An Agenda for Research of Uncovered Epidemiological Patterns of Tick-Borne Pathogens Affecting Human Health
Abstract
:1. Introduction
2. The Weather and the Ticks
- (i)
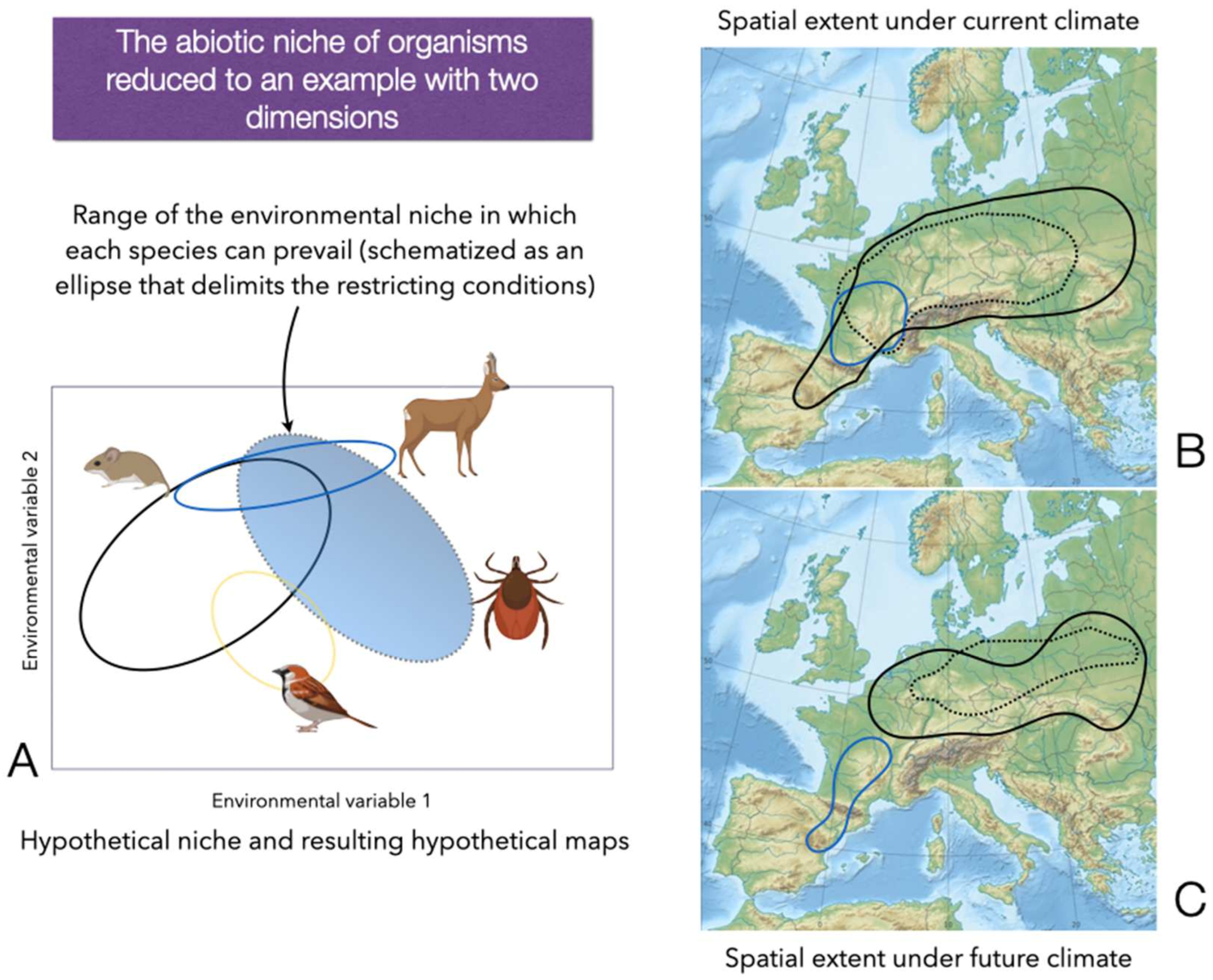
- The tick’s environmental niche is a hypervolume with a mathematical definition. A hypervolume is the intersection of the gradient of several climate variables that define the climate comfort for the tick. The capture and mathematical definition of such a niche for species affecting human health greatly improves calculations of risk and design of major strategies for protecting human health from TBP. To note, studies purely devoted to the evaluation of R0, the distribution of host and reservoirs, should also evaluate such a niche. The translation of these data to a map would result in an informative tool, but not in an instrument of decision. We do think that the interpretation of the findings obtained through the analysis of the limiting variables experienced by the tick is a priority.
