Global Trends of Lipid Metabolism Research in Epigenetics Field: A Bibliometric Analysis from 2012–2021
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Source and Search Strategy
2.2. Data Collection
2.3. Statistical Methods
3. Results
3.1. Annual Publication Outputs
3.2. Global Landscapes of Epigenetics in Lipid Metabolism
3.3. Major Output Contributors
3.4. Pivotal Journals
3.5. Trending Topics of Keywords
3.6. Analysis of Citations
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Chandel, N.S. Lipid Metabolism. Cold Spring Harb. Perspect. Biol. 2021, 13, a040576. [Google Scholar] [CrossRef] [PubMed]
- Remmerie, A.; Scott, C.L. Macrophages and lipid metabolism. Cell. Immunol. 2018, 330, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Bian, X.; Liu, R.; Meng, Y.; Xing, D.; Xu, D.; Lu, Z. Lipid metabolism and cancer. J. Exp. Med. 2021, 218, e20201606. [Google Scholar] [CrossRef] [PubMed]
- DeBerardinis, R.J.; Keshari, K.R. Metabolic analysis as a driver for discovery, diagnosis, and therapy. Cell 2022, 185, 2678–2689. [Google Scholar] [CrossRef] [PubMed]
- Olzmann, J.A.; Carvalho, P. Dynamics and functions of lipid droplets. Nat. Rev. Mol. Cell Biol. 2019, 20, 137–155. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Zhong, C.; Jin, S.; Zhang, Y.; Li, Y.; Xia, Q.; Cheng, J.; Fan, X.; Lin, H. Global Trends in Research of Lipid Metabolism in T lymphocytes from 1985 to 2022: A Bibliometric Analysis. Front. Immunol. 2022, 13, 884030. [Google Scholar] [CrossRef]
- Deans, C.; Maggert, K.A. What do you mean, “epigenetic”? Genetics 2015, 199, 887–896. [Google Scholar] [CrossRef] [Green Version]
- Poirier, S.; Samami, S.; Mamarbachi, M.; Demers, A.; Chang, T.-Y.; Vance, D.E.; Hatch, G.M.; Mayer, G. The Epigenetic Drug 5-Azacytidine Interferes with Cholesterol and Lipid Metabolism. J. Biol. Chem. 2014, 289, 18736–18751. [Google Scholar] [CrossRef] [Green Version]
- Du, H.; Zhao, Y.; Yin, Z.; Wang, D.W.; Chen, C. The role of miR-320 in glucose and lipid metabolism disorder-associated diseases. Int. J. Biol. Sci. 2021, 17, 402–416. [Google Scholar] [CrossRef]
