Body Composition Parameters Correlate to Depression Symptom Levels in Patients Treated with Hemodialysis and Peritoneal Dialysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Body Composition and Anthropometric Measurement
2.3. Clinical and Laboratory Data
2.4. Depression Assessment
2.5. Malnutrition Assessment
2.6. Charlson Comorbidity Index (CCI)
2.7. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jankowski, J.; Floege, J.; Fliser, D.; Böhm, M.; Marx, N. Cardiovascular Disease in Chronic Kidney Disease. Circulation 2021, 143, 1157–1172. [Google Scholar] [CrossRef] [PubMed]
- Kalantar-Zadeh, K.; Tortorici, A.R.; Chen, J.L.T.; Kamgar, M.; Lau, W.-L.; Moradi, H.; Rhee, C.M.; Streja, E.; Kovesdy, C.P. Dietary Restrictions in Dialysis Patients: Is There Anything Left to Eat? Semin. Dial. 2015, 28, 159–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naini, A.E.; Karbalaie, A.; Abedini, M.; Askari, G.; Moeinzadeh, F. Comparison of Malnutrition in Hemodialysis and Peritoneal Dialysis Patients and Its Relationship with Echocardiographic Findings. J. Res. Med. Sci. 2016, 21, 78. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-K.; Kim, S.G.; Oh, J.-E.; Lee, Y.-K.; Noh, J.-W.; Kim, H.J.; Song, Y.R. Impact of Sarcopenia on Long-Term Mortality and Cardiovascular Events in Patients Undergoing Hemodialysis. Korean J. Intern. Med. 2019, 34, 599–607. [Google Scholar] [CrossRef] [Green Version]
- Liabeuf, S.; Pepin, M.; Franssen, C.F.M.; Viggiano, D.; Carriazo, S.; Gansevoort, R.T.; Gesualdo, L.; Hafez, G.; Malyszko, J.; Mayer, C.; et al. Chronic Kidney Disease and Neurological Disorders: Are Uraemic Toxins the Missing Piece of the Puzzle? Nephrol. Dial. Transplant. 2021, 37, ii33–ii44. [Google Scholar] [CrossRef]
- Masson, P.; Webster, A.C.; Hong, M.; Turner, R.; Lindley, R.I.; Craig, J.C. Chronic Kidney Disease and the Risk of Stroke: A Systematic Review and Meta-Analysis. Nephrol. Dial. Transplant. 2015, 30, 1162–1169. [Google Scholar] [CrossRef] [Green Version]
- Palmer, S.; Vecchio, M.; Craig, J.C.; Tonelli, M.; Johnson, D.W.; Nicolucci, A.; Pellegrini, F.; Saglimbene, V.; Logroscino, G.; Fishbane, S.; et al. Prevalence of Depression in Chronic Kidney Disease: Systematic Review and Meta-Analysis of Observational Studies. Kidney Int. 2013, 84, 179–191. [Google Scholar] [CrossRef] [Green Version]
- Markaki, A.G.; Charonitaki, A.; Psylinakis, E.; Dimitropoulakis, P.; Spyridaki, A. Nutritional Status in Hemodialysis Patients Is Inversely Related to Depression and Introversion. Psychol. Health Med. 2019, 24, 1213–1219. [Google Scholar] [CrossRef]
- Finnegan-John, J.; Thomas, V.J. The Psychosocial Experience of Patients with End-Stage Renal Disease and Its Impact on Quality of Life: Findings from a Needs Assessment to Shape a Service. ISRN Nephrol. 2013, 2013, 308986. [Google Scholar] [CrossRef] [Green Version]
- Gebrie, M.H.; Ford, J. Depressive Symptoms and Dietary Non-Adherence among End Stage Renal Disease Patients Undergoing Hemodialysis Therapy: Systematic Review. BMC Nephrol. 2019, 20, 429. [Google Scholar] [CrossRef] [Green Version]
- Cukor, D.; Friedman, S. Number 2 Towards the Psychosocial Treatment of Depressed Patients on Dialysis. Internet J. Nephrol. 2004, 2. [Google Scholar]
- Lee, E. The Importance of Psychiatric Disorders in End-Stage Kidney Disease Patients. Kidney Res. Clin. Pract. 2022, 41, 133–135. [Google Scholar] [CrossRef] [PubMed]
- Suseł, J.; Batycka-Baran, A.; Reich, A.; Szepietowski, J. Uraemic Pruritus Markedly Affects the Quality of Life and Depressive Symptoms in Haemodialysis Patients with End-Stage Renal Disease. Acta Derm. Venereol. 2014, 94, 276–281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolak, E.; Radić, J.; Vučković, M.; Nenadić, D.B.; Begović, M.; Radić, M. Nutritional and Hydration Status and Adherence to Dietary Recommendations in Dalmatian Dialysis Patients. Nutrients 2022, 14, 3553. [Google Scholar] [CrossRef] [PubMed]
- Oki Data Corporation. Mc780—User Manual; Oki Data Corporation: Irving, TX, USA, 2015. [Google Scholar]
- Kohn, M.A.; Senyak, J. Sample Size Calculators. UCSF CTSI. 2021. Available online: https://sample-size.net/ (accessed on 20 December 2022).
