Health Risk Assessment in Children Occupationally and Para-Occupationally Exposed to Benzene Using a Reverse-Translation PBPK Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Study Population
2.3. Exposure Assessment
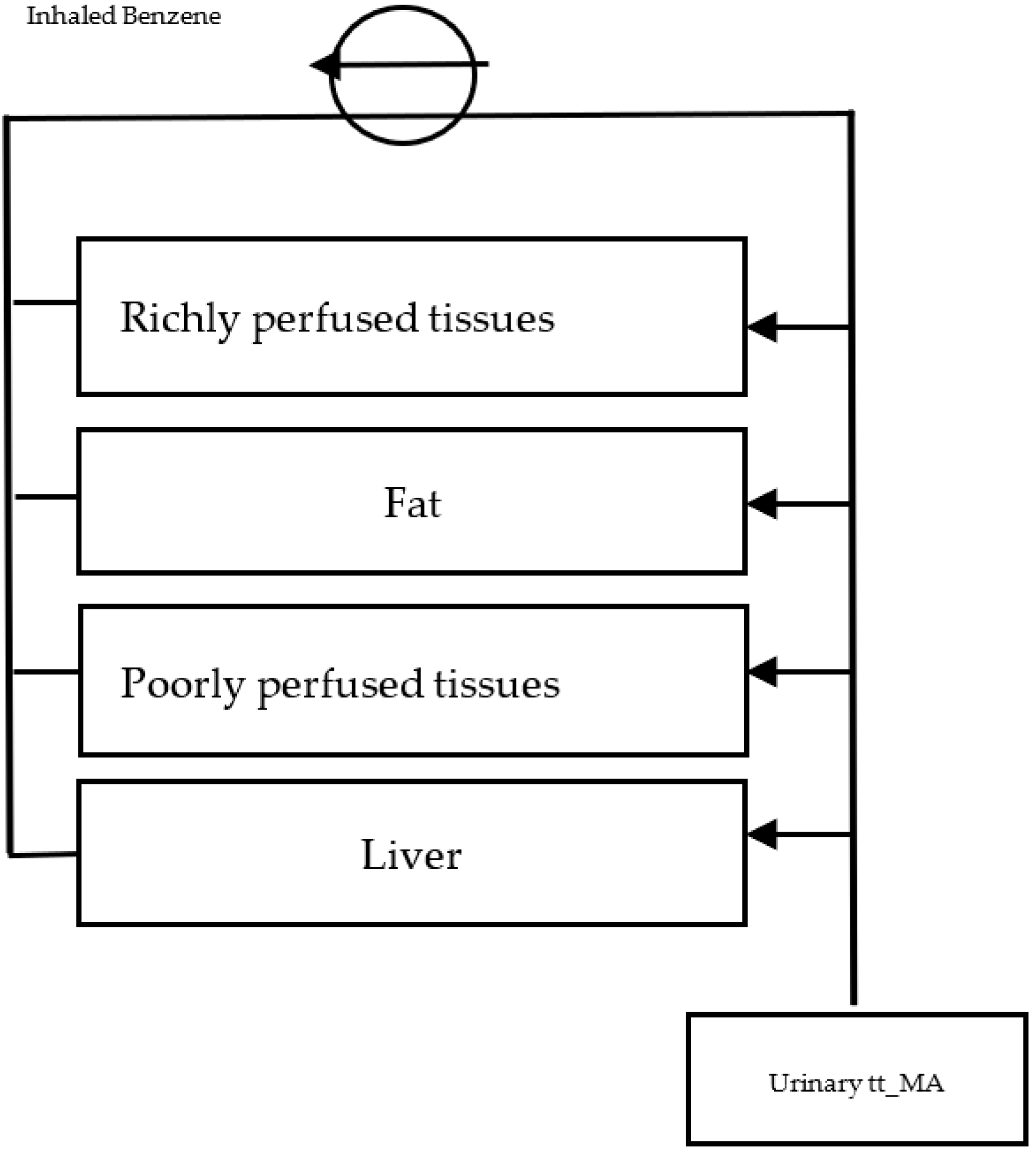
2.3.1. Reverse-Translation Toxicokinetic Model
2.3.2. Exposure Scenarios Evaluation
2.4. Risk Assessment
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- International Agency for Research on Cancer. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans; Benzene; International Agency for Research on Cancer: Lyon, France, 2019; Volume 120.