- Pardo, J.C.; de Porras, V.R.; Gil, J.; Font, A.; Puig-Domingo, M.; Jordà, M. Lipid Metabolism and Epigenetics Crosstalk in Prostate Cancer. Nutrients 2022, 14, 851. [Google Scholar] [CrossRef]
- Cooper, I.D. Bibliometrics basics. J. Med. Libr. Assoc. 2015, 103, 217–218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wallin, J.A. Bibliometric Methods: Pitfalls and Possibilities. Basic Clin. Pharmacol. Toxicol. 2005, 97, 261–275. [Google Scholar] [CrossRef]
- Dominissini, D.; Moshitch-Moshkovitz, S.; Schwartz, S.; Salmon-Divon, M.; Ungar, L.; Osenberg, S.; Cesarkas, K.; Jacob-Hirsch, J.; Amariglio, N.; Kupiec, M.; et al. Topology of the human and mouse m6A RNA methylomes revealed by m6A-seq. Nature 2012, 485, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.S.; Roundtree, I.A.; He, C. Post-transcriptional gene regulation by mRNA modifications. Nat. Rev. Mol. Cell Biol. 2017, 18, 31–42. [Google Scholar] [CrossRef] [Green Version]
- Meyer, K.D.; Saletore, Y.; Zumbo, P.; Elemento, O.; Mason, C.E.; Jaffrey, S.R. Comprehensive Analysis of mRNA Methylation Reveals Enrichment in 3′ UTRs and near Stop Codons. Cell 2012, 149, 1635–1646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sellitto, A.; Pecoraro, G.; Giurato, G.; Nassa, G.; Rizzo, F.; Saggese, P.; Martinez, C.; Scafoglio, C.; Tarallo, R. Regulation of Metabolic Reprogramming by Long Non-Coding RNAs in Cancer. Cancers 2021, 13, 3485. [Google Scholar] [CrossRef]
- Huo, M.; Zhang, J.; Huang, W.; Wang, Y. Interplay Among Metabolism, Epigenetic Modifications, and Gene Expression in Cancer. Front. Cell Dev. Biol. 2021, 9, 793428. [Google Scholar] [CrossRef]
- Yu, X.H.; Deng, W.Y.; Chen, J.J.; Xu, X.D.; Liu, X.X.; Chen, L.; Shi, M.W.; Liu, Q.X.; Tao, M.; Ren, K. LncRNA kcnq1ot1 promotes lipid accumulation and accelerates atherosclerosis via functioning as a ceRNA through the miR-452-3p/HDAC3/ABCA1 axis. Cell Death Dis. 2020, 11, 1043. [Google Scholar] [CrossRef]
