Biochar Facilitated Direct Interspecies Electron Transfer in Anaerobic Digestion to Alleviate Antibiotics Inhibition and Enhance Methanogenesis: A Review
Abstract
1. Introduction
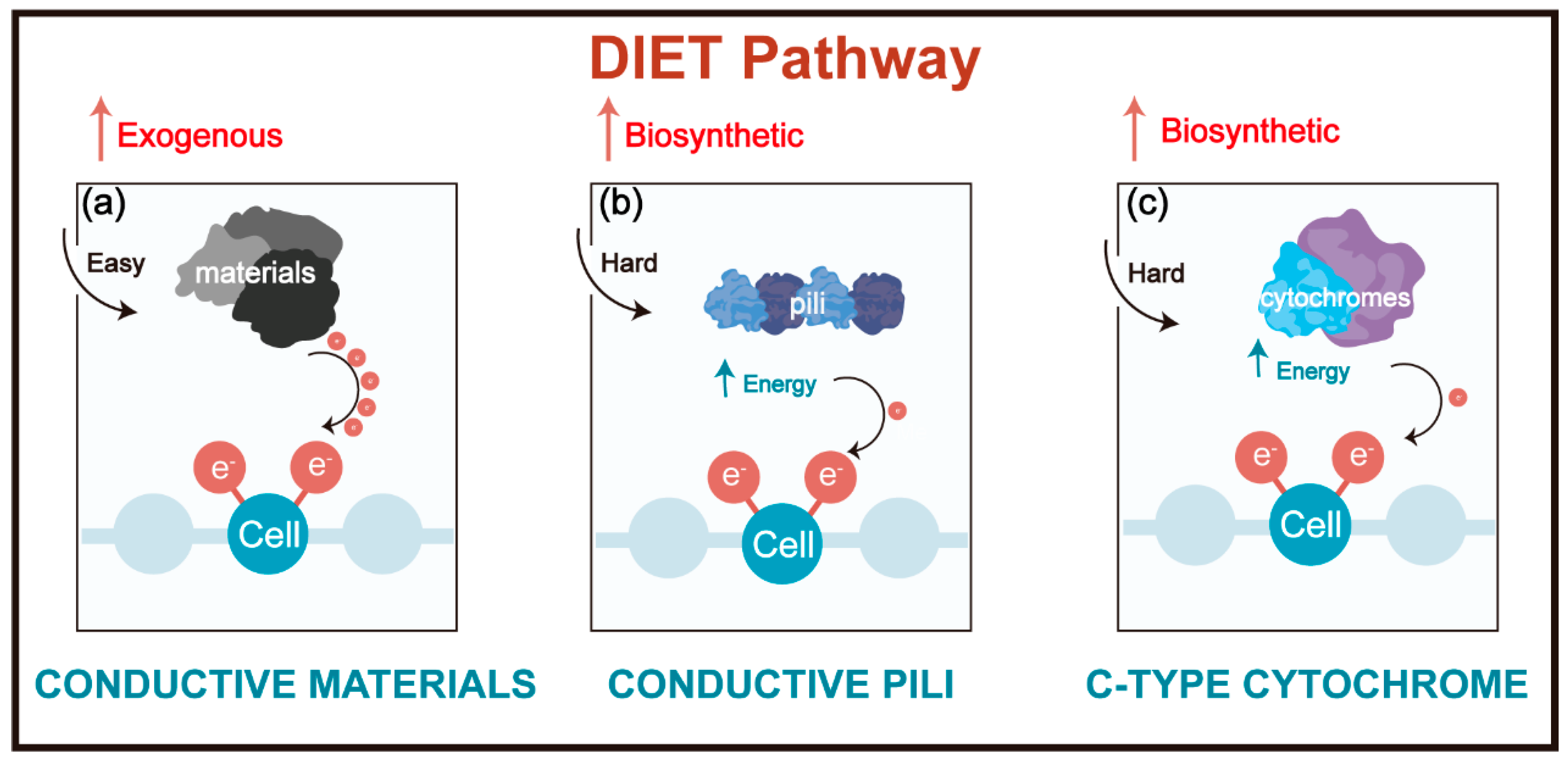
2. The Evolution of the DIET
3. Application of Biochar-Facilitated DIET for Alleviating Antibiotics Inhibition and Enhancing Methanogenesis
| Biochar Feedstock | Biochar Preparation/Dosage | Biochar Properties (Size, BET) | AD Feedstock | Experimental Scale | Operating Condition | Antibiotics (Concentration) | Removal Rate | AD Performance | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Sawdust | 500 °C, 1.5 h/ 15 g/L | 0.25–1 mm, 38.6 m2/g | Swine manure | Laboratory-scale | 35 °C 120 rpm | Tetracycline (0.5, 50 mg/L) | 79.7% 55% | CH4 production rate inhibited by 12.5% under 50 mg/L TC pressure | [25] |
| Rice husk | 300 °C, 2 h/ 5–10% of dry manure | 0.25 mm, 16.66 m2/g | Swine manure | Laboratory-scale | 37 °C 170 rpm | - | - | Methane yield increase 23.6–25.1% | [1] |
| Citrus peel | 500 °C, 1 h/ 1.5 g/g vs. of substrate | 0.25 mm, 6.6 m2/g | Sewage sludge Food waste | Laboratory-scale | 35 °C 120 rpm | - | - | Facilitated methane production (250.8 mL/g VS) and shortened lag time (3.5 days) | [63] |
| Pomelo peels | 600 °C, 2 h/ 0.5 g/L | 0.075 mm, 27.50 m2/g | Synthetic swine wastewater | Laboratory-scale | 35 °C HRT:22 h | Sulfadiazine Sulfamethazine (100 μg/L) | 25.77% 26.46% | Remove 95% of COD with average methane yield of 0.2 L/g COD | [55] |
| Fallen leaves | 300 °C, 2 h/ 15 g/L | 0.15 mm, 3.8 m2/g | Sewage sludge | Laboratory-scale | 37 °C 150 rpm | - | - | Shortened lag time and increased 45.9% methane production | [35] |
| Corn straw | 600 °C, 0.3 h/ 4% of TS | 0.5 mm, - | Corn straw | Pilot-scale | 38 °C 60 rpm | - | - | Biogas yield increase 19.42% | [64] |
| Wheat straw | 500 °C/ 10 g/L | 10–15 mm, - | Kitchen wastes Waste activated sludge | Laboratory-scale | 37 °C 30–40 rpm | - | - | Methane production increase 44% and methane production efficiency of 0.24 m3/kg COD | [2] |
| Cattle manure Sawdust | 300 °C 500 °C 700 °C, 1.5 h/ 15 g/L | 0.25–1 mm, 4.2–181.3 m2/g | Food wastes Sewage sludge | Laboratory-scale | 35 °C 120 rpm | - | - | Lag time decreased from 15.0 d to 1.1–3.0 d, maximum CH4 production rate increased from 4.0 mL/d to 10.4–13.9 mL/d | [62] |
| Sawdust | 500 °C, 1.5 h/ 15 g/L | 0.25–1 mm, 248.6 m2/g | Food waste Activated sludge | Laboratory-scale | 35 °C 30–40 rpm | - | - | Shortened lag time by 39.2–52.8% | [65] |