- (ii)
- There is a tendency to use the climate variables “as available” on the Internet without further verification or transformation. This is not a criticism of existing climate datasets, but a call of attention for epidemiologists working on ticks and TBP. For example, the seasons of the year are commonly calculated following astronomical dates; however, ticks do not adhere to an artificial construct that lacks an ecological background. Wettest, warmest, or driest quarters are calculated in a similar way. For example, studies [35] could not find “evidence supporting the superiority of the [current] calculation approach”. The superior performance of an approach based on a harmonic regression of monthly series of climate data, allowing the calculation of daily weather values has been demonstrated and validated [32] and scripts for calculations are freely available [33]. It is important to validate these methods or to provide solid variables that (a) have ecological meaning for ticks, (b) are not self-correlated, and (c) have been selected after an evaluation of the impact of the weather on the modelled tick.
- (iii)
- Species with large distributions may have “regional strains”, adapted to the prevailing climate, an extreme demonstrated so far for a few species [28,36]. The training of one single model, later projected to the full range of the species seems to not be the best modelling approach. On the other hand, an “-omics” approach to these hypothetical “races” or “strains” could probably provide key details regarding the transmission of TBP. Local strains of ticks, adapted to the prevailing climate, could gain contact with key reservoirs, circulating strains of a pathogen. As far as we know, this extreme has never been addressed in depth.
- (iv)
- Field surveys [37,38] proved that the trends of climate are pushing some tick species out of their “historical” limits or altering their periods of questing on hosts [39]. Nevertheless, studies of the combined impacts of the climate, the probable density and distribution of the hosts (that are affected also by the climate), and the landscape transformations, are available only for a few medically significant tick species. We think this is a promising field of research because of the need for a solid framework over which to build a new epidemiological approach.
3. Vertebrates: The Neglected Component of the Tick Niche
- (i)
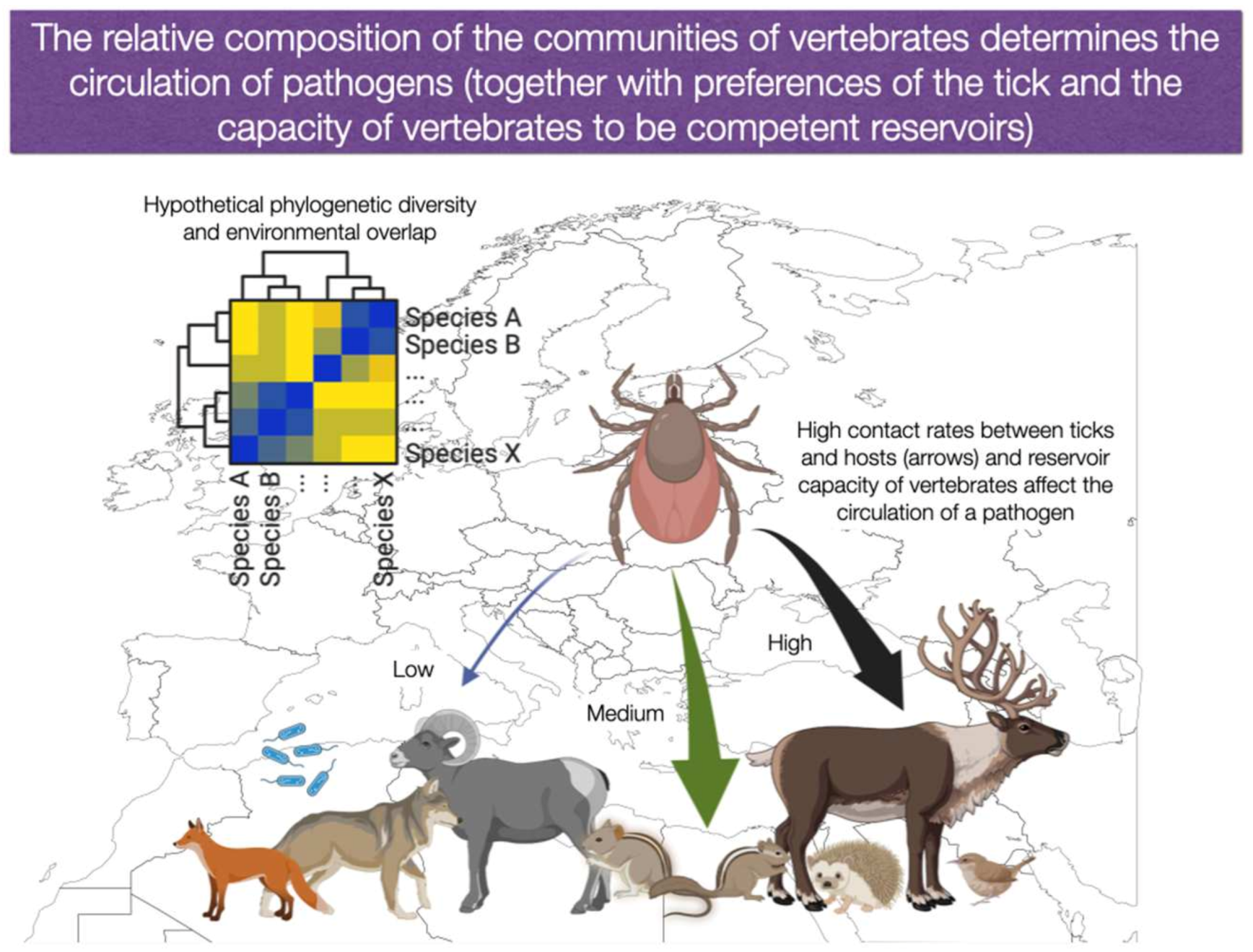
- It is necessary to continue generating knowledge on tick-hosts associations in the context of the community [60]. Other than prevalence values of single pathogens on each vertebrate, the focus should be on how a tick species could be “allocated” among the available vertebrates in the community, along the gradients of climate, landscape, and phylogenetic diversity of communities.