- Hu, Y.; Feng, Y.; Zhang, L.; Jia, Y.; Cai, D.; Qian, S.-B.; Du, M.; Zhao, R. GR-mediated FTO transactivation induces lipid accumulation in hepatocytes via demethylation of m6A on lipogenic mRNAs. RNA Biol. 2020, 17, 930–942. [Google Scholar] [CrossRef]
- Zhu, X.; Xia, M.; Gao, X. Update on genetics and epigenetics in metabolic associated fatty liver disease. Ther. Adv. Endocrinol. Metab. 2022, 13, 20420188221132138. [Google Scholar] [CrossRef]
- Hymel, E.; Fisher, K.W.; Farazi, P.A. Differential methylation patterns in lean and obese non-alcoholic steatohepatitis-associated hepatocellular carcinoma. BMC Cancer 2022, 22, 1276. [Google Scholar] [CrossRef] [PubMed]
- Jebari-Benslaiman, S.; Galicia-García, U.; Larrea-Sebal, A.; Olaetxea, J.R.; Alloza, I.; Vandenbroeck, K.; Benito-Vicente, A.; Martín, C. Pathophysiology of Atherosclerosis. Int. J. Mol. Sci. 2022, 23, 3346. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Pelisek, J.; Jin, Z.G. Atherosclerosis Is an Epigenetic Disease. Trends Endocrinol. Metab. 2018, 29, 739–742. [Google Scholar] [CrossRef]
- Zahr, T.; Liu, L.; Chan, M.; Zhou, Q.; Cai, B.; He, Y.; Aaron, N.; Accili, D.; Sun, L.; Qiang, L. PPARgamma (Peroxisome Proliferator-Activated Receptor gamma) Deacetylation Suppresses Aging-Associated Atherosclerosis and Hypercholesterolemia. Arterioscler. Thromb. Vasc. Biol. 2023, 43, 30–44. [Google Scholar] [CrossRef] [PubMed]
- Janani, C.; Kumari, B.R. PPAR gamma gene—A review. Diabetes Metab. Syndr. 2015, 9, 46–50. [Google Scholar] [CrossRef]
- Porcuna, J.; Mínguez-Martínez, J.; Ricote, M. The PPARalpha and PPARgamma Epigenetic Landscape in Cancer and Immune and Metabolic Disorders. Int. J. Mol. Sci. 2021, 22, 10573. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef] [Green Version]
- Esau, C.; Davis, S.; Murray, S.F.; Yu, X.X.; Pandey, S.K.; Pear, M.; Watts, L.; Booten, S.L.; Graham, M.; McKay, R.; et al. miR-122 regulation of lipid metabolism revealed by in vivo antisense targeting. Cell Metab. 2006, 3, 87–98. [Google Scholar] [CrossRef] [Green Version]