- Hsu, H.-J.; Yen, C.-H.; Chen, C.-K.; Wu, I.-W.; Lee, C.-C.; Sun, C.-Y.; Chang, S.-J.; Chou, C.-C.; Hsieh, M.-F.; Chen, C.-Y.; et al. Association between Uremic Toxins and Depression in Patients with Chronic Kidney Disease Undergoing Maintenance Hemodialysis. Gen. Hosp. Psychiatry 2013, 35, 23–27. [Google Scholar] [CrossRef]
- Ribeiro, H.; Ferreira, T.; Duarte, M.; Baião, V.; Inda-Filho, A.; Ferreira, A. Associação Entre Os Sintomas Depressivos e a Gordura Corporal Em Pacientes Com Doença Renal Crônica Em Hemodiálise. Rev. Psicol. Divers. Saúde 2021, 10, 407–414. [Google Scholar] [CrossRef]
- Barros, A.; da Costa, B.E.; Mottin, C.C.; d’Avila, D.O. Depression, Quality of Life, and Body Composition in Patients with End-Stage Renal Disease: A Cohort Study. Rev. Bras. Psiquiatr. 2016, 38, 301–306. [Google Scholar] [CrossRef] [Green Version]
- Gencer, F.; Yıldıran, H.; Erten, Y. Association of Malnutrition Inflammation Score With Anthropometric Parameters, Depression, and Quality of Life in Hemodialysis Patients. J. Am. Coll. Nutr. 2019, 38, 457–462. [Google Scholar] [CrossRef]
- Cindoğlu, Ç.; Beyazgül, B. Nutritional Status and Anxiety-Depression Relationship in Hemodialysis Patients. J. Surg. Med. 2021, 5, 429–432. [Google Scholar] [CrossRef]
- Budhram, B.; Sinclair, A.; Komenda, P.; Severn, M.; Sood, M.M. A Comparison of Patient-Reported Outcome Measures of Quality of Life By Dialysis Modality in the Treatment of Kidney Failure: A Systematic Review. Can. J. Kidney Health Dis. 2020, 7, 2054358120957431. [Google Scholar] [CrossRef]
- Tannor, E.K.; Archer, E.; Kapembwa, K.; van Schalkwyk, S.C.; Davids, M.R. Quality of Life in Patients on Chronic Dialysis in South Africa: A Comparative Mixed Methods Study. BMC Nephrol. 2017, 18, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| HD (n = 53) | PD (n = 20) | p * | |
|---|---|---|---|
| Basic characteristics | |||
| Sex (female), N (%) | 36 (68) | 11 (55) | 0.30 |
| Age (years), median (IQR) | 69 (56–76) | 54 (36–66) | 0.002 |
| Dialysis duration (months), median (IQR) | 46 (21–78) | 24 (8.5–36) | 0.001 |
| Presence of AH, N (%) | 34 (77) | 17 (85) | 0.74 |
| Presence of DM, N (%) | 14 (30) | 1 (5) | 0.03 |