- (ii)
- There is a growing need for sharing of available datasets of tick distribution data, not restricted only to the medically prominent species. Predictive models of possible occurrence need to be trained with accurate and reliable records and coordinates, which are mainly available for a few medically important tick species.
- (iii)
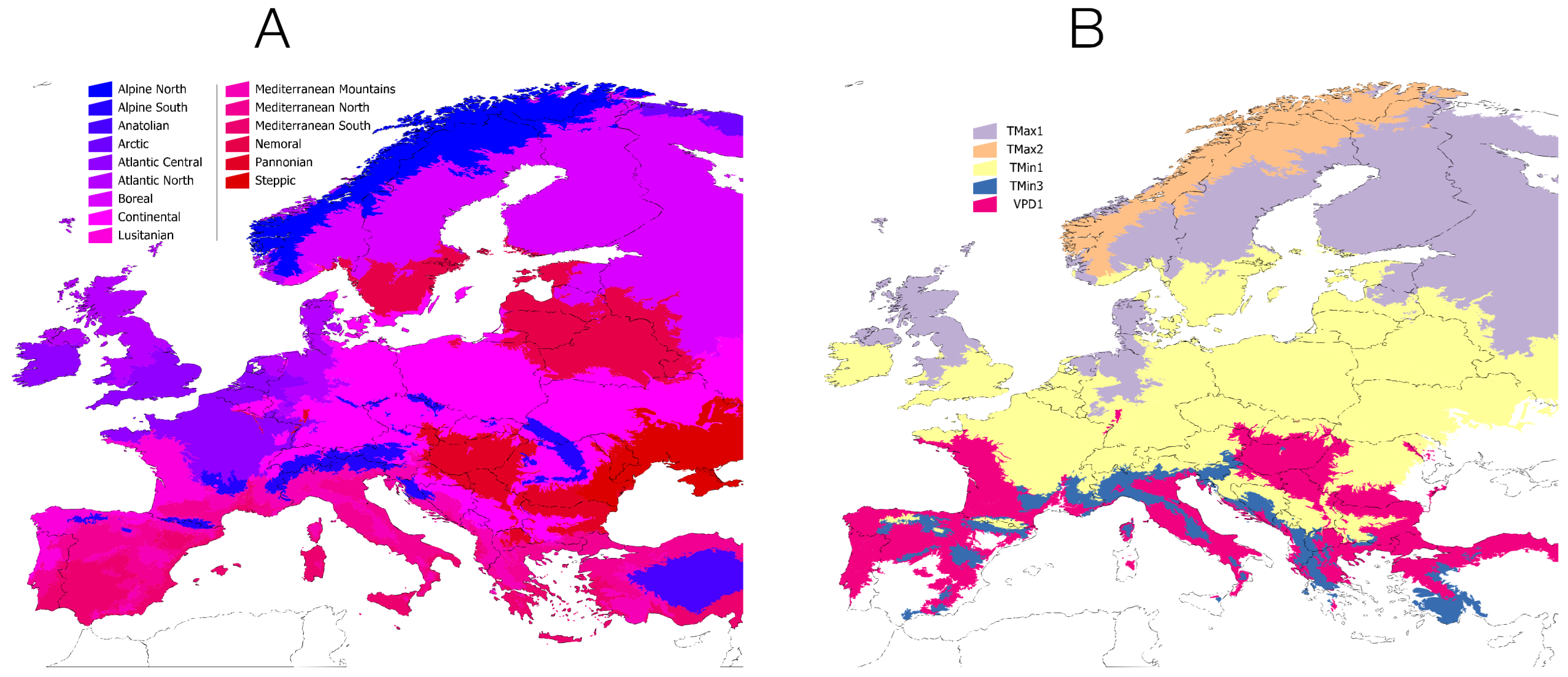
- Researchers on ticks and TBP imperiously need to know the estimated distribution of vertebrates in large areas. Good knowledge exists for countries such as the United States (through the GAP program: https://www.usgs.gov/programs/gap-analysis-project, last accessed on 1 November 2022), but this is lacking for most other countries. We urge to the preparation of digital atlases of distributions (actual and potential) of tick hosts and TBP reservoirs, together with their contributions to the circulation of TBP. The analysis of the resulting communities and the impact of phylogenetic relationships on the TBP circulation is a foreseeable outcome.