- Esau, C.; Kang, X.; Peralta, E.; Hanson, E.; Marcusson, E.G.; Ravichandran, L.V.; Sun, Y.; Koo, S.; Perera, R.J.; Jain, R.; et al. MicroRNA-143 Regulates Adipocyte Differentiation. J. Biol. Chem. 2004, 279, 52361–52365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rayner, K.; Sheedy, F.; Esau, C.C.; Hussain, F.N.; Temel, R.E.; Parathath, S.; van Gils, J.; Rayner, A.J.; Chang, A.N.; Suarez, Y.; et al. Antagonism of miR-33 in mice promotes reverse cholesterol transport and regression of atherosclerosis. J. Clin. Investig. 2011, 121, 2921–2931. [Google Scholar] [CrossRef] [PubMed]
- Castro, R.E.; Ferreira, D.M.; Afonso, M.B.; Borralho, P.M.; Machado, M.V.; Cortez-Pinto, H.; Rodrigues, C.M. miR-34a/SIRT1/p53 is suppressed by ursodeoxycholic acid in the rat liver and activated by disease severity in human non-alcoholic fatty liver disease. J. Hepatol. 2013, 58, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Schiano, C.; Benincasa, G.; Franzese, M.; Della Mura, N.; Pane, K.; Salvatore, M.; Napoli, C. Epigenetic-sensitive pathways in personalized therapy of major cardiovascular diseases. Pharmacol. Ther. 2020, 210, 107514. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Ni, S.; Zhang, H.; Fan, Y.; Xia, L.; Li, N. Silencing SGK1 alleviates osteoarthritis through epigenetic regulation of CREB1 and ABCA1 expression. Life Sci. 2020, 268, 118733. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yao, X.; Ma, M.; Ding, Y.; Zhang, H.; He, X.; Song, Z. Protective Effect of l-Theanine against DSS-Induced Colitis by Regulating the Lipid Metabolism and Reducing Inflammation via the NF-kappaB Signaling Pathway. J. Agric. Food. Chem. 2021, 69, 14192–14203. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Chen, H.; Li, D.; Ma, L. Effects of imbalance of lipid metabolism through NF-KB pathway on atherosclerosis and vascular aging in rats. Cell. Mol. Biol. 2022, 67, 144–150. [Google Scholar] [CrossRef]