| Presence of MD, N (%) | 14 (32) | 0 | 0.003 |
| Charlson Comorbidity Index, median (IQR) | 6 (4–8) | 4 (2–5.5) | <0.001 |
| Estimated 10-year survival (%), median (IQR) | 2 (0–53) | 53 (15–87) | 0.001 |
| Anthropometric parameters | |||
| Weight (kg), median (IQR) | 73.5 (66.3–84) | 80.75 (70.53–101.7) | 0.06 |
| Height (cm), median (IQR) | 175 (164–183) | 173 (171–178.75) | 0.89 |
| BMI (kg/m2), median (IQR) | 24.6 (22.4–27.45) | 25.65 (23.53–31.38) | 0.11 |
| Middle upper arm circumference (cm), median (IQR) | 28 (26.5–31.5) | 30.3 (26.63–33.75) | 0.26 |
| Waist circumference (cm), median (IQR) | 94 (88.5–101.5) | 98.5 (90.25–103.75) | 0.22 |
| Hip circumference (cm), median (IQR) | 102 (98.5–108.5) | 108.5 (97.5–114.5) | 0.06 |
| WHR, median (IQR) | 0.92 (0.86–0.97) | 0.9 (0.85–0.93) | 0.36 |
| WHtR, median (IQR) | 0.54 (0.5–0.59) | 0.56 (0.52–0.61) | 0.38 |
| Handgrip strength (kg), median (IQR) | 25.7 (17.5–32.3) | 31.3 (25.4–41.58) | 0.01 |
| Body composition | |||
| Total body water (kg), median (IQR) | 43.2 (36.55–50.75) | 42.2 (38.13–48.13) | 0.98 |
| Extracellular water (kg), median (IQR) | 18.2 (16.4–20.75) | 19.6 (17.28–20.88) | 0.36 |
| Intracellular water (kg), median (IQR) | 25 (20.4–29.75) | 23.7 (20.25–28.1) | 0.62 |
| Fat mass (kg), median (IQR) | 14.4 (7.25–21.05) | 19.3 (12.1–29.98) | 0.01 |
| Fat mass (%), median (IQR) | 18.5 (11.6–25.65) | 24.5 (16.68–33.2) | 0.03 |
| Fat-free mass (kg), median (IQR) | 61.9 (52.6–70.7) | 62.2 (55.3–69.38) | 0.65 |
| Visceral fat level, median (IQR) | 9 (8–11) | 8 (4.5–13.5) | 0.35 |
| Muscle mass (%), median (IQR) | 58.8 (49.95–67.2) | 59.1 (52.48–65.98) | 0.65 |
| Phase angle (°), median (IQR) | 4.8 (4.3–5.75) | 5.2 (4.73–5.88) | 0.04 |
| Trunk fat mass (kg), median (IQR) | 7.1 (3.4–11.3) | 9.6 (6.65–17.75) | 0.02 |
| Trunk fat mass (%), median (IQR) | 16.9 (9.5–23.9) | 22.3 (16.15–31.35) | 0.01 |
| SMI, median (IQR) | 7.93 (7.11–9.1) | 8.6 (7.6–9.37) | 0.25 |
| HD (n = 55) | PD (n = 20) | TOTAL (n= 75) | p * | |
|---|---|---|---|---|
| BDI-II score, median (IQR) | 7 (5–12) | 6 (4–9) | 7 (5–11) | 0.32 |
| Sociodemographic data, number (%) | ||||
| Education level | ||||
| Elementary school | 11 (20) | 4 (20) | 15 (20) | 0.19 |