- (iv)
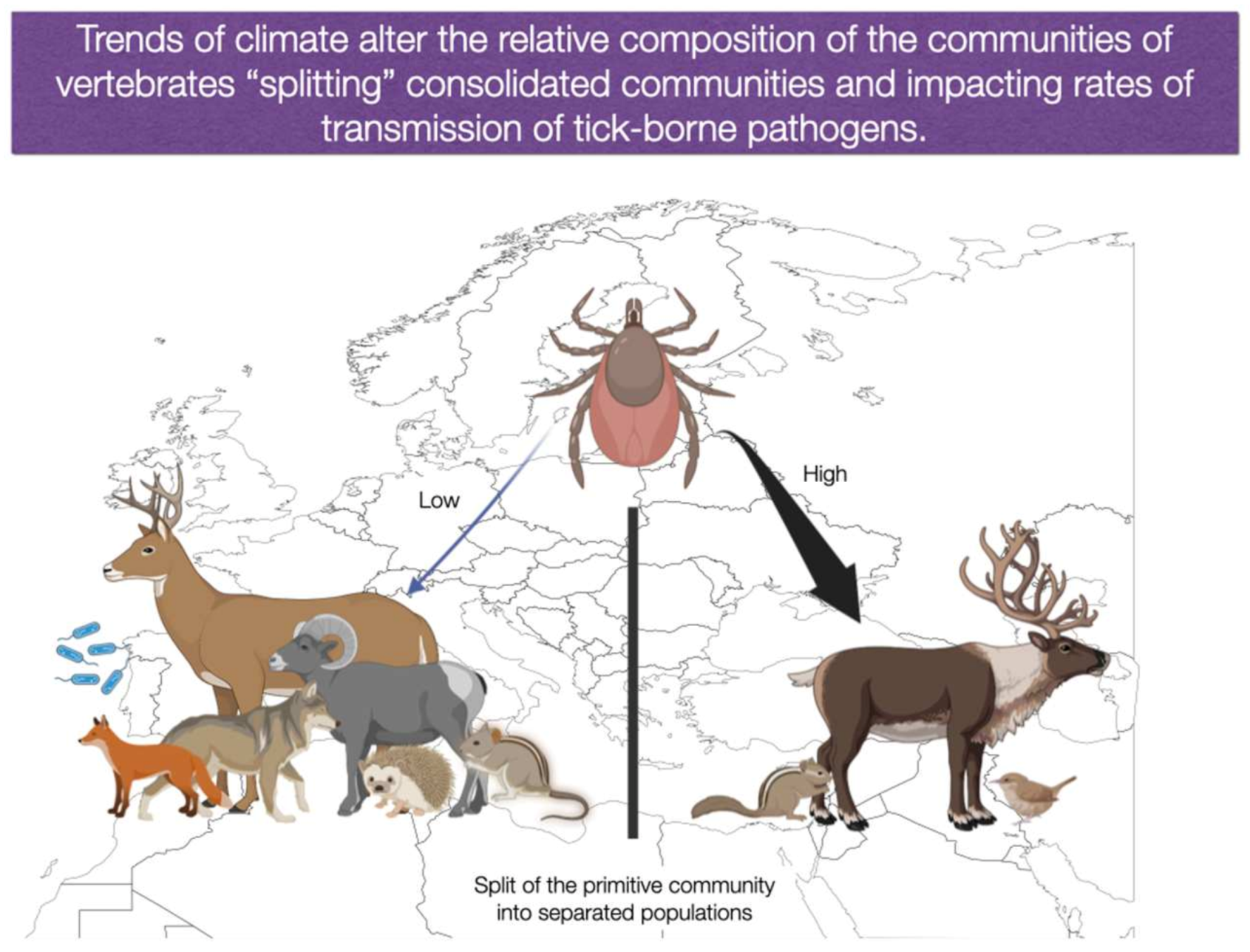
- We consider it of importance to analyse how changes in the circulation of TBP could happen under several restrictive variables, including both the direct effects of the climate, and the indirect effects derived of the lack of co-occurrence among vertebrates. Carefully designed field surveys in near sites exhibiting large changes of habitat (i.e., culture areas versus forest, etc.) or even an exhaustive bibliographic search could provide data, at least for the most studied species of ticks.
- (v)
- Although it may seem far from the focus of this paper, the study of the impact of the abiotic gradients of the landscape (climate, fragmentation, deforestation, competence phenomena) on the composition of the vertebrate community is a fundamental step towards a better understanding of the epidemiology of TBP. These studies are an urgent need for epidemiologists, stakeholders, and decision makers.
4. Analysing the Tick–Host–Pathogen Relationships
- (i)
- We firmly support a modelling approach of the expected environmental niche of vertebrates and tick(s) to fill gaps in not yet surveyed areas and obtain an indirect estimation of the contact rates using networks. Indexes of interactions obtained from these models could be calculated for ecological regions, allowing comparison among results from different regions, and testing its usefulness.
- (ii)
- An ecoregionalization of large areas, such as a continent, seems to be necessary for prospective studies of tick-borne pathogens [65]. Such division of the territory should be based on key habitat features (such as temperature, water deficit, forest density, etc.) known to affect both ticks and vertebrates.