| Sawdust | -/0.60 g/g vs. oily sludge | -, 840.68 m2/g | Naphthalene Starch Oily sludge | Laboratory-scale | 35 °C 110 rpm | - | - | Maximum CH4 yield (138.41 mL/g VS) was 2.19 times of control | [66] |
| Hickory wood chips | 900 °C/ 12 g/L | -,- | 3.2 g ethanol/L/d | Laboratory-scale | 36 °C | - | - | Methane production improved by 75% and specific methane production increased to 725 mL/g VS/d | [58] |
| Corncob | 500 °C, 1.5 h/ 4 g/L | 2–3 mm, 37.8 m2/g | Synthetic sewage | Laboratory-scale | 35 °C HRT:3.2 h | Tetracycline Triclocarban Triclosan Sulfamethoxazole (0.2 μg/L) | - | Improved the biotransformation potential of antibiotics, increasing the overall removal efficiency by 13.7% | [60] |
| Corncob | 500 °C, 1.5 h/ 2 g/L | 2–3 mm, 9.4 m2/g | Synthetic sewage | Laboratory-scale | 8 °C 10 °C HRT:4 h | - | - | CH4 production increased by over 15% at 10 °C | [67] |
| Corn straws | 900 °C, 1 h/ 10 g/L | 0.18 mm, - | Glucose Food waste | Laboratory-scale | 37 °C 80 rpm | - | - | Cumulative methane production improved by 42.07% | [68] |
| Apple tree branch | 550 °C/ 100% of dry sludge | 0.18 mm, 19.6 m2/g | Excess sludge | Laboratory-scale | 37 °C | - | - | Cumulative methane production was 172.3 mL/g COD, 30.2 times of control group | [69] |
| Cotton- wood | 700 °C, 0.6 h/ 8 g/L | 0.5 mm, 13.97 m2/g | Cornstalk | Pilot-scale | 45 °C 60 rpm | - | - | Improved the volumetric biogas production rate by 43.09% | [70] |
| Iron-rich sludge | 700 °C, 1.5 h/ 10 g/L | 0.048 mm, - | Synthetic wastewater | Laboratory-scale | 35 °C 140 rpm | - | - | Cumulative methane production of 486 mL/L and maximum methane production rate of 2.0 mL/h | [71] |
| Wood chip | 850 °C/ 8 g/L | -, 272 m2/g | Municipal Solid Waste | Pilot-scale | 51–53 °C - | - | - | Improved the methane content by 10% | [72] |
| Corn stalks | 500 °C, 2 h/ 5 g/L | 0.55 mm, - | Manure Corn stalk | Laboratory-scale | 37 °C | Sulfamethazine (60 and 120 mg/kg−1 of dry sludge) | 97.6% 96.8% | Cumulative biogas yield decreased by 7.2% and 8.7%, respectively | [26] |
| Excess sludge | 400 °C, 2 h/16% of dry sludge | -,- | Excess sludge | Laboratory-scale | 35 °C | - | - | CH4 production increased 54.5% | [73] |
| Blue algae | 450 °C, 2 h/ 10 g/L | 0.1–0.3 mm, 145.6 m2/g | Sludge hydrolysate with 1% and 4% (v/v) inoculation ratio | Laboratory-scale | 35 °C 120 rpm | - | - | Methane production increased by 12.2% and 17.5% and lag times shortened by 41.6% and 44.3%, respectively | [74] |