| Middle school | 34 (62) | 10 (50) | 44 (59) | |
| Intermediate education | 4 (7) | 5 (25) | 9 (12) | |
| Faculty education or higher | 6 (11) | 1 (5) | 7 (9) | |
| Working status | ||||
| Employed full-time | 2 (4) | 8 (40) | 53 (71) | <0.001 |
| Unemployed | 4 (7) | 2 (10) | 19 (25) | |
| Student | 0 | 1 (5) | 3 (4) | |
| Retired | 45 (82) | 9 (45) | 54 (72) | |
| Housewife | 1 (1.8) | 0 | 37 (49) | |
| Sick leave | 3 (5.5) | 0 | 37 (49) | |
| Inhabitant of | ||||
| Village | 11 (20) | 7 (35) | 18 (24) | 0.18 |
| Town/city | 44 (80) | 13 (65) | 57 (76) | |
| Monthly household budget | ||||
| <2850 kn | 8 (15) | 1 (5) | 9 (12) | 0.42 |
| 2850–5700 kn | 15 (27) | 3 (16) | 18 (24) | |
| 5700–8600 kn | 12 (22) | 7 (37) | 19 (26) | |
| 8600–11,400 kn | 11 (20) | 3 (16) | 14 (19) | |
| >11,400 kn | 9 (16.4) | 5 (26.3) | 14 (18.9) | |
| Number of people in household | ||||
| 1 | 10 (18) | 2 (10) | 12 (16) | 0.11 |
| 2 | 21 (38) | 8 (40) | 29 (39) | |
| 3 | 14 (25) | 1 (5) | 15 (20) | |
| 4 | 4 (7) | 4 (20) | 8 (11) | |
| 5 | 3 (5.5) | 3 (15) | 6 (8) | |
| 6 | 2 (3.6) | 2 (10) | 4 (5.3) | |
| 9 | 1 (2) | 0 | 1 (1) | |
| Physical activity intensity | ||||
| Extremely weak | 1 (2) | 3 (15) | 4 (5) | 0.10 |
| Weak | 22 (40) | 9 (45) | 31 (41) | |
| Moderate | 29 (53) | 8 (40) | 37 (49) | |
| Intense | 3 (5) | 0 | 3 (4) | |
| Number (%) | ||||
|---|---|---|---|---|
| BDI-II < 14 (n= 61) | BDI-II ≥ 14 (n = 12) | Total (n = 73) | p * | |
| Sex | ||||
| Male | 43 (70) | 4 (33) | 47 (64) | 0.02 |
| Female | 18 (30) | 8 (67) | 26 (36) | |
| Physical activity intensity | ||||
| Extremely weak | 2 (3) | 2 (17) | 4 (5) | 0.03 |
| Weak | 23 (38) | 8 (67) | 31 (42) | |
| Moderate | 33 (54) | 2 (17) | 35 (48) | |
| Intense | 3 (5) | 0 (0) | 3 (4) | |
| Nausea | 10 (16) | 6 (50) | 16 (22) | 0.02 |
| Have chewing/swallowing problem | 4 (7) | 4 (33) | 8 (11) | 0.02 |
| Gastrointestinal symptoms | ||||
| No symptoms with good appetite | 48 (79) | 5 (42) | 53 (73) | 0.02 |
| Mild symptoms | 11 (18) | 6 (50) | 17 (23) | |
| Frequent diarrhea or vomiting or severe anorexia | (3) | 1 (8) | 3 (4) | |
| Nutritionally related functional impairment | ||||
| Normal-to-improved functional capacity | 36 (59) | 0 | 36 (49) | <0.001 |