- Geethangili, M.; Lin, C.-W.; Mersmann, H.J.; Ding, S.T. Methyl Brevifolincarboxylate Attenuates Free Fatty Acid-Induced Lipid Metabolism and Inflammation in Hepatocytes through AMPK/NF-kappaB Signaling Pathway. Int. J Mol. Sci. 2021, 22, 10062. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Tian, Y.; Zuo, Y.; Zhang, X.; Gao, Y.; Wang, P.; Sun, L.; Zhang, H.; Liang, H. Nicotinamide riboside ameliorates high-fructose-induced lipid metabolism disorder in mice via improving FGF21 resistance in the liver and white adipose tissue. Food Funct. 2022, 13, 12400–12411. [Google Scholar] [CrossRef]
- Zang, Y.; Fan, L.; Chen, J.; Huang, R.; Qin, H. Improvement of Lipid and Glucose Metabolism by Capsiate in Palmitic Acid-Treated HepG2 Cells via Activation of the AMPK/SIRT1 Signaling Pathway. J. Agric. Food Chem. 2016, 66, 6772–6781. [Google Scholar] [CrossRef]
- Okuno, Y.; Fukuhara, A.; Hashimoto, E.; Kobayashi, H.; Kobayashi, S.; Otsuki, M.; Shimomura, I. Oxidative Stress Inhibits Healthy Adipose Expansion Through Suppression of SREBF1-Mediated Lipogenic Pathway. Diabetes 2018, 67, 1113–1127. [Google Scholar] [CrossRef] [Green Version]
- Guo, J.; Ren, W.; Li, A.; Ding, Y.; Guo, W.; Su, D.; Hu, C.; Xu, K.; Chen, H.; Xu, X.; et al. Fat Mass and Obesity-Associated Gene Enhances Oxidative Stress and Lipogenesis in Nonalcoholic Fatty Liver Disease. Dig. Dis. Sci. 2013, 58, 1004–1009. [Google Scholar] [CrossRef]
- Geula, S.; Moshitch-Moshkovitz, S.; Dominissini, D.; Mansour, A.A.; Kol, N.; Salmon-Divon, M.; Hershkovitz, V.; Peer, E.; Mor, N.; Manor, Y.S.; et al. m6A mRNA methylation facilitates resolution of naïve pluripotency toward differentiation. Science 2015, 347, 1002–1006. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Yu, J.; Frazier, K.; Weng, X.; Li, Y.; Cham, C.M.; Dolan, K.; Zhu, X.; Hubert, N.; Tao, Y.; et al. Circadian Clock Regulation of Hepatic Lipid Metabolism by Modulation of m6A mRNA Methylation. Cell Rep. 2018, 25, 1816–1828.e4. [Google Scholar] [CrossRef] [Green Version]