- D’Andrea, M.A.; Reddy, G.K. Health Risks Associated with Benzene Exposure in Children: A Systematic Review. Glob. Pediatr. Health 2018, 5, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Filippini, T.; Hatch, E.E.; Rothman, K.J.; Heck, J.E.; Park, A.S.; Crippa, A.; Orsini, N.; Vinceti, M. Association between Outdoor Air Pollution and Childhood Leukemia: A Systematic Review and Dose-Response Meta-Analysis. Environ. Health Perspect. 2019, 127, 46002. [Google Scholar] [CrossRef] [Green Version]
- Landrigan, P.J.; Goldman, L.R. Children’s Vulnerability To Toxic Chemicals: A Challenge And Opportunity To Strengthen Health And Environmental Policy. Health Aff. 2011, 30, 842–850. [Google Scholar] [CrossRef] [Green Version]
- Gale, R.P.; Opelz, G. Commentary: Does Immune Suppression Increase Risk of Developing Acute Myeloid Leukemia? Leukemia 2012, 26, 422–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janitz, A.E.; Campbell, J.E.; Magzamen, S.; Pate, A.; Stoner, J.A.; Peck, J.D. Benzene and Childhood Acute Leukemia in Oklahoma. Environ. Res. 2017, 158, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Pyatt, D.; Hays, S. A Review of the Potential Association between Childhood Leukemia and Benzene. Chem.-Biol. Interact. 2010, 184, 151–164. [Google Scholar] [CrossRef]
- Carlos-Wallace, F.M.; Zhang, L.; Smith, M.T.; Rader, G.; Steinmaus, C. Parental, In Utero, and Early-Life Exposure to Benzene and the Risk of Childhood Leukemia: A Meta-Analysis. Am. J. Epidemiol. 2016, 183, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Badham, H.J.; Renaud, S.J.; Wan, J.; Winn, L.M. Benzene-Initiated Oxidative Stress: Effects on Embryonic Signaling Pathways. Chem.-Biol. Interact. 2010, 184, 218–221. [Google Scholar] [CrossRef]
- McHale, C.M.; Zhang, L.; Smith, M.T. Current Understanding of the Mechanism of Benzene-Induced Leukemia in Humans: Implications for Risk Assessment. Carcinogenesis 2012, 33, 240–252. [Google Scholar] [CrossRef] [Green Version]
- North, C.M.; Rooseboom, M.; Kocabas, N.A.; Schnatter, A.R.; Faulhammer, F.; Williams, S.D. Modes of Action Considerations in Threshold Expectations for Health Effects of Benzene. Toxicol. Lett. 2020, 334, 78–86. [Google Scholar] [CrossRef]
- La Cruz-Góngora, V.D.; Martínez-Tapia, B.; Shamah-Levy, T.; Villalpando, S. Nutritional status of iron, vitamin B12, vitamin A and anemia in Mexican children: Results from the Ensanut 2018–19. Salud Pública Méx. 2021, 63, 359–370. [Google Scholar] [CrossRef] [PubMed]
- Cancer Today. Available online: http://gco.iarc.fr/today/home (accessed on 15 August 2022).
- GBD Compare|IHME Viz Hub. Available online: http://vizhub.healthdata.org/gbd-compare (accessed on 2 July 2021).