| Occasional difficulty with baseline ambulation | 24 (39) | 12 (100) | 36 (49) | |
| Bed/chair- ridden, or little to no physical activity | 1 (2) | 0 | 1 (1) | |
| Fat mass (kg), (IQR) | 14.4 (8.6–22.2) | 17.6 (13.4–40.5) | 14.7 (9.3–24.7) | 0.04 |
| Fat mass (%), (IQR) | 17.9 (12.5–27) | 23.6 (20.4–37.3) | 20.2 (13.7–27.8) | 0.01 |
| Na (umol/L) | 138 (136–140) | 135.5 (133.5–137) | 138 (135–139) | 0.01 |
| Cl (umol/L) | 99 (97–101) | 96.5 (95–99) | 99 (96–101) | 0.01 |
| Beck Depression Inventory (BDI-II) | |||
|---|---|---|---|
| Median (IQR) | |||
| HD (n = 53) | PD (n = 20) | p * | |
| 1. SADNESS | 0 (0–0) | 0 (0–0) | 0.06 |
| 2. PESIMISM | 1 (0–1) | 0 (0–0) | 0.002 |
| 3. PAST FAILURE | 0 (0–0) | 0 (0–0) | 0.18 |
| 4. LOSS OF PLEASURE | 0 (0–1) | 0 (0–1) | 0.89 |
| 5. GUILTY FEELINGS | 0 (0–0) | 0 (0–0) | 0.64 |
| 6. PUNISHMENT FEELING | 0 (0–0) | 0 (0–0) | 0.41 |
| 7. SELF-DISLIKE | 0 (0–0) | 0 (0–0) | 0.14 |
| 8. SELF-CRITICALNESS | 0 (0–0.5) | 0 (0–1) | 0.62 |
| 9. SUICIDAL THOUGHTS | 0 (0–0) | 0 (0–0) | 0.56 |
| 10. CRYING | 0 (0–0) | 0 (0–0) | 0.94 |
| 11. AGITATION | 0 (0–1) | 0 (0–1) | 0.30 |
| 12. LOSS OF INTEREST | 0 (0–1) | 0 (0–1) | 0.98 |
| 13. INDECIDIVENESS | 0 (0–0.5) | 0 (0–0) | 0.46 |
| 14. WORTHLESSNESS | 0 (0–0) | 0 (0–0) | 0.26 |
| 15. LOSS OF ENERGY | 1 (1–1) | 1 (0–1) | 0.46 |
| 16. CHANGES IN SLEEP | 1 (0–1) | 1 (0–1) | 0.42 |
| 17. IRRITABILITY | 0 (0–1) | 0 (0–1) | 0.72 |
| 18. CHANGES IN APPETITE | 0 (0–1) | 1 (0–1) | 0.09 |
| 19. CONCENTRATION | 0 (0–1) | 0 (0–1) | 0.41 |
| 20. TIREDNESS | 1 (1–1) | 1 (0–1) | 0.25 |
| 21. LOSS OF INTEREST IN SEX | 1.5 (0–3) | 1 (0–1) | 0.04 |
| HD (n = 55) | PD (n = 20) | |
|---|---|---|
| Parameters | Rho (p-Value *) | Rho (p-Value *) |
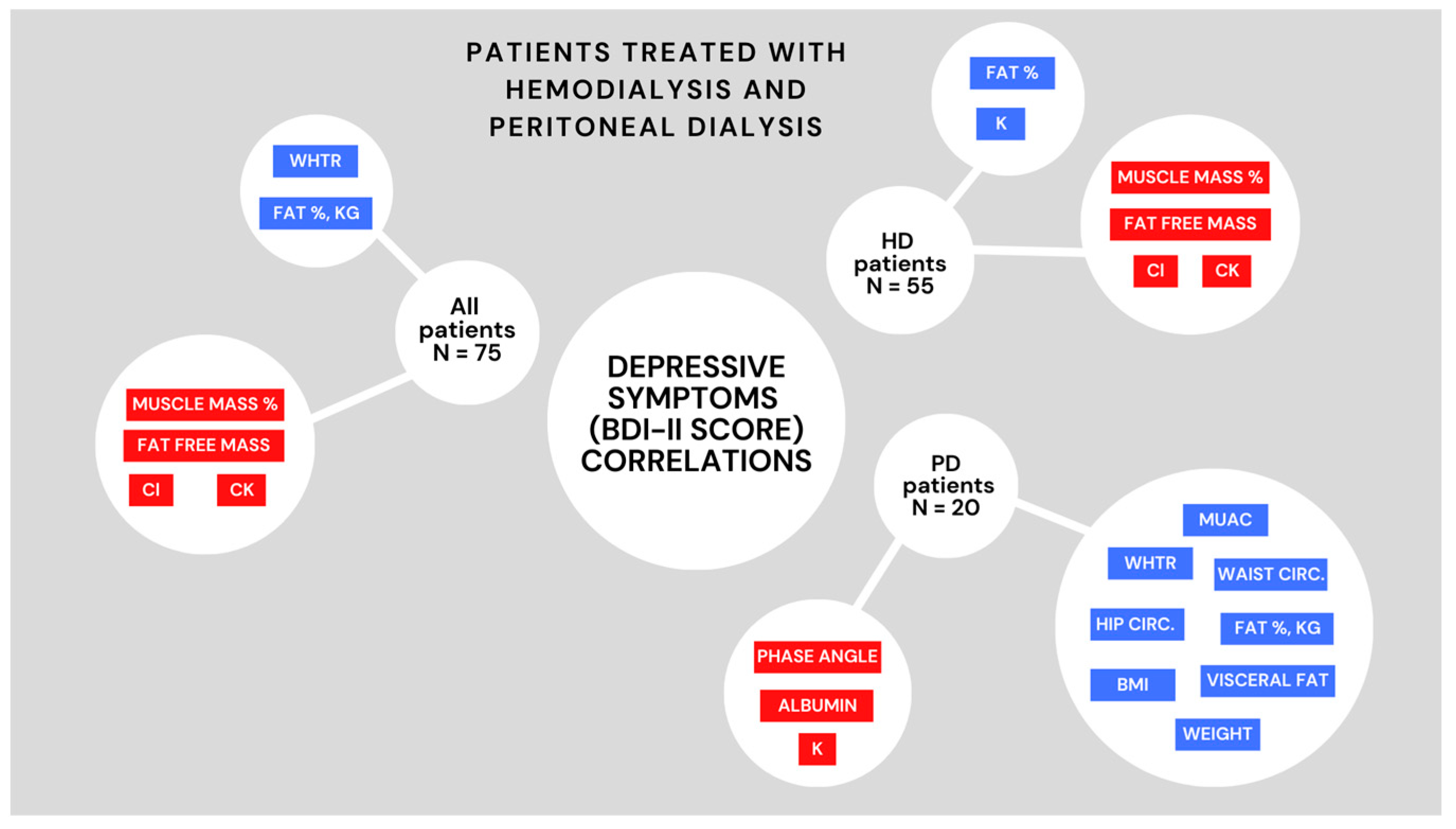
| Middle upper arm circumference (cm) | −0.119 (0.40) | 0.506 (0.03) |
| Waist circumference (cm) | −0.096 (0.50) | 0.495 (0.04) |
| Hip circumference (cm) | 0.127 (0.38) | 0.501 (0.03) |
| WHtR | 0.174 (0.22) | 0.503 (0.03) |
| Height (cm) | −0.430 (<0.001) | −0.242 (0.33) |
| Weight (kg) | −0.210 (0.13) | 0.533 (0.02) |
| BMI (kg/m2) | 0.080 (0.57) | 0.508 (0.03) |
| Fat mass (%) | 0.319 (0.02) | 0.547 (0.02) |