| Wood pellets | 700–800 °C/ 15 g/L | 0.2–0.3 mm, 193 m2/g | Food waste | Pilot-scale | 55 °C | - | - | Improved the average methane production by 37% | [75] |
| Discarded fruitwood | 550 °C, 2 h/ 3.3% of dry sludge | 0.3–0.45 mm, 206 m2/g | Chicken manure | Laboratory-scale | 35 °C | - | - | Maximum cumulative methane production of 294 mL/g vs. manure | [76] |
| Pine sawdust | 900 °C, 0.33 h/15 g/L | 0.025 mm, 265 m2/g | Food wastes | Laboratory-scale | 37 °C | - | - | Increase 46.9% cumulative methane production and 43.0% daily methane production rate | [77] |
4. Mechanisms of Biochar-Facilitated DIET for Alleviating Antibiotics Inhibition and Enhancing Methanogenesis
4.1. Electrochemical Mechanism of Biochar-Facilitated DIET
4.1.1. Electrical Conductivity
4.1.2. Redox-Active Characteristics
4.1.3. Electron Transfer System Activity
4.2. Microbial Mechanism of Biochar-Facilitated DIET
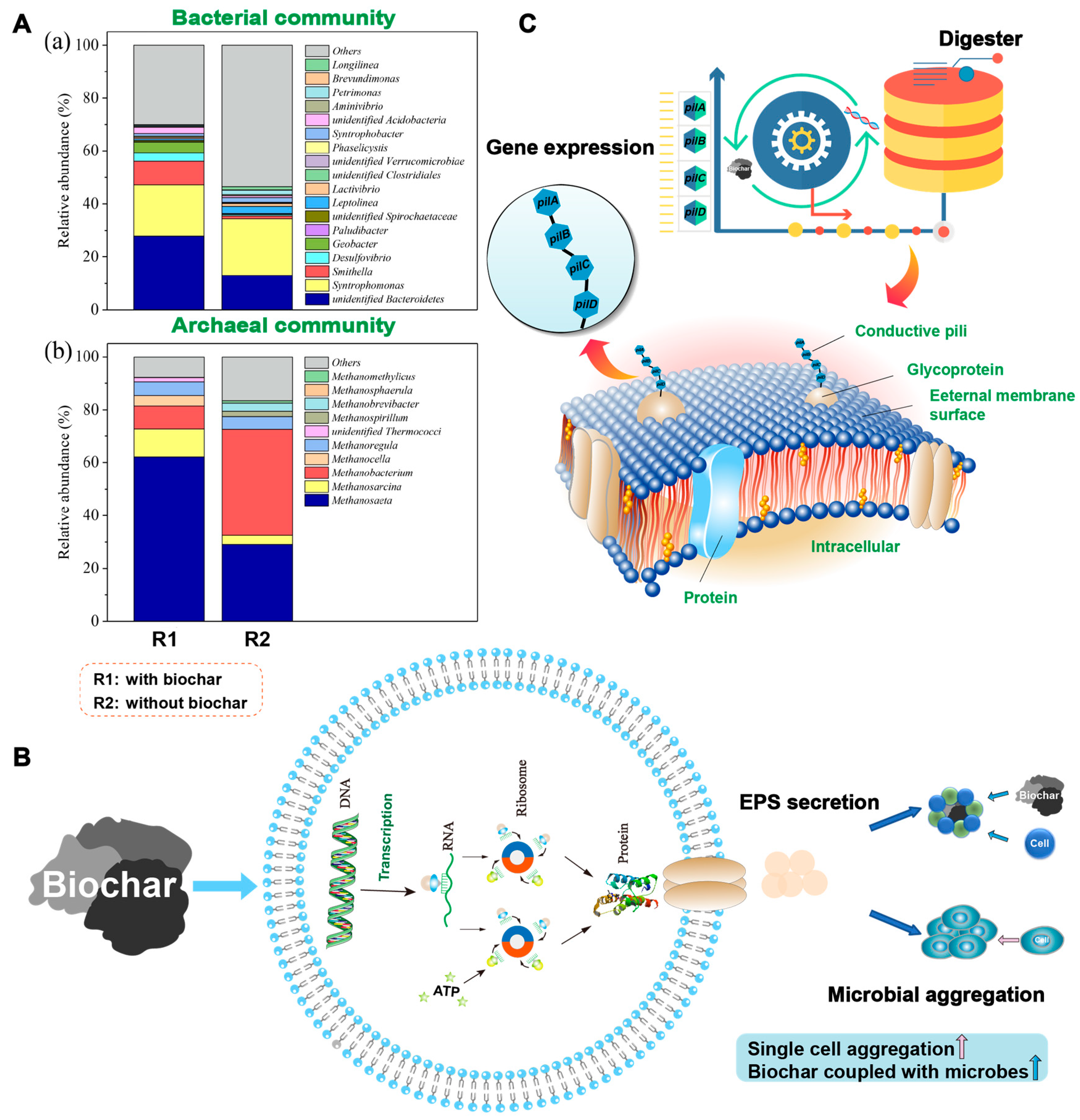
4.2.1. Enhanced Abundance of Potential DIET Microorganisms
4.2.2. Facilitated Microbial Aggregation
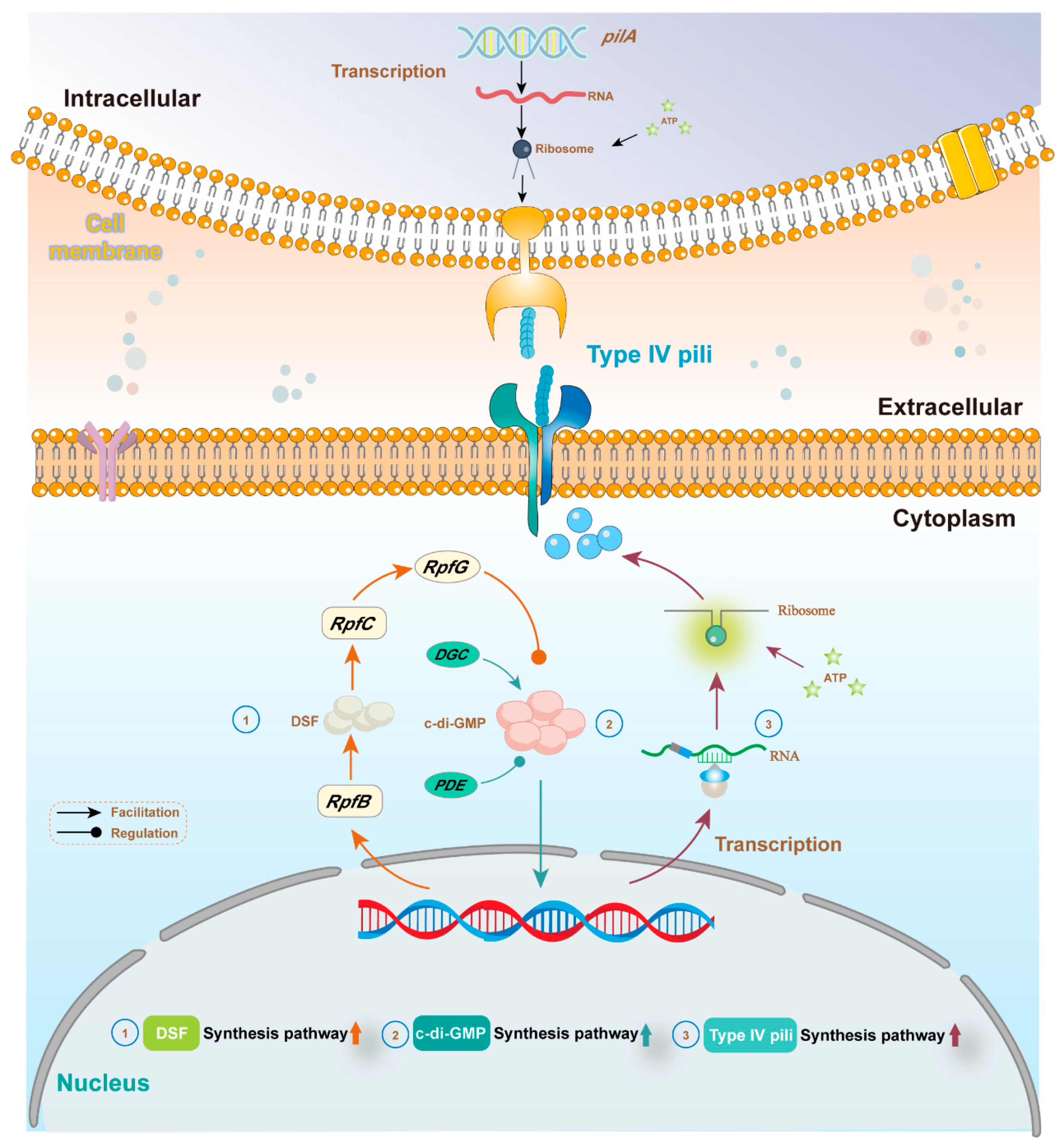
4.2.3. Regulated DIET-Associated Gene Expression
5. Challenges of Biochar in Practical Application
6. Conclusions and Prospects
- Development of investigation methods. It is necessary to integrate bioinformatic analysis, in situ spectroelectrochemical characterization, and material morphological characterization into a comprehensive analytical tool to further explore DIET strains. Concurrently, the coupling of molecular simulation and density functional theory calculations with conventional microbial analysis is also recommended to explore the potential DIET mechanisms in depth [120].
- Mechanisms of antibiotic removal. There is a lack of in-depth research on the mechanism of enhancing biodegradation of antibiotics with biochar-facilitated DIET. The construction of biohybrids of biochar and methanogens (e.g., Methanosarcina barkeri) explores the mechanism of antibiotics degradation by the DIET process, and the role of antibiotics during electron storage and redistribution at the biotic-abiotic interface [121].
- Prediction of machine learning models. It is challenging to obtain the optimal operating conditions such as feedstock type, pyrolysis temperature, and biochar dose for biochar-enhanced AD performance due to the existence of a complex bioconversion process. Employing multitask models constructed with machine learning algorithms provides an in-depth comprehension of key factors for biochar to facilitate DIET-enhanced methanogenesis and alleviate antibiotic suppression [122].