- (iii)
- Ecological studies on vertebrates suggest that while keystone species’ effects on an organism’s persistence are intuitive, their manifestation in complex natural communities is context-dependent and difficult to predict [66]. An empirical evaluation of this concept using a network of tick-vertebrate-pathogens in a target area has never been performed.
- (iv)
- One important challenge in the epidemiology of TBP is to understand whether the network constructs could explain which attributes of the vertebrates regulate their relative competence as reservoirs in situations simulating real communities. This is another promising field of research, bringing together both field and in silico approaches.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Johnson, N.; Phipps, L.P.; Hansford, K.M.; Folly, A.J.; Fooks, A.R.; Medlock, J.M.; Mansfield, K.L. One Health approach to tick and tick-borne disease surveillance in the United Kingdom. Int. J. Environ. Res. Public Health 2022, 19, 5833. [Google Scholar] [CrossRef] [PubMed]
- Dantas-Torres, F.; Chomel, B.B.; Otranto, D. Ticks and tick-borne diseases: A One Health perspective. Trends Parasitol. 2012, 28, 437. [Google Scholar] [CrossRef] [PubMed]
- Tomassone, L.; Berriatua, E.; De Sousa, R.; Duscher, G.G.; Mihalca, A.D.; Silaghi, C.; Zintl, A. Neglected vector-borne zoonoses in Europe: Into the wild. Vet. Parasitol. 2018, 251, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ehrmann, S.; Ruyts, S.C.; Scherer-Lorenzen, M.; Bauhus, J.; Brunet, J.; Cousins, S.A.; Liira, J. Habitat properties are key drivers of Borrelia burgdorferi (sl) prevalence in Ixodes ricinus populations of deciduous forest fragments. Parasites Vectors 2018, 11, 23. [Google Scholar] [CrossRef] [PubMed]
- Jung Kjær, L.; Soleng, A.; Edgar, K.S.; Lindstedt, H.E.H.; Paulsen, K.M.; Andreassen, Å.K.; Bødker, R. Predicting the spatial abundance of Ixodes ricinus ticks in southern Scandinavia using environmental and climatic data. Sci. Rep. 2019, 9, 18144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung Kjær, L.; Soleng, A.; Edgar, K.S.; Lindstedt, H.E.H.; Paulsen, K.M.; Andreassen, Å.K.; Bødker, R. Spatial data of Ixodes ricinus instar abundance and nymph pathogen prevalence, Scandinavia, 2016–2017. Sci. Data 2020, 7, 238. [Google Scholar]
- Estrada-Peña, A.; Fernández-Ruiz, N. Is composition of vertebrates an indicator of the prevalence of tick-borne pathogens? Infect. Ecol. Epidemiol. 2022, 12, 2025647. [Google Scholar] [CrossRef]
- Morávková, B.V. Analysis of Genetic Diversity of Borrelia burgdorferi Sensu Lato and Borrelia miyamotoi across Central and Eastern Europe Using Multilocus Sequence Typing (MLST) in Range of Slovakia and Data from the MLST Database with Focus on Central and Eastern Europe. Ph.D. Thesis, Faculty of Science, University of South Bohemia, Cěské Budějovice, Czech Republic, 2022. [Google Scholar]
- Mysterud, A.; Stigum, V.M.; Linløkken, H.; Herland, A.; Viljugrein, H. How general are generalist parasites? The small mammal part of the Lyme disease transmission cycle in two ecosystems in northern Europe. Oecologia 2019, 190, 115. [Google Scholar] [CrossRef]
- Fabri, N.D.; Sprong, H.; Hofmeester, T.R.; Heesterbeek, H.; Donnars, B.F.; Widemo, F.; Cromsigt, J.P. Wild ungulate species differ in their contribution to the transmission of Ixodes ricinus-borne pathogens. Parasites Vectors 2021, 14, 360. [Google Scholar] [CrossRef]
- Jones, K.E.; Bielby, J.; Cardillo, P.; Fritz, S.A.; O’Dell, J.; Orme, C.D.L.; Safi, K.; Sechrest, W.; Boakes, E.; Carbone, C.; et al. PanTHERIA: A species-level database of life history, ecology, and geography of extant and recently extinct mammals. Ecology 2009, 90, 2648. [Google Scholar] [CrossRef] [Green Version]
- Sullivan, B.L.; Wood, C.L.; Iliff, M.J.; Bonney, R.E.; Fink, D.; Kelling, S. eBird: A citizen-based bird observation network in the biological sciences. Biol. Conserv. 2009, 142, 2282–2292. [Google Scholar] [CrossRef]
- Valavi, R.; Guillera-Arroita, G.; Lahoz-Monfort, J.J.; Elith, J. Predictive performance of presence-only species distribution models: A benchmark study with reproducible code. Ecol. Monogr. 2022, 92, e01486. [Google Scholar] [CrossRef]