| Fat mass (kg) | 0.219 (0.12) | 0.528 (0.02) |
| Visceral fat | 0.042 (0.77) | 0.479 (0.04) |
| Fat-free mass (kg) | −0.398 (<0.001) | 0.184 (0.47) |
| Muscle mass (%) | −0.398 (<0.001) | 0.184 (0.47) |
| Phase angle (°) | −0.32 (0.02) | −0.489 (0.04) |
| Creatine kinase (mmol/L) | −0.476 (<0.001) | 0 (>0.99) |
| Serum albumin (g/L) | 0.064 (0.65) | −0.527 (0.02) |
| Potassium (mmol/L) | 0.284 (0.04) | −0.506 (0.03) |
| Chloride (mmol/L) | −0.340 (0.02) | −0.376 (0.14) |
| Predictors | BDI-II Multivariate Linear Regression | |||
|---|---|---|---|---|
| Adjusted for Age and Sex | ||||
| ß | p * | 95% CI | ||
| HD | ||||
| RL FatP | 0.23 | 0.003 | 0.081–0.376 | R = 0.612 R2 = 0.374 Corrected R2 = 0.329 |
| MIS score | 0.96 | 0.004 | 0.322–1.605 | |
| CK | −0.04 | 0.03 | −0.071–(−0.004) | |
| PD | - | - | - | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vučković, M.; Radić, J.; Kolak, E.; Nenadić, D.B.; Begović, M.; Radić, M. Body Composition Parameters Correlate to Depression Symptom Levels in Patients Treated with Hemodialysis and Peritoneal Dialysis. Int. J. Environ. Res. Public Health 2023, 20, 2285. https://doi.org/10.3390/ijerph20032285
Vučković M, Radić J, Kolak E, Nenadić DB, Begović M, Radić M. Body Composition Parameters Correlate to Depression Symptom Levels in Patients Treated with Hemodialysis and Peritoneal Dialysis. International Journal of Environmental Research and Public Health. 2023; 20(3):2285. https://doi.org/10.3390/ijerph20032285
Chicago/Turabian StyleVučković, Marijana, Josipa Radić, Ela Kolak, Dora Bučan Nenadić, Mirna Begović, and Mislav Radić. 2023. "Body Composition Parameters Correlate to Depression Symptom Levels in Patients Treated with Hemodialysis and Peritoneal Dialysis" International Journal of Environmental Research and Public Health 20, no. 3: 2285. https://doi.org/10.3390/ijerph20032285
APA StyleVučković, M., Radić, J., Kolak, E., Nenadić, D. B., Begović, M., & Radić, M. (2023). Body Composition Parameters Correlate to Depression Symptom Levels in Patients Treated with Hemodialysis and Peritoneal Dialysis. International Journal of Environmental Research and Public Health, 20(3), 2285. https://doi.org/10.3390/ijerph20032285