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, S.; Chen, Z.Q.; Wen, Q.X. Impacts of biochar on anaerobic digestion of swine manure: Methanogenesis and antibiotic resistance genes dissemination. Bioresour. Technol. 2021, 324, 124679. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, M.S.; Che, X.R.; Li, C.; Liang, D.D.; Zhou, H.; Liu, L.F.; Zhao, Z.Q.; Zhang, Y.B. Biochar stimulates growth of novel species capable of direct interspecies electron transfer in anaerobic digestion via ethanol-type fermentation. Environ. Res. 2020, 189, 109983. [Google Scholar] [CrossRef] [PubMed]
- Sahota, S.; Shah, G.; Ghosh, P.; Kapoor, R.; Sengupta, S.; Singh, P.; Vijay, V.; Sahay, A.; Vijay, V.K.; Thakur, I.S. Review of trends in biogas upgradation technologies and future perspectives. Bioresour. Technol. Rep. 2018, 1, 79–88. [Google Scholar] [CrossRef]
- Arif, S.; Liaquat, R.; Adil, M. Applications of materials as additives in anaerobic digestion technology. Renew. Sustain. Energy Rev. 2018, 97, 354–366. [Google Scholar] [CrossRef]
- Patel, S.K.S.; Das, D.; Kim, S.C.; Cho, B.-K.; Kalia, V.C.; Lee, J.-K. Integrating strategies for sustainable conversion of waste biomass into dark-fermentative hydrogen and value-added products. Renew. Sustain. Energy Rev. 2021, 150, 111491. [Google Scholar] [CrossRef]
- Sahoo, K.K.; Sinha, A.; Das, D. Process engineering strategy for improved methanol production in Methylosinus trichosporium through enhanced mass transfer and solubility of methane and carbon dioxide. Bioresour. Technol. 2023, 371, 128603. [Google Scholar] [CrossRef]
- Nobu, M.K.; Narihiro, T.; Mei, R.; Kamagata, Y.; Lee, P.K.H.; Lee, P.-H.; McInerney, M.J.; Liu, W.-T. Catabolism and interactions of uncultured organisms shaped by eco-thermodynamics in methanogenic bioprocesses. Microbiome 2020, 8, 111. [Google Scholar] [CrossRef]
- Song, X.; Liu, J.; Jiang, Q.; Zhang, P.; Shao, Y.; He, W.; Feng, Y. Enhanced electron transfer and methane production from low-strength wastewater using a new granular activated carbon modified with nano-Fe3O4. Chem. Eng. J. 2019, 374, 1344–1352. [Google Scholar] [CrossRef]
- Zheng, Z.; Cai, Y.; Zhang, Y.; Zhao, Y.; Gao, Y.; Cui, Z.; Hu, Y.; Wang, X. The effects of C/N (10–25) on the relationship of substrates, metabolites, and microorganisms in “inhibited steady-state” of anaerobic digestion. Water Res. 2021, 188, 116466. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, P.; Zhang, J.; He, Y.; Tong, Y.W. Micro–Nano Magnetite-Loaded Biochar Enhances Interspecies Electron Transfer and Viability of Functional Microorganisms in Anaerobic Digestion. ACS Sustain. Chem. Eng. 2022, 10, 2811–2821. [Google Scholar] [CrossRef]
- He, P.; Wu, G.; Tang, R.; Ji, P.; Yuan, S.; Wang, W.; Hu, Z. Influence of arsanilic acid, Cu2+, PO43– and their interaction on anaerobic digestion of pig manure. Front. Environ. Sci. Eng. 2017, 12, 9. [Google Scholar] [CrossRef]
- Rani, J.; Pandey, K.P.; Kushwaha, J.; Priyadarsini, M.; Dhoble, A.S. Antibiotics in anaerobic digestion: Investigative studies on digester performance and microbial diversity. Bioresour. Technol. 2022, 361, 127662. [Google Scholar] [CrossRef] [PubMed]
- Douafer, H.; Andrieu, V.; Phanstiel, O.I.V.; Brunel, J.M. Antibiotic Adjuvants: Make Antibiotics Great Again! J. Med. Chem. 2019, 62, 8665–8681. [Google Scholar] [CrossRef] [PubMed]
- Aydin, S.; Ince, B.; Ince, O. Application of real-time PCR to determination of combined effect of antibiotics on Bacteria, Methanogenic Archaea, Archaea in anaerobic sequencing batch reactors. Water Res. 2015, 76, 88–98. [Google Scholar] [CrossRef]
- Qiao, M.; Ying, G.-G.; Singer, A.C.; Zhu, Y.-G. Review of antibiotic resistance in China and its environment. Environ. Int. 2018, 110, 160–172. [Google Scholar] [CrossRef]
- Chen, J.; Tong, T.; Jiang, X.; Xie, S. Biodegradation of sulfonamides in both oxic and anoxic zones of vertical flow constructed wetland and the potential degraders. Environ. Pollut. 2020, 265, 115040. [Google Scholar] [CrossRef]
- Gaballah, M.S.; Guo, J.; Sun, H.; Aboagye, D.; Sobhi, M.; Muhmood, A.; Dong, R. A review targeting veterinary antibiotics removal from livestock manure management systems and future outlook. Bioresour. Technol. 2021, 333, 125069. [Google Scholar] [CrossRef]
- Oberoi, A.S.; Jia, Y.; Zhang, H.; Khanal, S.K.; Lu, H. Insights into the Fate and Removal of Antibiotics in Engineered Biological Treatment Systems: A Critical Review. Environ. Sci. Technol. 2019, 53, 7234–7264. [Google Scholar] [CrossRef]
- Khurana, P.; Pulicharla, R.; Kaur Brar, S. Antibiotic-metal complexes in wastewaters: Fate and treatment trajectory. Environ. Int. 2021, 157, 106863. [Google Scholar] [CrossRef]
- Summers, Z.M.; Fogarty, H.E.; Leang, C.; Franks, A.E.; Malvankar, N.S.; Lovley, D.R. Direct Exchange of Electrons Within Aggregates of an Evolved Syntrophic Coculture of Anaerobic Bacteria. Science 2010, 330, 1413–1415. [Google Scholar] [CrossRef]
- Liu, X.; Huang, L.; Rensing, C.; Ye, J.; Nealson, K.H.; Zhou, S. Syntrophic interspecies electron transfer drives carbon fixation and growth by Rhodopseudomonas palustris under dark, anoxic conditions. Sci. Adv. 2021, 7, eabh1852. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.-Y.; Ding, L.-J.; Zama, E.F.; Liu, P.-P.; Hozzein, W.N.; Zhu, Y.-G. Biochar Modulates Methanogenesis through Electron Syntrophy of Microorganisms with Ethanol as a Substrate. Environ. Sci. Technol. 2018, 52, 12198–12207. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zhang, D.; Dai, L.; Dong, B.; Dai, X. Magnetite Triggering Enhanced Direct Interspecies Electron Transfer: A Scavenger for the Blockage of Electron Transfer in Anaerobic Digestion of High-Solids Sewage Sludge. Environ. Sci. Technol. 2018, 52, 7160–7169. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Yuan, Y.; Yu, L.; Zhang, B.; Li, J.; Liu, T.; Yu, Z.; Zhou, S. Biochar enhances bioelectrochemical remediation of pentachlorophenol-contaminated soils via long-distance electron transfer. J. Hazard. Mater. 2020, 391, 122213. [Google Scholar] [CrossRef]
- Wang, G.; Chu, Y.; Zhu, J.; Sheng, L.; Liu, G.; Xing, Y.; Fu, P.; Li, Q.; Chen, R. Multi-faceted influences of biochar addition on swine manure digestion under tetracycline antibiotic pressure. Bioresource Technology 2022, 346, 126352. [Google Scholar] [CrossRef]
- Zhang, Y.; Cao, L.; Fu, H.; Zhang, M.; Meng, J.; Althakafy, J.T.; Abo-Dief, H.M.; El-Bahy, S.M.; Zhang, Y.; Wei, H.; et al. Effect of sulfamethazine on anaerobic digestion of manure mediated by biochar. Chemosphere 2022, 306, 135567. [Google Scholar] [CrossRef]