- Leal, Y.A.; Torres, J.; Gamboa, R.; Mantilla-Morales, A.; Piña-Sanchez, P.; Arrieta, O.; Bonifaz, L.; Meneses, A.; Duque, C.; Piñeros, M. Cancer Incidence in Merida, Mexico 2015–2018: First Report from the Population-Based Cancer Registry. Arch. Med. Res. 2022, 53, 859–866. [Google Scholar] [CrossRef] [PubMed]
- Metayer, C.; Dahl, G.; Wiemels, J.; Miller, M. Childhood Leukemia: A Preventable Disease. Pediatrics 2016, 138, S45–S55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérez-Herrera, N.; Díaz de León-Martínez, L.; Flores-Ramírez, R.; Barbier, O.; Ortega-Romero, M.; May-Euán, F.; Saldaña-Villanueva, K.; Perera-Rios, J.; Pérez-Vázquez, F.J. Evaluation of Benzene Exposure and Early Biomarkers of Kidney Damage in Children Exposed to Solvents Due to Precarious Work in Ticul, Yucatán, México. Ann. Glob. Health 2019, 85, 94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arcega-Cabrera, F.; Fargher, L.F.; Oceguera-Vargas, I.; Noreña-Barroso, E.; Yánez-Estrada, L.; Alvarado, J.; González, L.; Moo-Puc, R.; Pérez-Herrera, N.; Quesadas-Rojas, M.; et al. Water Consumption as Source of Arsenic, Chromium, and Mercury in Children Living in Rural Yucatan, Mexico: Blood and Urine Levels. Bull. Environ. Contam. Toxicol. 2017, 99, 452–459. [Google Scholar] [CrossRef]
- Azari, M.R.; Hosseini, V.; Jafari, M.J.; Asadi, P.; Mousavion, M.A. Evaluation of Occupational Exposure of Shoe Makers to Benzene and Toluene Compounds in Shoe Manufacturing Workshops in East Tehran. Tanaffos 2012, 11, 43–49. [Google Scholar]
- Dowty, B.J.; Laseter, J.L.; Storer, J. The Transplacental Migration and Accumulation in Blood of Volatile Organic Constituents. Pediatr. Res. 1976, 10, 696–701. [Google Scholar] [CrossRef]
- INEGI México en Cifras. Available online: https://www.inegi.org.mx/app/areasgeograficas/ (accessed on 2 August 2022).
- Geografía (INEGI), I.N. de E. y Directorio Nacional de Unidades Económicas. DENUE. Available online: https://www.inegi.org.mx/app/mapa/denue/default.aspx (accessed on 6 September 2021).
- Ducos, P.; Gaudin, R.; Bel, J.; Maire, C.; Francin, J.M.; Robert, A.; Wild, P. trans,trans-Muconic Acid, a Reliable Biological Indicator for the Detection of Individual Benzene Exposure down to the ppm Level. Int. Arch. Occup. Environ. Heath 1992, 64, 309–313. [Google Scholar] [CrossRef]
- Majumdar, D.; Dutta, C.; Sen, S. Inhalation Exposure or Body Burden? Better Way of Estimating Risk–An Application of PBPK Model. Environ. Toxicol. Pharmacol. 2016, 41, 54–61. [Google Scholar] [CrossRef]
- Valcke, M.; Krishnan, K. Assessing the Impact of the Duration and Intensity of Inhalation Exposure on the Magnitude of the Variability of Internal Dose Metrics in Children and Adults. Inhal. Toxicol. 2011, 23, 863–877. [Google Scholar] [CrossRef]
- Valcke, M.; Krishnan, K. Characterization of the Human Kinetic Adjustment Factor for the Health Risk Assessment of Environmental Contaminants: Human Kinetic Adjustment Factor (HKAF) in Health Risk Assessment. J. Appl. Toxicol. 2014, 34, 227–240. [Google Scholar] [CrossRef] [PubMed]
- Perelló, C.M.; Barroso, C.S.-T.; Jiménez, D.R.; Rodríguez, R.; Fernández, J.L.; Juan, G.F.; Figuerola, J.; Freixedas, F.G. Relación entre la percepción del consumo oral de líquidos y el volumen urinario en población sana. Pediatr. Aten. Primaria 2017, 19, 223–229. [Google Scholar]
- United States Environmental Protection Agency. Exposure Factors Interactive Resource for Scenarios Tool (ExpoFIRST), Version 2.1. Available online: https://cfpub.epa.gov/ncea/efp/recordisplay.cfm?deid=344928 (accessed on 2 August 2022).
- US EPA. Benzene CASRN 71-43-2|DTXSID3039242|IRIS|US EPA, ORD. Available online: https://cfpub.epa.gov/ncea/iris2/chemicallanding.cfm?substance_nmbr=276 (accessed on 9 March 2022).