- Schmitz, O.J.; Miller, J.R.; Trainor, A.M.; Abrahms, B. Towards a community ecology of landscapes: Predicting multiple predator–prey interactions across geographic space. Ecology 2017, 98, 2281. [Google Scholar] [CrossRef] [PubMed]
- Dobson, A.D.; Finnie, T.J.; Randolph, S.E. A modified matrix model to describe the seasonal population ecology of the European tick Ixodes ricinus. J. Appl. Ecol. 2011, 48, 1017. [Google Scholar] [CrossRef]
- Wu, X.; Duvvuri, V.R.; Lou, Y.; Ogden, N.H.; Pelcat, Y.; Wu, J. Developing a temperature-driven map of the basic reproductive number of the emerging tick vector of Lyme disease Ixodes scapularis in Canada. J. Theor. Biol. 2013, 319, 50. [Google Scholar] [CrossRef] [PubMed]
- Guisan, A.; Thuiller, W. Predicting species distribution: Offering more than simple habitat models. Ecol. Lett. 2005, 8, 993. [Google Scholar] [CrossRef]
- Elith, J.; Graham, C.; Valavi, R.; Abegg, M.; Bruce, C.; Ferrier, S.; Zimmermann, N.E. Presence-only and presence-absence data for comparing species distribution modelling methods. Biodivers. Inform. 2020, 15, 69. [Google Scholar] [CrossRef]
- Saupe, E.E.; Barve, V.; Myers, C.E.; Soberón, J.; Barve, N.; Hensz, C.M.; Lira-Noriega, A. Variation in niche and distribution model performance: The need for a priori assessment of key causal factors. Ecol. Mod. 2012, 237, 11. [Google Scholar] [CrossRef]
- Barve, N.; Barve, V.; Jiménez-Valverde, A.; Lira-Noriega, A.; Maher, S.P.; Peterson, A.T.; Villalobos, F. The crucial role of the accessible area in ecological niche modelling and species distribution modelling. Ecol. Model. 2011, 222, 1810. [Google Scholar] [CrossRef]
- Dumas, A.; Bouchard, C.; Lindsay, L.R.; Ogden, N.H.; Leighton, P.A. Fine-scale determinants of the spatiotemporal distribution of Ixodes scapularis in Quebec (Canada). Ticks Tick Borne Dis. 2022, 13, 101833. [Google Scholar] [CrossRef]
- Dormann, C.F. Promising the future? Global change projections of species distributions. Basic Appl. Ecol. 2007, 8, 387. [Google Scholar] [CrossRef]
- Austin, M.P. Spatial prediction of species distribution: An interface between ecological theory and statistical modelling. Ecol. Mod. 2002, 157, 101. [Google Scholar] [CrossRef] [Green Version]
- Thuiller, W.; Brotons, L.; Araújo, M.B.; Lavorel, S. Effects of restricting environmental range of data to project current and future species distributions. Ecography 2004, 27, 165. [Google Scholar] [CrossRef]
- Ashcroft, M.B.; French, K.O.; Chisholm, L.A. An evaluation of environmental factors affecting species distributions. Ecol. Mod. 2011, 222, 524. [Google Scholar] [CrossRef] [Green Version]
- Bedia, J.; Herrera, S.; Gutiérrez, J.M. Dangers of using global bioclimatic datasets for ecological niche modelling. Limitations for future climate projections. Glob. Planet. Chang. 2013, 107, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Jetz, W.; McGeoch, M.A.; Guralnick, R.; Ferrier, S.; Beck, J.; Costello, M.J.; Turak, E. Essential biodiversity variables for mapping and monitoring species populations. Nat. Ecol. Evol. 2019, 3, 539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nolzen, H.; Brugger, K.; Reichold, A.; Brock, J.; Lange, M.; Thulke, H.H. Model-based extrapolation of ecological systems under future climate scenarios: The example of Ixodes ricinus ticks. PLoS ONE 2022, 17, e0267196. [Google Scholar] [CrossRef] [PubMed]
- Estrada-Peña, A.; Thuiller, W. An assessment of the effect of data partitioning on the performance of modelling algorithms for habitat suitability for ticks. Med. Vet. Entomol. 2008, 22, 248–257. [Google Scholar] [CrossRef]
- Kearney, M.; Porter, W.P.; Williams, C.; Ritchie, S.; Hoffmann, A.A. Integrating biophysical models and evolutionary theory to predict climatic impacts on species’ ranges: The dengue mosquito Aedes aegypti in Australia. Funct. Ecol. 2009, 23, 528–538. [Google Scholar] [CrossRef]
- Porter, W.P.; Gates, D.M. Thermodynamic equilibria of animals with environment. Ecol. Monogr. 1969, 39, 227–244. [Google Scholar] [CrossRef]
- Estrada-Peña, A.; Estrada-Sánchez, A.; de la Fuente, J. A global set of Fourier-transformed remotely sensed covariates for the description of abiotic niche in epidemiological studies of tick vector species. Parasites Vectors 2014, 7, 302. [Google Scholar] [CrossRef]