- Huang, L.; Liu, X.; Ye, Y.; Chen, M.; Zhou, S. Evidence for the coexistence of direct and riboflavin-mediated interspecies electron transfer in Geobacter co-culture. Environ. Microbiol. 2020, 22, 243–254. [Google Scholar] [CrossRef]
- Ueki, T.; Nevin, K.P.; Rotaru, A.-E.; Wang, L.-Y.; Ward, J.E.; Woodard, T.L.; Lovley, D.R. Geobacter Strains Expressing Poorly Conductive Pili Reveal Constraints on Direct Interspecies Electron Transfer Mechanisms. mBio 2018, 9, e01273-18. [Google Scholar] [CrossRef]
- Liu, X.; Zhuo, S.Y.; Rensing, C.; Zhou, S.G. Syntrophic growth with direct interspecies electron transfer between pili-free Geobacter species. ISME J. 2018, 12, 2142–2151. [Google Scholar] [CrossRef]
- Lovley, D.R. Electrically conductive pili: Biological function and potential applications in electronics. Curr. Opin. Electrochem. 2017, 4, 190–198. [Google Scholar] [CrossRef]
- Wang, C.; Liu, Y.; Wang, C.Q.; Xing, B.; Zhu, S.D.; Huang, J.J.; Xu, X.Y.; Zhu, L. Biochar facilitates rapid restoration of methanogenesis by enhancing direct interspecies electron transfer after high organic loading shock. Bioresour. Technol. 2021, 320, 124360. [Google Scholar] [CrossRef]
- Chiappero, M.; Norouzi, O.; Hu, M.; Demichelis, F.; Berruti, F.; Di Maria, F.; Mašek, O.; Fiore, S. Review of biochar role as additive in anaerobic digestion processes. Renew. Sustain. Energy Rev. 2020, 131, 110037. [Google Scholar] [CrossRef]
- Zhao, W.; Yang, H.; He, S.; Zhao, Q.; Wei, L. A review of biochar in anaerobic digestion to improve biogas production: Performances, mechanisms and economic assessments. Bioresour. Technol. 2021, 341, 125797. [Google Scholar] [CrossRef] [PubMed]
- Hoang, A.T.; Goldfarb, J.L.; Foley, A.M.; Lichtfouse, E.; Kumar, M.; Xiao, L.; Ahmed, S.F.; Said, Z.; Luque, R.; Bui, V.G.; et al. Production of biochar from crop residues and its application for anaerobic digestion. Bioresour. Technol. 2022, 363, 127970. [Google Scholar] [CrossRef] [PubMed]
- Bu, J.; Hu, B.-B.; Wu, H.-Z.; Zhu, M.-J. Improved methane production with redox-active/conductive biochar amendment by establishing spatial ecological niche and mediating electron transfer. Bioresour. Technol. 2022, 351, 127072. [Google Scholar] [CrossRef]
- Cui, Y.; Mao, F.; Zhang, J.; He, Y.; Tong, Y.W.; Peng, Y. Biochar enhanced high-solid mesophilic anaerobic digestion of food waste: Cell viability and methanogenic pathways. Chemosphere 2021, 272, 129863. [Google Scholar] [CrossRef]
- Wang, W.; Lee, D.-J. Direct interspecies electron transfer mechanism in enhanced methanogenesis: A mini-review. Bioresour. Technol. 2021, 330, 124980. [Google Scholar] [CrossRef] [PubMed]
- Reguera, G.; McCarthy, K.D.; Mehta, T.; Nicoll, J.S.; Tuominen, M.T.; Lovley, D.R. Extracellular electron transfer via microbial nanowires. Nature 2005, 435, 1098–1101. [Google Scholar] [CrossRef]
- Steidl, R.J.; Lampa-Pastirk, S.; Reguera, G. Mechanistic stratification in electroactive biofilms of Geobacter sulfurreducens mediated by pilus nanowires. Nat. Commun. 2016, 7, 12217. [Google Scholar] [CrossRef]
- Li, Y.; Tang, Y.; Xiong, P.; Zhang, M.; Deng, Q.; Liang, D.; Zhao, Z.; Feng, Y.; Zhang, Y. High-efficiency methanogenesis via kitchen wastes served as ethanol source to establish direct interspecies electron transfer during anaerobic Co-digestion with waste activated sludge. Water Res. 2020, 176, 115763. [Google Scholar] [CrossRef]
- Yin, Q.; Gu, M.; Hermanowicz, S.W.; Hu, H.; Wu, G. Potential interactions between syntrophic bacteria and methanogens via type IV pili and quorum-sensing systems. Environ. Int. 2020, 138, 105650. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.Q.; Wang, J.F.; Li, Y.; Zhu, T.T.; Yu, Q.L.; Wang, T.T.; Liang, S.; Zhang, Y.B. Why do DIETers like drinking: Metagenomic analysis for methane and energy metabolism during anaerobic digestion with ethanol. Water Res. 2020, 171, 14. [Google Scholar] [CrossRef]
- Wang, F.; Gu, Y.; O’Brien, J.P.; Yi, S.M.; Yalcin, S.E.; Srikanth, V.; Shen, C.; Vu, D.; Ing, N.L.; Hochbaum, A.I.; et al. Structure of Microbial Nanowires Reveals Stacked Hemes that Transport Electrons over Micrometers. Cell 2019, 177, 361–369.e310. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhan, J.; Liu, L.; Gan, F.; Ye, J.; Nealson, K.H.; Rensing, C.; Zhou, S. In Situ Spectroelectrochemical Characterization Reveals Cytochrome-Mediated Electric Syntrophy in Geobacter Coculture. Environ. Sci. Technol. 2021, 55, 10142–10151. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Hsu, L.H.H.; Kavanagh, P.; Barriere, F.; Lens, P.N.L.; Lapinsonniere, L.; Lienhard, J.H.; Schroder, U.; Jiang, X.C.; Leech, D. The ins and outs of microorganism-electrode electron transfer reactions. Nat. Rev. Chem. 2017, 1, 13. [Google Scholar] [CrossRef]
- Costa, N.L.; Clarke, T.A.; Philipp, L.-A.; Gescher, J.; Louro, R.O.; Paquete, C.M. Electron transfer process in microbial electrochemical technologies: The role of cell-surface exposed conductive proteins. Bioresour. Technol. 2018, 255, 308–317. [Google Scholar] [CrossRef]
- Lin, R.; Cheng, J.; Ding, L.; Murphy, J.D. Improved efficiency of anaerobic digestion through direct interspecies electron transfer at mesophilic and thermophilic temperature ranges. Chem. Eng. J. 2018, 350, 681–691. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Z.; Lu, T.; Liu, J.; Wang, Y.; Shen, P.; Wei, Y. Response and mechanisms of the performance and fate of antibiotic resistance genes to nano-magnetite during anaerobic digestion of swine manure. J. Hazard. Mater. 2019, 366, 192–201. [Google Scholar] [CrossRef]
- Kassab, G.; Khater, D.; Odeh, F.; Shatanawi, K.; Halalsheh, M.; Arafah, M.; van Lier, J.B. Impact of Nanoscale Magnetite and Zero Valent Iron on the Batch-Wise Anaerobic Co-Digestion of Food Waste and Waste-Activated Sludge. Water 2020, 12, 1283. [Google Scholar] [CrossRef]
- Wang, J.; He, W.; Wang, T.; Li, M.; Li, X. Sucrose-modified iron nanoparticles for highly efficient microbial production of hyaluronic acid by Streptococcus zooepidemicus. Colloids Surf. B Biointerfaces 2021, 205, 111854. [Google Scholar] [CrossRef]
- Deng, Y.; Zhang, K.; Zou, J.; Li, X.; Wang, Z.; Hu, C. Electron shuttles enhanced the removal of antibiotics and antibiotic resistance genes in anaerobic systems: A review. Front. Microbiol. 2022, 13, 4589. [Google Scholar] [CrossRef]
- Baek, G.; Kim, J.; Kim, J.; Lee, C. Role and Potential of Direct Interspecies Electron Transfer in Anaerobic Digestion. Energies 2018, 11, 107. [Google Scholar] [CrossRef]