- Xie, W.; Ma, L.L.; Xu, Y.Q.; Wang, B.H.; Li, S.M. METTL3 inhibits hepatic insulin sensitivity via N6-methyladenosine modification of Fasn mRNA and promoting fatty acid metabolism. Biochem. Biophys. Res. Commun. 2019, 518, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Takemoto, S.; Nakano, M.; Fukami, T.; Nakajima, M. m6A modification impacts hepatic drug and lipid metabolism properties by regulating carboxylesterase 2. Biochem. Pharmacol. 2021, 193, 114766. [Google Scholar] [CrossRef]
- Bridges, M.C.; Daulagala, A.C.; Kourtidis, A. LNCcation: lncRNA localization and function. J. Cell Biol. 2021, 220, e202009045. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Wang, J.; He, H.; Huang, Z.; Wu, S.; Chen, C.; Liu, W.; Xie, L.; Tao, Y.; Cong, L.; et al. Close interactions between lncRNAs, lipid metabolism and ferroptosis in cancer. Int. J. Biol. Sci. 2021, 17, 4493–4513. [Google Scholar] [CrossRef]



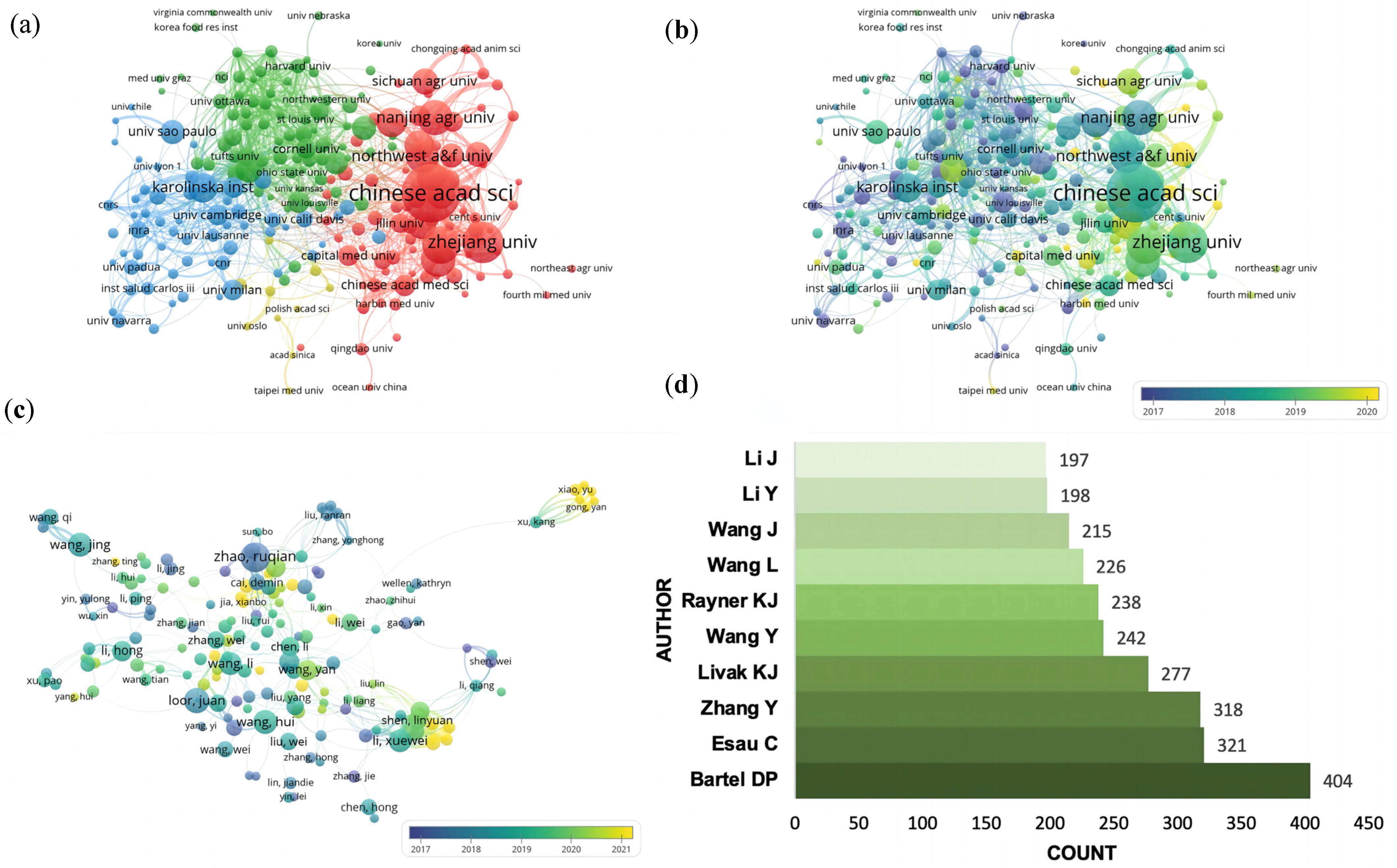
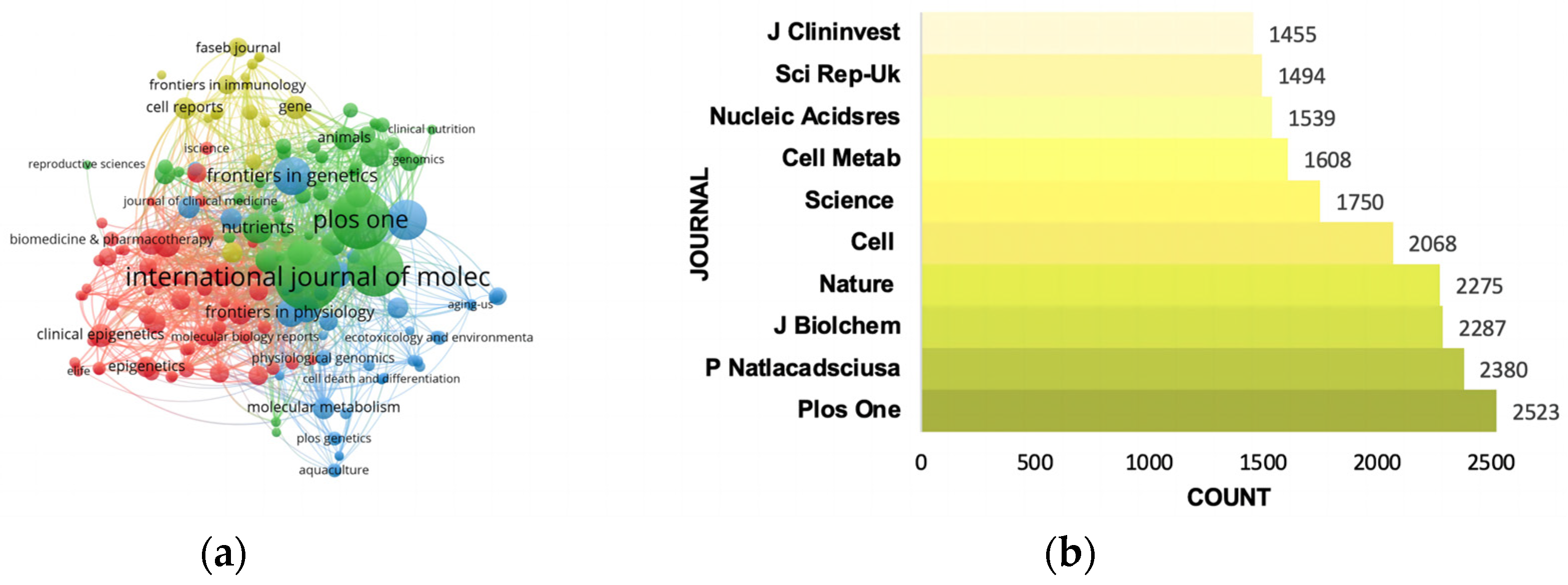


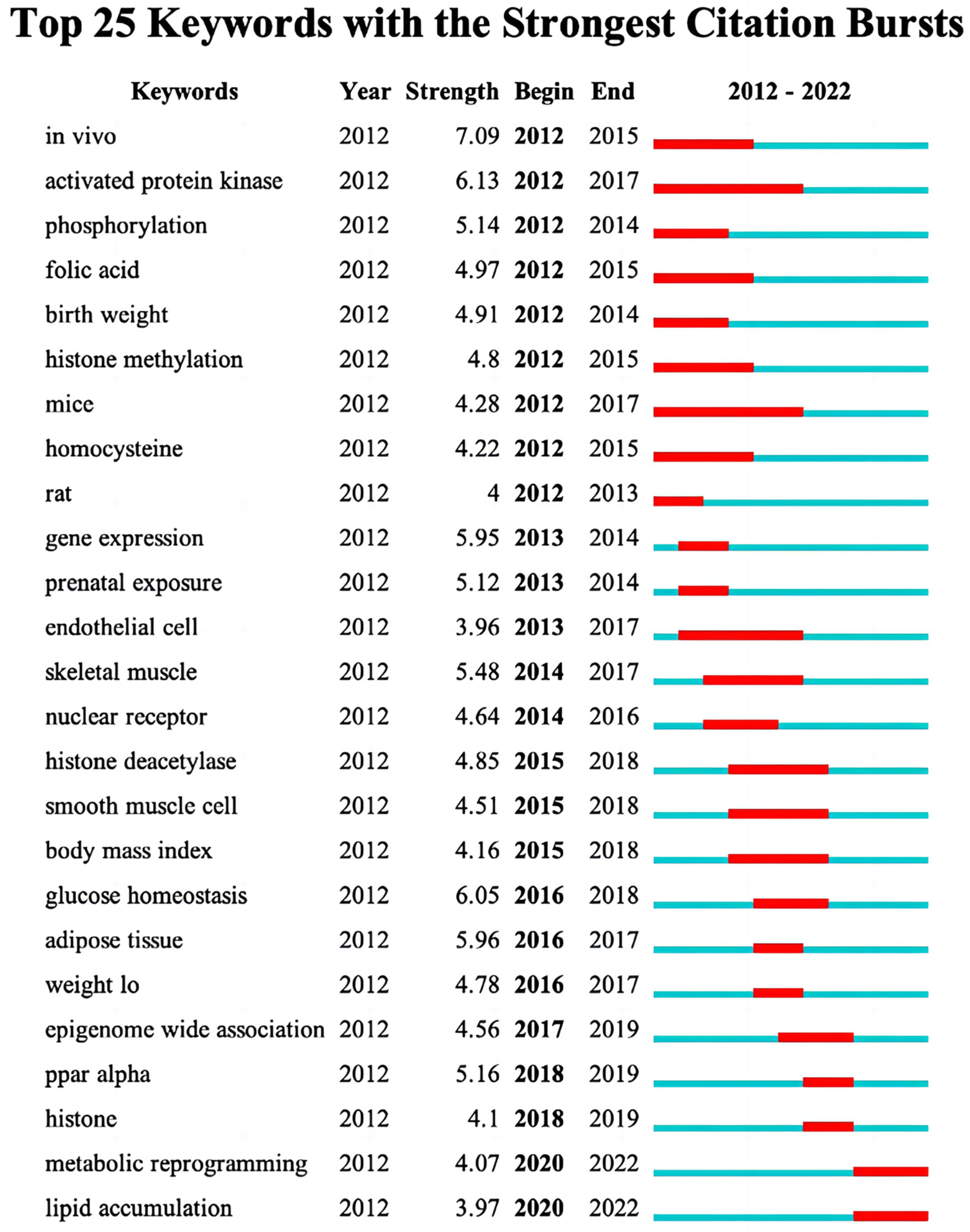
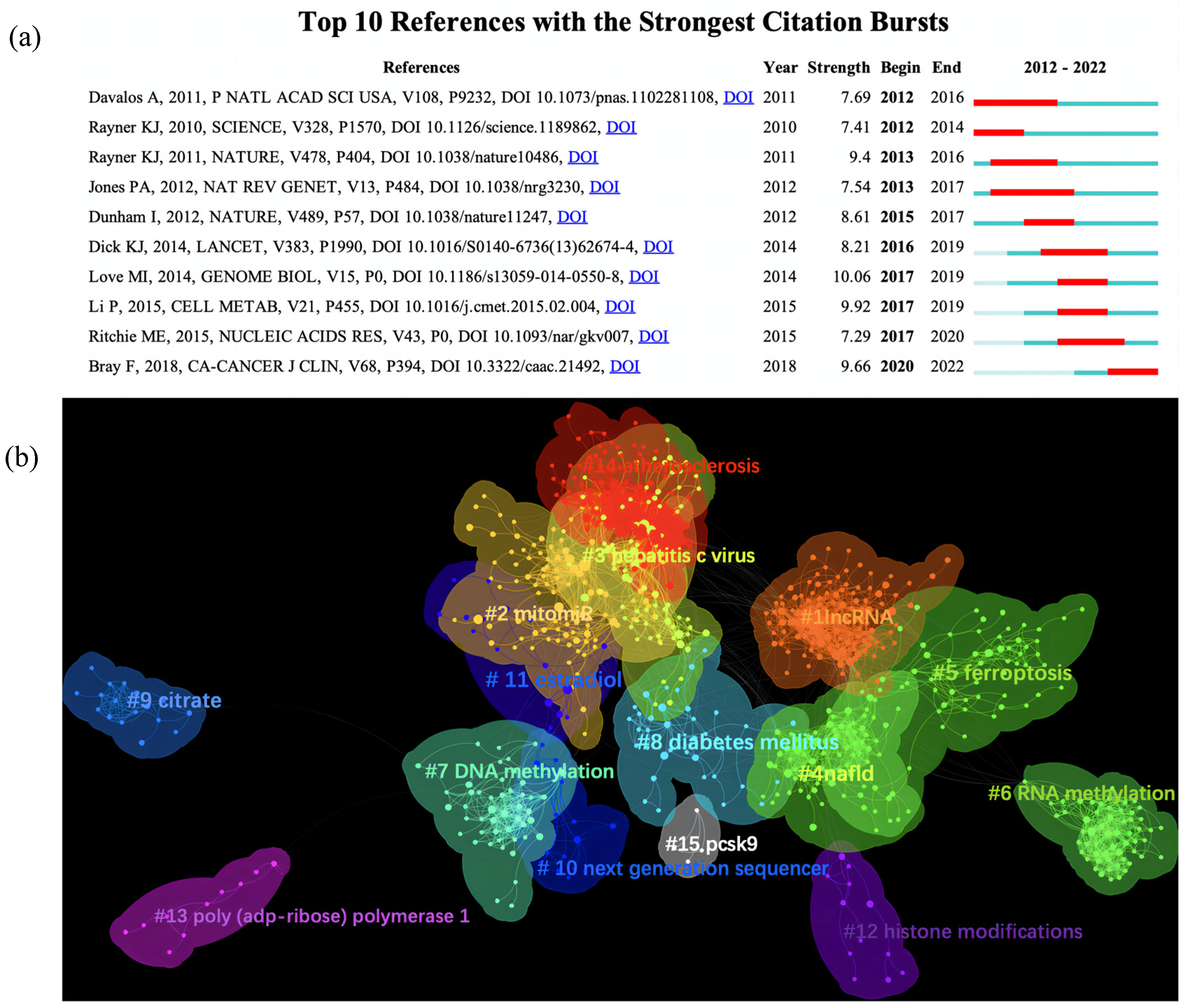
| RANK | Country | Documents | % of Total | Centrality | Half-Life |
|---|---|---|---|---|---|
| 1 | China | 1382 | 26.69 | 0.22 | 7.5 |
| 2 | USA | 1049 | 20.26 | 0.41 | 5.5 |
| 3 | Italy | 206 | 3.98 | 0.09 | 6.5 |
| 4 | Germany | 193 | 3.73 | 0.17 | 6.5 |
| 5 | Spain | 184 | 3.55 | 0.09 | 6.5 |
| 6 | England | 171 | 3.30 | 0.18 | 6.5 |
| 7 | Canada | 169 | 3.26 | 0.12 | 5.5 |
| 8 | France | 159 | 3.07 | 0.09 | 5.5 |
| 9 | Japan | 125 | 2.41 | 0.05 | 6.5 |
| 10 | India | 114 | 2.20 | 0.03 | 6.5 |
| Rank | Institutions | Documents | Citations | Centrality | Half-Life | Total Link Strength |
|---|---|---|---|---|---|---|
| 1 | Chinese Academy of Sciences | 106 | 2987 | 0.16 | 6.5 | 157 |
| 2 | Zhejiang University | 69 | 1597 | 0.06 | 4.5 | 71 |
| 3 | Fudan University | 60 | 1375 | 0.06 | 4.5 | 76 |
| 4 | Nanjing Agricultural University | 56 | 1525 | 0.02 | 4.5 | 30 |