- Estrada-Peña, A.; de la Fuente, J.D. Data from: Species interactions in occurrence data for a community of tick-transmitted pathogens. Sci. Data 2016, 3, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Estrada-Peña, A.; Alexander, N.; Wint, G.R. Perspectives on modelling the distribution of ticks for large areas: So far so good? Parasites Vectors 2016, 9, 179. [Google Scholar] [CrossRef] [Green Version]
- Bede-Fazekas, Á.; Somodi, I. The way bioclimatic variables are calculated has impact on potential distribution models. Methods Ecol. Evol. 2020, 11, 1559–1570. [Google Scholar] [CrossRef]
- Gilbert, L.; Aungier, J.; Tomkins, J.L. Climate of origin affects tick (Ixodes ricinus) host-seeking behavior in response to temperature: Implications for resilience to climate change? Ecol. Evol. 2014, 4, 1186–1198. [Google Scholar] [CrossRef] [PubMed]
- Jore, S.; Viljugrein, H.; Hofshagen, M.; Brun-Hansen, H.; Kristoffersen, A.B.; Nygård, K.; Ytrehus, B. Multisource analysis reveals latitudinal and altitudinal shifts in range of Ixodes ricinus at its northern distribution limit. Parasites Vectors 2011, 4, 84. [Google Scholar] [CrossRef] [Green Version]
- Jaenson, T.G.; Jaenson, D.G.; Eisen, L.; Petersson, E.; Lindgren, E. Changes in the geographical distribution and abundance of the tick Ixodes ricinus during the past 30 years in Sweden. Parasites Vectors 2012, 5, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosa, R.; Pugliese, A. Effects of tick population dynamics and host densities on the persistence of tick-borne infections. Math. Biosci. 2007, 208, 216–240. [Google Scholar] [CrossRef]
- Vellend, M.; Geber, M.A. Connections between species diversity and genetic diversity. Ecol. Lett. 2005, 8, 767–781. [Google Scholar] [CrossRef]
- Gravel, D.; Canham, C.D.; Beaudet, M.; Messier, C. Reconciling niche and neutrality: The continuum hypothesis. Ecol. Lett. 2006, 9, 399–409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Götzenberger, L.; de Bello, F.; Bråthen, K.A.; Davison, J.; Dubuis, A.; Guisan, A.; Zobel, M. Ecological assembly rules in plant communities—Approaches, patterns and prospects. Biol. Rev. 2012, 87, 111–127. [Google Scholar] [CrossRef] [PubMed]
- Takumi, K.; Sprong, H.; Hofmeester, T.R. Impact of vertebrate communities on Ixodes ricinus-borne disease risk in forest areas. Parasites Vectors 2019, 12, 434. [Google Scholar] [CrossRef] [Green Version]
- Estrada-Peña, A.; Sprong, H.; Cabezas-Cruz, A.; de la Fuente, J.; Ramo, A.; Coipan, E.C. Nested coevolutionary networks shape the ecological relationships of ticks, hosts, and the Lyme disease bacteria of the Borrelia burgdorferi sl complex. Parasites Vectors 2016, 9, 517. [Google Scholar] [CrossRef] [Green Version]
- Jaarsma, R.I.; Sprong, H.; Takumi, K.; Kazimirova, M.; Silaghi, C.; Mysterud, A.; Estrada-Peña, A. Anaplasma phagocytophilum evolves in geographical and biotic niches of vertebrates and ticks. Parasites Vectors 2019, 12, 328. [Google Scholar] [CrossRef] [PubMed]
- Estrada-Peña, A.; Binder, L.C.; Nava, S.; Szabo, M.P.; Labruna, M.B. Exploring the ecological and evolutionary relationships between Rickettsia and hard ticks in the Neotropical region. Ticks Tick Borne Dis. 2021, 12, 101754. [Google Scholar] [CrossRef] [PubMed]
- Ogden, N.H.; Mechai, S.; Margos, G. Changing geographic ranges of ticks and tick-borne pathogens: Drivers, mechanisms and consequences for pathogen diversity. Front. Cell. Infect. Microbiol. 2013, 3, 46–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Millins, C.; Dickinson, E.R.; Isakovic, P.; Gilbert, L.; Wojciechowska, A.; Paterson, V.; Biek, R. Landscape structure affects the prevalence and distribution of a tick-borne zoonotic pathogen. Parasites Vectors 2018, 11, 621. [Google Scholar] [CrossRef]
- Menge, B.A.; Berlow, E.L.; Blanchette, C.A.; Navarrete, S.A.; Yamada, S.B. The keystone species concept: Variation in interaction strength in a rocky intertidal habitat. Ecol. Monogr. 1994, 64, 249. [Google Scholar] [CrossRef]
- Hofmeester, T.R.; Coipan, E.C.; Van Wieren, S.E.; Prins, H.H.T.; Takken, W.; Sprong, H. Few vertebrate species dominate the Borrelia burgdorferi sl life cycle. Environ. Res. Let. 2016, 11, 043001. [Google Scholar]
- Estrada-Peña, A.; Nava, S.; Tarragona, E.; de la Fuente, J.; Guglielmone, A.A. A community approach to the Neotropical ticks-hosts interactions. Sci. Rep. 2020, 10, 9269. [Google Scholar] [CrossRef]