- D’Andrea, M.A.; Reddy, G.K. Health Effects of Benzene Exposure among Children Following a Flaring Incident at the British Petroleum Refinery in Texas City. Pediatr. Hematol. Oncol. 2014, 31, 1–10. [Google Scholar] [CrossRef] [PubMed]
- D’Andrea, M.A.; Reddy, G.K. Adverse Health Effects of Benzene Exposure Among Children Following a Flaring Incident at the British Petroleum Refinery in Texas City. Clin. Pediatr. 2016, 55, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.R.; Yoo, C.I.; Lee, J.H.; Kim, S.-R.; Kim, Y. Hematological Changes of Children Exposed to Volatile Organic Compounds Containing Low Levels of Benzene. Sci. Total Environ. 2002, 299, 237–245. [Google Scholar] [CrossRef]
- Guo, H.; Ahn, S.; Zhang, L. Benzene-Associated Immunosuppression and Chronic Inflammation in Humans: A Systematic Review. Occup. Environ. Med. 2020, 78, 377–384. [Google Scholar] [CrossRef]
- Azkur, A.K.; Akdis, M.; Azkur, D.; Sokolowska, M.; van de Veen, W.; Brüggen, M.; O’Mahony, L.; Gao, Y.; Nadeau, K.; Akdis, C.A. Immune Response to SARS-CoV-2 and Mechanisms of Immunopathological Changes in COVID-19. Allergy 2020, 75, 1564–1581. [Google Scholar] [CrossRef]
- Cui, X.; Zhao, Z.; Zhang, T.; Guo, W.; Guo, W.; Zheng, J.; Zhang, J.; Dong, C.; Na, R.; Zheng, L.; et al. A Systematic Review and Meta-analysis of Children with Coronavirus Disease 2019 (COVID-19). J. Med. Virol. 2021, 93, 1057–1069. [Google Scholar] [CrossRef]
- Encuesta Nacional de Salud y Nutrición. Available online: https://ensanut.insp.mx/index.php (accessed on 23 November 2022).
- Allali, S.; Brousse, V.; Sacri, A.-S.; Chalumeau, M.; de Montalembert, M. Anemia in Children: Prevalence, Causes, Diagnostic Work-up, and Long-Term Consequences. Expert Rev. Hematol. 2017, 10, 1023–1028. [Google Scholar] [CrossRef]
- Rothman, N.; Li, G.-L.; Dosemeci, M.; Bechtold, W.E.; Marti, G.E.; Wang, Y.-Z.; Linet, M.; Xi, L.; Lu, W.; Smith, M.T.; et al. Hematotoxocity among Chinese Workers Heavily Exposed to Benzene. Am. J. Ind. Med. 1996, 29, 236–246. [Google Scholar] [CrossRef]
- Muñoz-Aguirre, P.; Huerta-Gutierrez, R.; Zamora, S.; Mohar, A.; Vega-Vega, L.; Hernández-Ávila, J.E.; Morales-Carmona, E.; Zapata-Tarres, M.; Bautista-Arredondo, S.; Perez-Cuevas, R.; et al. Acute Lymphoblastic Leukaemia Survival in Children Covered by Seguro Popular in Mexico: A National Comprehensive Analysis 2005–2017. Health Syst. Reform 2021, 7, e1914897. [Google Scholar] [CrossRef]
- Mumtaz, M.; Fisher, J.; Blount, B.; Ruiz, P. Application of Physiologically Based Pharmacokinetic Models in Chemical Risk Assessment. J. Toxicol. 2012, 2012, 904603. [Google Scholar] [CrossRef] [PubMed]
- Flores-Hernández, C.; Castillo, D.P.; Huerta-Franco, M.-R.; Hernández, J.; Cappacione, K.; Vargas-Luna, M. Psycho-Toxicologic Assessment of Workers with Chronic Exposure to Organic Solvents in the Leather and Shoe Industries. Cienc. Trab. 2012, 43, 129–134. [Google Scholar]
- Vermeulen, R.; Lan, Q.; Li, G.; Rappaport, S.M.; Kim, S.; van Wendel de Joode, B.; Shen, M.; Bohong, X.; Smith, M.T.; Zhang, L.; et al. Assessment of Dermal Exposure to Benzene and Toluene in Shoe Manufacturing by Activated Carbon Cloth Patches. J. Environ. Monit. 2006, 8, 1143. [Google Scholar] [CrossRef]
- OECD. Guidance Document on the Characterisation, Validation and Reporting of Physiologically Based Kinetic (PBK) Models for Regulatory Purposes, OECD Series on Testing and Assessment, No. 331; Environment, Health and Safety, Environment Directorate, OECD: Paris, France, 2021.
