression of Claudin-2. J. Immunol. 2017, 199, 2976–2984. [Google Scholar] [CrossRef] [Green Version]
- Lipkin, M.; Sherlock, P.; Bell, B.M. Generation Time of Epithelial Cells in the Human Colon. Nature 1962, 195, 175–177. [Google Scholar] [CrossRef] [PubMed]
- Hering, N.A.; Andres, S.; Fromm, A.; van Tol, E.A.; Amasheh, M.; Mankertz, J.; Fromm, M.; Schulzke, J.D. Transforming growth factor-beta, a whey protein component, strengthens the intestinal barrier by upregulating claudin-4 in HT-29/B6 cells. J. Nutr. 2011, 141, 783–789. [Google Scholar] [CrossRef] [Green Version]
- Oshima, T.; Miwa, H.; Joh, T. Changes in the expression of claudins in active ulcerative colitis. J. Gastroenterol. Hepatol. 2008, 23 (Suppl. S2), S146–S150. [Google Scholar] [CrossRef]
- Kucharzik, T.; Walsh, S.V.; Chen, J.; Parkos, C.A.; Nusrat, A. Neutrophil Transmigration in Inflammatory Bowel Disease Is Associated with Differential Expression of Epithelial Intercellular Junction Proteins. Am. J. Pathol. 2001, 159, 2001–2009. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Z.; Bian, C.; Luo, Z.; Guille, C.; Ogunrinde, E.; Wu, J.; Zhao, M.; Fitting, S.; Kamen, D.L.; Oates, J.C.; et al. Progesterone decreases gut permeability through upregulating occludin expression in primary human gut tissues and Caco-2 cells. Sci. Rep. 2019, 9, 8367. [Google Scholar] [CrossRef] [Green Version]
- Taman, H.; Fenton, C.G.; Hensel, I.V.; Anderssen, E.; Florholmen, J.; Paulssen, R.H. Transcriptomic Landscape of Treatment—Naïve Ulcerative Colitis. J. Crohn Colitis 2018, 12, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yang, G.; Geng, X.R.; Cao, Y.; Li, N.; Ma, L.; Chen, S.; Yang, P.C.; Liu, Z. Microbial products induce claudin-2 to compromise gut epithelial barrier function. PLoS ONE 2013, 8, e68547. [Google Scholar] [CrossRef]
- Tsai, P.Y.; Zhang, B.; He, W.Q.; Zha, J.M.; Odenwald, M.A.; Singh, G.; Tamura, A.; Shen, L.; Sailer, A.; Yeruva, S.; et al. IL-22 Upregulates Epithelial Claudin-2 to Drive Diarrhea and Enteric Pathogen Clearance. Cell Host Microbe 2017, 21, 671–681. [Google Scholar] [CrossRef] [Green Version]
- Roberts, J.L.; Liu, G.; Darby, T.M.; Fernandes, L.M.; Diaz-Hernandez, M.E.; Jones, R.M.; Drissi, H. Bifidobacterium adolescentis supplementation attenuates fracture-induced systemic sequelae. Biomed. Pharmacother. 2020, 132, 110831. [Google Scholar] [CrossRef]
- Shastri, S.; Shinde, T.; Sohal, S.S.; Gueven, N.; Eri, R. Idebenone Protects against Acute Murine Colitis via Antioxidant and Anti-Inflammatory Mechanisms. Int. J. Mol. Sci. 2020, 21, 484. [Google Scholar] [CrossRef] [Green Version]
- Segui, J.; Gironella, M.; Sans, M.; Granell, S.; Gil, F.; Gimeno, M.; Coronel, P.; Pique, J.M.; Panes, J. Superoxide dismutase ameliorates TNBS-induced colitis by reducing oxidative stress, adhesion molecule expression, and leukocyte recruitment into the inflamed intestine. J. Leukoc. Biol. 2004, 76, 537–544. [Google Scholar] [CrossRef] [Green Version]
- Damiani, C.R.; Benetton, C.A.; Stoffel, C.; Bardini, K.C.; Cardoso, V.H.; Di Giunta, G.; Pinho, R.A.; Dal-Pizzol, F.; Streck, E.L. Oxidative stress and metabolism in animal model of colitis induced by dextran sulfate sodium. J. Gastroenterol. Hepatol. 2007, 22, 1846–1851. [Google Scholar] [CrossRef]
- Miao, L.; St Clair, D.K. Regulation of superoxide dismutase genes: Implications in disease. Free Radic. Biol. Med. 2009, 47, 344–356. [Google Scholar] [CrossRef] [Green Version]
- Martinez, F.O.; Helming, L.; Milde, R.; Varin, A.; Melgert, B.N.; Draijer, C.; Thomas, B.; Fabbri, M.; Crawshaw, A.; Ho, L.P.; et al. Genetic programs expressed in resting and IL-4 alternatively activated mouse and human macrophages: Similarities and differences. Blood 2013, 121, e57–e69. [Google Scholar] [CrossRef]