- Xu, S.; Zhang, W.; Zuo, L.; Qiao, Z.; He, P. Comparative facilitation of activated carbon and goethite on methanogenesis from volatile fatty acids. Bioresour. Technol. 2020, 302, 122801. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Zhao, Z.; Zhang, Y. Potential of direct interspecies electron transfer in synergetic enhancement of methanogenesis and sulfate removal in an up-flow anaerobic sludge blanket reactor with magnetite. Sci. Total Environ. 2019, 677, 299–306. [Google Scholar] [CrossRef]
- Cheng, D.; Ngo, H.H.; Guo, W.; Chang, S.W.; Nguyen, D.D.; Nguyen, Q.A.; Zhang, J.; Liang, S. Improving sulfonamide antibiotics removal from swine wastewater by supplying a new pomelo peel derived biochar in an anaerobic membrane bioreactor. Bioresour. Technol. 2021, 319, 124160. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Li, J.; Wang, Y.; Yang, C. Impacts of different biochar types on the anaerobic digestion of sewage sludge. RSC Adv. 2019, 9, 42375–42386. [Google Scholar] [CrossRef]
- Li, Y.Q.; Wang, Z.X.; Jiang, Z.L.; Feng, L.; Pan, J.T.; Zhu, M.Y.; Ma, C.J.; Jing, Z.M.; Jiang, H.; Zhou, H.J.; et al. Bio-based carbon materials with multiple functional groups and graphene structure to boost methane production from ethanol anaerobic digestion. Bioresour. Technol. 2022, 344, 9. [Google Scholar] [CrossRef]
- Qi, Q.; Sun, C.; Zhang, J.; He, Y.; Wah Tong, Y. Internal enhancement mechanism of biochar with graphene structure in anaerobic digestion: The bioavailability of trace elements and potential direct interspecies electron transfer. Chem. Eng. J. 2021, 406, 126833. [Google Scholar] [CrossRef]
- Wang, M.; Zhao, Z.; Zhang, Y. Magnetite-contained biochar derived from fenton sludge modulated electron transfer of microorganisms in anaerobic digestion. J. Hazard. Mater. 2021, 403, 123972. [Google Scholar] [CrossRef]
- Lei, Z.; Zhang, S.; Wang, L.; Li, Q.; Li, Y.-Y.; Wang, X.C.; Chen, R. Biochar enhances the biotransformation of organic micropollutants (OMPs) in an anaerobic membrane bioreactor treating sewage. Water Res. 2022, 223, 118974. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Wu, C.; Chai, C.; Cao, S.; Bai, X.; Ma, K.; Jin, X.; Shi, X.; Jin, P. Adsorption of micropollutants from wastewater using iron and nitrogen co-doped biochar: Performance, kinetics and mechanism studies. J. Hazard. Mater. 2022, 424, 127606. [Google Scholar] [CrossRef]
- Wang, G.J.; Gao, X.; Li, Q.; Zhao, H.X.; Liu, Y.Z.; Wang, X.C.C.; Chen, R. Redox-based electron exchange capacity of biowaste-derived biochar accelerates syntrophic phenol oxidation for methanogenesis via direct interspecies electron transfer. J. Hazard. Mater. 2020, 390, 121726. [Google Scholar] [CrossRef]
- Jiang, Q.; Chen, Y.; Yu, S.; Zhu, R.; Zhong, C.; Zou, H.; Gu, L.; He, Q. Effects of citrus peel biochar on anaerobic co-digestion of food waste and sewage sludge and its direct interspecies electron transfer pathway study. Chem. Eng. J. 2020, 398, 125643. [Google Scholar] [CrossRef]
- Shen, R.; Yao, Z.; Yu, J.; Luo, J.; Zhao, L. Enhanced performance and microbial communities of anaerobic digestion with biochar in pilot-scale continuous stirred-tank reactors: Effects of substrate concentration and hydraulic retention time. Sustain. Energy Fuels 2022, 6, 5324–5336. [Google Scholar] [CrossRef]
- Wang, G.J.; Li, Q.; Gao, X.; Wang, X.C.C. Synergetic promotion of syntrophic methane production from anaerobic digestion of complex organic wastes by biochar: Performance and associated mechanisms. Bioresour. Technol. 2018, 250, 812–820. [Google Scholar] [CrossRef]
- Shi, Y.; Liu, M.; Li, J.; Yao, Y.; Tang, J.; Niu, Q. The dosage-effect of biochar on anaerobic digestion under the suppression of oily sludge: Performance variation, microbial community succession and potential detoxification mechanisms. J. Hazard. Mater. 2022, 421, 126819. [Google Scholar] [CrossRef] [PubMed]
- Lei, Z.; Ma, Y.; Wang, J.; Wang, X.C.C.; Li, Q.; Chen, R. Biochar addition supports high digestion performance and low membrane fouling rate in an anaerobic membrane bioreactor under low temperatures. Bioresour. Technol. 2021, 330, 8. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Feng, L.; Li, Y.; Han, Y.; Zhou, H.; Pan, J. The role of electrochemical properties of biochar to promote methane production in anaerobic digestion. J. Clean Prod. 2022, 362, 132296. [Google Scholar] [CrossRef]
- Yan, P.; Zhao, Y.; Zhang, H.; Chen, S.; Zhu, W.; Yuan, X.; Cui, Z. A comparison and evaluation of the effects of biochar on the anaerobic digestion of excess and anaerobic sludge. Sci. Total Environ. 2020, 736, 139159. [Google Scholar] [CrossRef]
- Shen, R.; Jing, Y.; Feng, J.; Zhao, L.; Yao, Z.; Yu, J.; Chen, J.; Chen, R. Simultaneous carbon dioxide reduction and enhancement of methane production in biogas via anaerobic digestion of cornstalk in continuous stirred-tank reactors: The influences of biochar, environmental parameters, and microorganisms. Bioresour. Technol. 2021, 319, 124146. [Google Scholar] [CrossRef]
- Che, L.; Yang, B.; Tian, Q.; Xu, H. Iron-based biochar derived from waste-activated sludge enhances anaerobic digestion of synthetic salty organic wastewater for methane production. Bioresour. Technol. 2022, 345, 126465. [Google Scholar] [CrossRef]
- Bona, D.; Beggio, G.; Weil, T.; Scholz, M.; Bertolini, S.; Grandi, L.; Baratieri, M.; Schievano, A.; Silvestri, S.; Pivato, A. Effects of woody biochar on dry thermophilic anaerobic digestion of organic fraction of municipal solid waste. J. Environ. Manag. 2020, 267, 110633. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Meng, Q.; Wu, F.; Zhang, C.; Tan, X.; Wan, C. Enhanced biogas production in anaerobic digestion of sludge medicated by biochar prepared from excess sludge: Role of persistent free radicals and electron mediators. Bioresour. Technol. 2022, 347, 126422. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Zhang, C.; Wu, P.; Ding, P.; Zhang, Y.; Cui, M.-h.; Liu, H. Algae biochar enhanced methanogenesis by enriching specific methanogens at low inoculation ratio during sludge anaerobic digestion. Bioresour. Technol. 2021, 338, 125493. [Google Scholar] [CrossRef]
- Zhang, L.; Lim, E.Y.; Loh, K.-C.; Ok, Y.S.; Lee, J.T.E.; Shen, Y.; Wang, C.-H.; Dai, Y.; Tong, Y.W. Biochar enhanced thermophilic anaerobic digestion of food waste: Focusing on biochar particle size, microbial community analysis and pilot-scale application. Energy Convers. Manag. 2020, 209, 112654. [Google Scholar] [CrossRef]