- DOF-Diario Oficial de La Federación. Available online: https://www.dof.gob.mx/nota_detalle.php?codigo=5314833&fecha=20/09/2013#gsc.tab=0 (accessed on 11 December 2022).
- Edokpolo, B.; Yu, Q.; Connell, D. Health Risk Assessment of Ambient Air Concentrations of Benzene, Toluene and Xylene (BTX) in Service Station Environments. IJERPH 2014, 11, 6354–6374. [Google Scholar] [CrossRef] [Green Version]
- Chaiklieng, S.; Suggaravetsiri, P.; Autrup, H. Risk Assessment on Benzene Exposure among Gasoline Station Workers. IJERPH 2019, 16, 2545. [Google Scholar] [CrossRef] [Green Version]
- Demirel, G.; Özden, Ö.; Döğeroğlu, T.; Gaga, E.O. Personal Exposure of Primary School Children to BTEX, NO2 and Ozone in Eskişehir, Turkey: Relationship with Indoor/Outdoor Concentrations and Risk Assessment. Sci. Total Environ. 2014, 473, 537–548. [Google Scholar] [CrossRef] [PubMed]
- Spycher, B.D.; Lupatsch, J.E.; Huss, A.; Rischewski, J.; Schindera, C.; Spoerri, A.; Vermeulen, R.; Kuehni, C.E. Parental Occupational Exposure to Benzene and the Risk of Childhood Cancer: A Census-Based Cohort Study. Environ. Int. 2017, 108, 84–91. [Google Scholar] [CrossRef]
- Heck, J.E.; He, D.; Contreras, Z.A.; Ritz, B.; Olsen, J.; Hansen, J. Parental Occupational Exposure to Benzene and the Risk of Childhood and Adolescent Acute Lymphoblastic Leukaemia: A Population-Based Study. Occup. Environ. Med. 2019, 76, 527–529. [Google Scholar] [CrossRef]

| Exposure Scenario | Chronic Exposure (CE) (mg/m3) | Hazard Quotient (EC/RfC a) | Carcinogenic Risk (CE.IUR b) |
|---|---|---|---|
| (a) Children: 6 to 12 years old exposed 4 h/day | 0.80 | 26.7 | 2–6 × 10−3 |
| (b) Children: 6 to 12 years old exposed 8 h/day | 1.60 | 53.3 | 4–10 × 10−3 |
| (c) Adults: projection for 8 h/day exposure during 70 years of life | 1.11 | 37 | 2–9 × 10−3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pech, K.; Pérez-Herrera, N.; Vértiz-Hernández, Á.A.; Lajous, M.; Farías, P. Health Risk Assessment in Children Occupationally and Para-Occupationally Exposed to Benzene Using a Reverse-Translation PBPK Model. Int. J. Environ. Res. Public Health 2023, 20, 2275. https://doi.org/10.3390/ijerph20032275
Pech K, Pérez-Herrera N, Vértiz-Hernández ÁA, Lajous M, Farías P. Health Risk Assessment in Children Occupationally and Para-Occupationally Exposed to Benzene Using a Reverse-Translation PBPK Model. International Journal of Environmental Research and Public Health. 2023; 20(3):2275. https://doi.org/10.3390/ijerph20032275
Chicago/Turabian StylePech, Kristal, Norma Pérez-Herrera, Ángel Antonio Vértiz-Hernández, Martín Lajous, and Paulina Farías. 2023. "Health Risk Assessment in Children Occupationally and Para-Occupationally Exposed to Benzene Using a Reverse-Translation PBPK Model" International Journal of Environmental Research and Public Health 20, no. 3: 2275. https://doi.org/10.3390/ijerph20032275
APA StylePech, K., Pérez-Herrera, N., Vértiz-Hernández, Á. A., Lajous, M., & Farías, P. (2023). Health Risk Assessment in Children Occupationally and Para-Occupationally Exposed to Benzene Using a Reverse-Translation PBPK Model. International Journal of Environmental Research and Public Health, 20(3), 2275. https://doi.org/10.3390/ijerph20032275