- Li, B.; Alli, R.; Vogel, P.; Geiger, T.L. IL-10 modulates DSS-induced colitis through a macrophage-ROS-NO axis. Mucosal Immunol. 2014, 7, 869–878. [Google Scholar] [CrossRef] [Green Version]
- Qasimi, P.; Ming-Lum, A.; Ghanipour, A.; Ong, C.J.; Cox, M.E.; Ihle, J.; Cacalano, N.; Yoshimura, A.; Mui, A.L. Divergent mechanisms utilized by SOCS3 to mediate interleukin-10 inhibition of tumor necrosis factor alpha and nitric oxide production by macrophages. J. Biol. Chem. 2006, 281, 6316–6324. [Google Scholar] [CrossRef] [Green Version]
- Qin, H.; Holdbrooks, A.T.; Liu, Y.; Reynolds, S.L.; Yanagisawa, L.L.; Benveniste, E.N. SOCS3 deficiency promotes M1 macrophage polarization and inflammation. J. Immunol. 2012, 189, 3439–3448. [Google Scholar] [CrossRef] [Green Version]
- Honma, K.; Udono, H.; Kohno, T.; Yamamoto, K.; Ogawa, A.; Takemori, T.; Kumatori, A.; Suzuki, S.; Matsuyama, T.; Yui, K. Interferon regulatory factor 4 negatively regulates the production of proinflammatory cytokines by macrophages in response to LPS. Proc. Natl. Acad. Sci. USA 2005, 102, 16001–16006. [Google Scholar] [CrossRef] [Green Version]
- Zhu, W.; Jin, Z.; Yu, J.; Liang, J.; Yang, Q.; Li, F.; Shi, X.; Zhu, X.; Zhang, X. Baicalin ameliorates experimental inflammatory bowel disease through polarization of macrophages to an M2 phenotype. Int. Immunopharmacol. 2016, 35, 119–126. [Google Scholar] [CrossRef]
- Yang, T.N.; Li, X.N.; Wang, Y.X.; Ma, X.Y.; Li, J.L. Disrupted microbiota-barrier-immune interaction in phthalates-mediated barrier defect in the duodenum. Chemosphere 2022, 308, 136275. [Google Scholar] [CrossRef]
- Chiu, K.K.; Bashir, S.T.; Abdel-Hamid, A.M.; Clark, L.V.; Laws, M.J.; Cann, I.; Nowak, R.A.; Flaws, J.A. Isolation of DiNP-Degrading Microbes from the Mouse Colon and the Influence DiNP Exposure Has on the Microbiota, Intestinal Integrity, and Immune Status of the Colon. Toxics 2022, 10, 75. [Google Scholar] [CrossRef]
- Chiu, K.; Bashir, S.T.; Nowak, R.A.; Mei, W.; Flaws, J.A. Subacute exposure to di-isononyl phthalate alters the morphology, endocrine function, and immune system in the colon of adult female mice. Sci. Rep. 2020, 10, 18788. [Google Scholar] [CrossRef]

| Alpha Diversities | Control | DEHP D1 | DEHP D2 | DEHP D3 |
|---|---|---|---|---|
| Shannon | 1.88 | 1.91 | 2.04 | 2.25 |
| Simpson | 0.28 | 0.22 | 0.2 | 0.16 |
| pd | 13.24 | 12.69 | 12.39 | 12.07 |
| Alpha Diversities | No DINP Control | DINP D1 | DINP D2 | DINP D3 |
|---|---|---|---|---|
| Shannon | 2.59 | 2.44 | 2.52 | 2.72 |
| Simpson | 0.13 | 0.17 | 0.15 | 0.12 |
| pd | 15.53 | 16.49 | 16.54 | 15.53 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shum, T.-F.; Wang, L.; Chiou, J. Impact of Plasticizer on the Intestinal Epithelial Integrity and Tissue-Repairing Ability within Cells in the Proximity of the Human Gut Microbiome. Int. J. Environ. Res. Public Health 2023, 20, 2152. https://doi.org/10.3390/ijerph20032152
Shum T-F, Wang L, Chiou J. Impact of Plasticizer on the Intestinal Epithelial Integrity and Tissue-Repairing Ability within Cells in the Proximity of the Human Gut Microbiome. International Journal of Environmental Research and Public Health. 2023; 20(3):2152. https://doi.org/10.3390/ijerph20032152
Chicago/Turabian StyleShum, Tim-Fat, Liwen Wang, and Jiachi Chiou. 2023. "Impact of Plasticizer on the Intestinal Epithelial Integrity and Tissue-Repairing Ability within Cells in the Proximity of the Human Gut Microbiome" International Journal of Environmental Research and Public Health 20, no. 3: 2152. https://doi.org/10.3390/ijerph20032152