| 5 | Shanghai Jiao Tong University | 55 | 1334 | 0.03 | 3.5 | 68 |
| 6 | Northwest A&F University | 53 | 829 | 0.01 | 3.5 | 28 |
| 7 | Karolinska Institute | 46 | 1405 | 0.16 | 3.5 | 73 |
| 8 | University of Illinois | 46 | 1084 | 0.02 | 4.5 | 41 |
| 9 | Chinese Academy of Agricultural Sciences | 46 | 662 | 0.02 | 2.5 | 27 |
| 10 | University of Chinese Academy of Sciences | 45 | 828 | 0.05 | 6.5 | 81 |
| Rank | Author | Affiliation | H-Index | Documents | Citations | Total Link Strength |
|---|---|---|---|---|---|---|
| 1 | Fernandez-Hernando, Carlos | Yale University | 61 | 27 | 1572 | 79 |
| 2 | Zhao, Ruqian | Tianjin Medical University | 33 | 21 | 443 | 35 |
| 3 | Loor, Juan J | University of Illinois Urbana-Champaign | 58 | 17 | 381 | 26 |
| 4 | Wang, Jing | Dalian Maritime University | 84 | 16 | 185 | 23 |
| 5 | Wang, Hui | Jilin University | 99 | 16 | 160 | 16 |
| 6 | Li, Xuewei | Chinese Academy of Sciences | 30 | 15 | 265 | 84 |
| 7 | Wang, Lili | Qingdao University of Science & Technology | 116 | 15 | 370 | 10 |
| 8 | Zhu, Li | Zhejiang University | 113 | 13 | 239 | 84 |
| 9 | Suarez, Yajaira | Yale University | 51 | 13 | 739 | 56 |
| 10 | Goedeke, Leigh | Yale University | 27 | 13 | 893 | 54 |
| Rank | Journals | Counts | JCR Partitions | Impact Factor (2021) |
|---|---|---|---|---|
| 1 | International Journal of Molecular Sciences | 137 | Q1 | 6.208 |
| 2 | Plos One | 112 | Q2 | 3.752 |
| 3 | Scientific Reports | 102 | Q2 | 4.997 |
| 4 | BMC Genomics | 59 | Q2 | 4.558 |
| 5 | Frontiers In Genetics | 52 | Q2 | 4.772 |
| 6 | Nutrients | 36 | Q1 | 6.706 |
| 7 | Cells | 34 | Q2 | 7.666 |
| 8 | Frontiers In Physiology | 32 | Q1 | 4.755 |
| 9 | Nature Communications | 31 | Q1 | 17.694 |
| 10 | Frontiers In Endocrinology | 29 | Q1 | 6.055 |
| Rank | Title | Journal | First Author | Year |
|---|---|---|---|---|
| 1 | Feedback modulation of cholesterol metabolism by the lipid-responsive non-coding RNA LeXis | Nature | Sallam T | 2016 |