- Estrada-Peña, A.; de La Fuente, J.; Ostfeld, R.S.; Cabezas-Cruz, A. Interactions between tick and transmitted pathogens evolved to minimise competition through nested and coherent networks. Sci. Rep. 2015, 5, 10361. [Google Scholar] [CrossRef] [Green Version]
- Norberg, A.; Abrego, N.; Blanchet, F.G.; Adler, F.R.; Anderson, B.J.; Anttila, J.; Ovaskainen, O. A comprehensive evaluation of predictive performance of 33 species distribution models at species and community levels. Ecol. Monogr. 2019, 89, e01370. [Google Scholar] [CrossRef]
- Clark, J.S.; Gelfand, A.E.; Woodall, C.W.; Zhu, K. More than the sum of the parts: Forest climate response from joint species distribution models. Ecol. Appl. 2013, 24, 990–999. [Google Scholar] [CrossRef] [PubMed]
- Harris, D.J. Generating realistic assemblages with a joint species distribution model. Methods Ecol. Evol. 2015, 6, 465–473. [Google Scholar] [CrossRef]
- Ovaskainen, O.; Roy, D.B.; Fox, R.; Anderson, B.J. Uncovering hidden spatial structure in species communities with spatially explicit joint species distribution models. Methods Ecol. Evol. 2016, 7, 428. [Google Scholar] [CrossRef] [Green Version]
- Kraft, N.J.; Adler, P.B.; Godoy, O.; James, E.C.; Fuller, S.; Levine, J.M. Community assembly, coexistence and the environmental filtering metaphor. Funct. Ecol. 2015, 29, 592. [Google Scholar] [CrossRef]
- Wisz, M.S.; Pottier, J.; Kissling, W.D.; Pellissier, L.; Lenoir, J.; Damgaard, C.F.; Svenning, J.C. The role of biotic interactions in shaping distributions and realised assemblages of species: Implications for species distribution modelling. Biol. Rev. 2013, 88, 15–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tikhonov, G.; Abrego, N.; Dunson, D.; Ovaskainen, O. Using joint species distribution models for evaluating how species-to-species associations depend on the environmental context. Methods Ecol. Evol. 2017, 8, 443–452. [Google Scholar] [CrossRef] [Green Version]
- Nava, S.; Guglielmone, A.A. A meta-analysis of host specificity in Neotropical hard ticks (Acari: Ixodidae). Bull. Entomol. Res. 2013, 103, 216–224. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.H.; Han, B.A. Data-driven predictions and novel hypotheses about zoonotic tick vectors from the genus Ixodes. BMC Ecol. 2018, 18, 7. [Google Scholar] [CrossRef] [Green Version]
- Canard, E.F.; Mouquet, N.; Mouillot, D.; Stanko, M.; Miklisova, D.; Gravel, D. Empirical evaluation of neutral interactions in host-parasite networks. Am. Nat. 2014, 183, 468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delmas, E.; Besson, M.; Brice, M.H.; Burkle, L.A.; Dalla Riva, G.V.; Fortin, M.J.; Poisot, T. Analysing ecological networks of species interactions. Biol. Rev. 2019, 94, 16. [Google Scholar] [CrossRef] [PubMed]
- Metzger, M.J.; Bunce, R.G.H.; Jongman, R.H.; Mücher, C.A.; Watkins, J.W. A climatic stratification of the environment of Europe. Glob. Ecol. Biogeogr. 2005, 14, 549. [Google Scholar] [CrossRef]
- Ippoliti, C.; Candeloro, L.; Gilbert, M.; Goffredo, M.; Mancini, G.; Curci, G.; Conte, A. Defining ecological regions in Italy based on a multivariate clustering approach: A first step towards a targeted vector borne disease surveillance. PLoS ONE 2019, 14, e0219072. [Google Scholar] [CrossRef] [Green Version]
- Wolcott, K.A.; Margos, G.; Fingerle, V.; Becker, N.S. Host association of Borrelia burgdorferi sensu lato: A review. Ticks Tick Borne Dis. 2021, 12, 101766. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Estrada-Peña, A.; Fernández-Ruiz, N. An Agenda for Research of Uncovered Epidemiological Patterns of Tick-Borne Pathogens Affecting Human Health. Int. J. Environ. Res. Public Health 2023, 20, 2206. https://doi.org/10.3390/ijerph20032206
Estrada-Peña A, Fernández-Ruiz N. An Agenda for Research of Uncovered Epidemiological Patterns of Tick-Borne Pathogens Affecting Human Health. International Journal of Environmental Research and Public Health. 2023; 20(3):2206. https://doi.org/10.3390/ijerph20032206
Chicago/Turabian StyleEstrada-Peña, Agustín, and Natalia Fernández-Ruiz. 2023. "An Agenda for Research of Uncovered Epidemiological Patterns of Tick-Borne Pathogens Affecting Human Health" International Journal of Environmental Research and Public Health 20, no. 3: 2206. https://doi.org/10.3390/ijerph20032206