- Ma, J.; Chen, F.; Xue, S.; Pan, J.; Khoshnevisan, B.; Yang, Y.; Liu, H.; Qiu, L. Improving anaerobic digestion of chicken manure under optimized biochar supplementation strategies. Bioresour. Technol. 2021, 325, 124697. [Google Scholar] [CrossRef] [PubMed]
- Sugiarto, Y.; Sunyoto, N.M.S.; Zhu, M.; Jones, I.; Zhang, D. Effect of biochar addition on microbial community and methane production during anaerobic digestion of food wastes: The role of minerals in biochar. Bioresour. Technol. 2021, 323, 124585. [Google Scholar] [CrossRef]
- Barua, S.; Zakaria, B.S.; Dhar, B.R. Enhanced methanogenic co-degradation of propionate and butyrate by anaerobic microbiome enriched on conductive carbon fibers. Bioresour. Technol. 2018, 266, 259–266. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, Y.; Yu, Q.; Dang, Y.; Li, Y.; Quan, X. Communities stimulated with ethanol to perform direct interspecies electron transfer for syntrophic metabolism of propionate and butyrate. Water Res. 2016, 102, 475–484. [Google Scholar] [CrossRef]
- Park, J.H.; Kang, H.J.; Park, K.H.; Park, H.D. Direct interspecies electron transfer via conductive materials: A perspective for anaerobic digestion applications. Bioresour. Technol. 2018, 254, 300–311. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, Y.; Quan, X.; Zhang, Y. Towards engineering application: Potential mechanism for enhancing anaerobic digestion of complex organic waste with different types of conductive materials. Water Res. 2017, 115, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zhao, Z.; Zhang, Y. Using straw as a bio-ethanol source to promote anaerobic digestion of waste activated sludge. Bioresour. Technol. 2019, 286, 121388. [Google Scholar] [CrossRef]
- Zhu, Y.; Jin, Z.; Yu, Q.; Zhao, Z.; Zhang, Y. Alleviating acid inhibition in anaerobic digestion of food waste: Coupling ethanol-type fermentation with biochar addition. Environ. Res. 2022, 212, 113355. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Q.; Liu, Y.; Gao, X.Y.; Chen, H.; Xu, X.Y.; Zhu, L. Role of biochar in the granulation of anaerobic sludge and improvement of electron transfer characteristics. Bioresour. Technol. 2018, 268, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Wang, Z.; Liu, K.; Si, B.; Yang, G.; Tian, C.; Zhang, Y. Multi-cycle anaerobic digestion of hydrothermal liquefaction aqueous phase: Role of carbon and iron based conductive materials in inhibitory compounds degradation, microbial structure shaping, and interspecies electron transfer regulation. Chem. Eng. J. 2023, 454, 140019. [Google Scholar] [CrossRef]
- Martins, G.; Salvador, A.F.; Pereira, L.; Alves, M.M. Methane Production and Conductive Materials: A Critical Review. Environ. Sci. Technol. 2018, 52, 10241–10253. [Google Scholar] [CrossRef]
- Gabhi, R.S.; Kirk, D.W.; Jia, C.Q. Preliminary investigation of electrical conductivity of monolithic biochar. Carbon 2017, 116, 435–442. [Google Scholar] [CrossRef]
- Gabhi, R.; Basile, L.; Kirk, D.W.; Giorcelli, M.; Tagliaferro, A.; Jia, C.Q. Electrical conductivity of wood biochar monoliths and its dependence on pyrolysis temperature. Biochar 2020, 2, 369–378. [Google Scholar] [CrossRef]
- Guo, W.; Li, Y.; Zhao, K.; Xu, Q.; Jiang, H.; Zhou, H. Performance and Microbial Community Analysis of Anaerobic Digestion of Vinegar Residue with Adding of Acetylene Black or Hydrochar. Waste Biomass Valorization 2020, 11, 3315–3325. [Google Scholar] [CrossRef]
- Zhang, P.; Zheng, S.; Liu, J.; Wang, B.; Liu, F.; Feng, Y. Surface properties of activated sludge-derived biochar determine the facilitating effects on Geobacter co-cultures. Water Res. 2018, 142, 441–451. [Google Scholar] [CrossRef]
- Kluepfel, L.; Keiluweit, M.; Kleber, M.; Sander, M. Redox Properties of Plant Biomass-Derived Black Carbon (Biochar). Environ. Sci. Technol. 2014, 48, 5601–5611. [Google Scholar] [CrossRef]
- Sui, M.; Li, Y.; Jiang, Y.; Wang, L.; Zhang, W.; Sathishkumar, K.; Zakaria, H. Sediment-based biochar facilitates highly efficient nitrate removal: Physicochemical properties, biological responses and potential mechanism. Chem. Eng. J. 2021, 405, 126645. [Google Scholar] [CrossRef]
- Lu, C.X.; Shen, Y.W.; Li, C.; Zhu, N.W.; Yuan, H.P. Redox-Active Biochar and Conductive Graphite Stimulate Methanogenic Metabolism in Anaerobic Digestion of Waste-Activated Sludge: Beyond Direct Interspecies Electron Transfer. ACS Sustain. Chem. Eng. 2020, 8, 12626–12636. [Google Scholar] [CrossRef]
- Zhao, Z.; Cao, Y.; Li, S.; Zhang, Y. Effects of biowaste-derived biochar on the electron transport efficiency during anaerobic acid orange 7 removal. Bioresour. Technol. 2021, 320, 124295. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.J.; Li, Q.; Li, Y.; Xing, Y.; Yao, G.F.; Liu, Y.Z.; Chen, R.; Wang, X.C.C. Redox-active biochar facilitates potential electron tranfer between syntrophic partners to enhance anaerobic digestion under high organic loading rate. Bioresour. Technol. 2020, 298, 9. [Google Scholar] [CrossRef]
- Burboa-Charis, V.A.; Alvarez, L.H. Methane production from antibiotic bearing swine wastewater using carbon-based materials as electrons’ conduits during anaerobic digestion. Int. J. Energy Res. 2020, 44, 10996–11005. [Google Scholar] [CrossRef]
- Qin, Y.; Yin, X.; Xu, X.; Yan, X.; Bi, F.; Wu, W. Specific surface area and electron donating capacity determine biochar’s role in methane production during anaerobic digestion. Bioresour. Technol. 2020, 303, 122919. [Google Scholar] [CrossRef]
- Liu, H.; Xu, Y.; Li, L.; Yuan, S.; Geng, H.; Tang, Y.; Dai, X. A novel green composite conductive material enhancing anaerobic digestion of waste activated sludge via improving electron transfer and metabolic activity. Water Res. 2022, 220, 118687. [Google Scholar] [CrossRef]
- Cheng, J.; Li, H.; Ding, L.; Zhou, J.; Song, W.; Li, Y.-Y.; Lin, R. Improving hydrogen and methane co-generation in cascading dark fermentation and anaerobic digestion: The effect of magnetite nanoparticles on microbial electron transfer and syntrophism. Chem. Eng. J. 2020, 397, 125394. [Google Scholar] [CrossRef]
- Li, Q.; Xu, M.; Wang, G.; Chen, R.; Qiao, W.; Wang, X. Biochar assisted thermophilic co-digestion of food waste and waste activated sludge under high feedstock to seed sludge ratio in batch experiment. Bioresour. Technol. 2018, 249, 1009–1016. [Google Scholar] [CrossRef]
- Yin, Q.; Yang, S.; Wang, Z.; Xing, L.; Wu, G. Clarifying electron transfer and metagenomic analysis of microbial community in the methane production process with the addition of ferroferric oxide. Chem. Eng. J. 2018, 333, 216–225. [Google Scholar] [CrossRef]