| 2 | m6A RNA methylation promotes XIST-mediated transcriptional repression | Nature | Patil DP | 2016 |
| 3 | Long Noncoding RNA HULC Modulates Abnormal Lipid Metabolism in Hepatoma Cells through an miR-9-Mediated RXRA Signaling Pathway | Cancer Res | Cui M | 2015 |
| 4 | Circadian Clock Regulation of Hepatic Lipid Metabolism by Modulation of m6A mRNA Methylation | Cell Rep | Zhong X | 2018 |
| 5 | Long noncoding RNAs regulate adipogenesis | P Natl Acad Sci USA | Sun L | 2013 |
| 6 | Genome-wide identification of microRNAs regulating cholesterol and triglyceride homeostasis | Nat Med | Wagschal A | 2015 |
| 7 | miR-27b inhibits LDLR and ABCA1 expression but does not influence plasma and hepatic lipid levels in mice | Atherosclerosis | Goedeke L | 2015 |
| 8 | Identification of a novel human long non-coding RNA that regulates hepatic lipid metabolism by inhibiting SREBP-1c | Int J Biol Sci | Li D | 2017 |
| 9 | Long noncoding RNA MALAT1 promotes hepatic steatosis and insulin resistance by increasing nuclear SREBP-1c protein stability | Sci Rep-UK | Yan CF | 2016 |
| 10 | An integrated encyclopedia of DNA elements in the human genome | Nature | Dunham I | 2012 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Huang, Y.; Lu, S.; Yuan, D.; Liu, J. Global Trends of Lipid Metabolism Research in Epigenetics Field: A Bibliometric Analysis from 2012–2021. Int. J. Environ. Res. Public Health 2023, 20, 2382. https://doi.org/10.3390/ijerph20032382
Liu H, Huang Y, Lu S, Yuan D, Liu J. Global Trends of Lipid Metabolism Research in Epigenetics Field: A Bibliometric Analysis from 2012–2021. International Journal of Environmental Research and Public Health. 2023; 20(3):2382. https://doi.org/10.3390/ijerph20032382
Chicago/Turabian StyleLiu, Hanqi, Yanqing Huang, Shanshan Lu, Didi Yuan, and Junwen Liu. 2023. "Global Trends of Lipid Metabolism Research in Epigenetics Field: A Bibliometric Analysis from 2012–2021" International Journal of Environmental Research and Public Health 20, no. 3: 2382. https://doi.org/10.3390/ijerph20032382