- Van Steendam, C.; Smets, I.; Skerlos, S.; Raskin, L. Improving anaerobic digestion via direct interspecies electron transfer requires development of suitable characterization methods. Curr. Opin. Biotechnol. 2019, 57, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Basak, B.; Hwang, J.-H.; Salama, E.-S.; Chatterjee, P.K.; Jeon, B.-H. Microbial Symbiosis: A Network towards Biomethanation. Trends Microbiol. 2020, 28, 968–984. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, R.; Wang, H.; Yang, K. Direct interspecies electron transfer stimulated by granular activated carbon enhances anaerobic methanation efficiency from typical kitchen waste lipid-rapeseed oil. Sci. Total Environ. 2020, 704, 135282. [Google Scholar] [CrossRef]
- Yan, W.; Shen, N.; Xiao, Y.; Chen, Y.; Sun, F.; Kumar Tyagi, V.; Zhou, Y. The role of conductive materials in the start-up period of thermophilic anaerobic system. Bioresour. Technol. 2017, 239, 336–344. [Google Scholar] [CrossRef]
- Sathishkumar, K.; Li, Y.; Sanganyado, E. Electrochemical behavior of biochar and its effects on microbial nitrate reduction: Role of extracellular polymeric substances in extracellular electron transfer. Chem. Eng. J. 2020, 395, 125077. [Google Scholar] [CrossRef]
- Bu, J.; Wei, H.-L.; Wang, Y.-T.; Cheng, J.-R.; Zhu, M.-J. Biochar boosts dark fermentative H2 production from sugarcane bagasse by selective enrichment/colonization of functional bacteria and enhancing extracellular electron transfer. Water Res. 2021, 202, 117440. [Google Scholar] [CrossRef]
- Fulaz, S.; Vitale, S.; Quinn, L.; Casey, E. Nanoparticle–Biofilm Interactions: The Role of the EPS Matrix. Trends Microbiol. 2019, 27, 915–926. [Google Scholar] [CrossRef]
- Wang, F.; Wang, J.; Han, Y.; Lu, J.; Zan, S.; Du, M. In Situ Biogas Upgrading and Fertilizer Recovery in Anaerobic Digestion from Laminaria Hydrothermal Carbonization Process Water by Fe-Modified Hydrochar. ACS Sustain. Chem. Eng. 2020, 8, 13623–13633. [Google Scholar] [CrossRef]
- Li, X.; Chu, S.; Wang, P.; Li, K.; Su, Y.; Wu, D.; Xie, B. Potential of biogas residue biochar modified by ferric chloride for the enhancement of anaerobic digestion of food waste. Bioresour. Technol. 2022, 360, 127530. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, L.; Guo, B.; Zhou, Y.; Gao, M.; Sharaf, A.; Liu, Y. Granular activated carbon stimulated microbial physiological changes for enhanced anaerobic digestion of municipal sewage. Chem. Eng. J. 2020, 400, 125838. [Google Scholar] [CrossRef]
- Lei, Y.; Sun, D.; Dang, Y.; Feng, X.; Huo, D.; Liu, C.; Zheng, K.; Holmes, D.E. Metagenomic analysis reveals that activated carbon aids anaerobic digestion of raw incineration leachate by promoting direct interspecies electron transfer. Water Res. 2019, 161, 570–580. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-H.; Park, J.-H.; Seong, H.; Sul, W.J.; Jin, K.-H.; Park, H.-D. Metagenomic insight into methanogenic reactors promoting direct interspecies electron transfer via granular activated carbon. Bioresour. Technol. 2018, 259, 414–422. [Google Scholar] [CrossRef]
- Lin, R.; Cheng, J.; Zhang, J.; Zhou, J.; Cen, K.; Murphy, J.D. Boosting biomethane yield and production rate with graphene: The potential of direct interspecies electron transfer in anaerobic digestion. Bioresour. Technol. 2017, 239, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Ai, Y.; Jin, J.; Hayat, T.; Alsaedi, A.; Zhuang, L.; Wang, X. Efficient elimination of organic and inorganic pollutants by biochar and biochar-based materials. Biochar 2020, 2, 47–64. [Google Scholar] [CrossRef]
- Xiang, L.; Liu, S.; Ye, S.; Yang, H.; Song, B.; Qin, F.; Shen, M.; Tan, C.; Zeng, G.; Tan, X. Potential hazards of biochar: The negative environmental impacts of biochar applications. J. Hazard. Mater. 2021, 420, 126611. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, Z.; Zhu, S.; Li, S.; Wei, C. Effects of biochar on anaerobic treatment systems: Some perspectives. Bioresour. Technol. 2023, 367, 128226. [Google Scholar] [CrossRef]
- Gao, X.; Cheng, H.-Y.; Del Valle, I.; Liu, S.; Masiello, C.A.; Silberg, J.J. Charcoal Disrupts Soil Microbial Communication through a Combination of Signal Sorption and Hydrolysis. ACS Omega 2016, 1, 226–233. [Google Scholar] [CrossRef]
- Lu, Y.; Xie, Q.; Tang, L.; Yu, J.; Wang, J.; Yang, Z.; Fan, C.; Zhang, S. The reduction of nitrobenzene by extracellular electron transfer facilitated by Fe-bearing biochar derived from sewage sludge. J. Hazard. Mater. 2021, 403, 123682. [Google Scholar] [CrossRef]
- Yun, S.; Fang, W.; Du, T.; Hu, X.; Huang, X.; Li, X.; Zhang, C.; Lund, P.D. Use of bio-based carbon materials for improving biogas yield and digestate stability. Energy 2018, 164, 898–909. [Google Scholar] [CrossRef]
- Hu, A.; Ye, J.; Ren, G.; Qi, Y.; Chen, Y.; Zhou, S. Metal-Free Semiconductor-Based Bio-Nano Hybrids for Sustainable CO2-to-CH4 Conversion with High Quantum Yield. Angew. Chem. Int. Ed. 2022, 61, e202206508. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, L.; Li, C.; Tian, H.; Ning, J.; Zhang, J.; Tong, Y.W.; Wang, X. Data-Driven Based In-Depth Interpretation and Inverse Design of Anaerobic Digestion for CH4-Rich Biogas Production. ACS ES&T Eng. 2022, 2, 642–652. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, K.; Deng, Y.; Liu, Z.; Feng, Y.; Hu, C.; Wang, Z. Biochar Facilitated Direct Interspecies Electron Transfer in Anaerobic Digestion to Alleviate Antibiotics Inhibition and Enhance Methanogenesis: A Review. Int. J. Environ. Res. Public Health 2023, 20, 2296. https://doi.org/10.3390/ijerph20032296
Zhang K, Deng Y, Liu Z, Feng Y, Hu C, Wang Z. Biochar Facilitated Direct Interspecies Electron Transfer in Anaerobic Digestion to Alleviate Antibiotics Inhibition and Enhance Methanogenesis: A Review. International Journal of Environmental Research and Public Health. 2023; 20(3):2296. https://doi.org/10.3390/ijerph20032296
Chicago/Turabian StyleZhang, Kaoming, Yuepeng Deng, Zhiquan Liu, Yiping Feng, Chun Hu, and Zhu Wang. 2023. "Biochar Facilitated Direct Interspecies Electron Transfer in Anaerobic Digestion to Alleviate Antibiotics Inhibition and Enhance Methanogenesis: A Review" International Journal of Environmental Research and Public Health 20, no. 3: 2296. https://doi.org/10.3390/ijerph20032296
APA StyleZhang, K., Deng, Y., Liu, Z., Feng, Y., Hu, C., & Wang, Z. (2023). Biochar Facilitated Direct Interspecies Electron Transfer in Anaerobic Digestion to Alleviate Antibiotics Inhibition and Enhance Methanogenesis: A Review. International Journal of Environmental Research and Public Health, 20(3), 2296. https://doi.org/10.3390/ijerph20032296








