Are Fermented Foods Effective against Inflammatory Diseases?
Abstract
1. Introduction
2. Kombucha
3. Fermented Turmeric
4. Fermented Tea
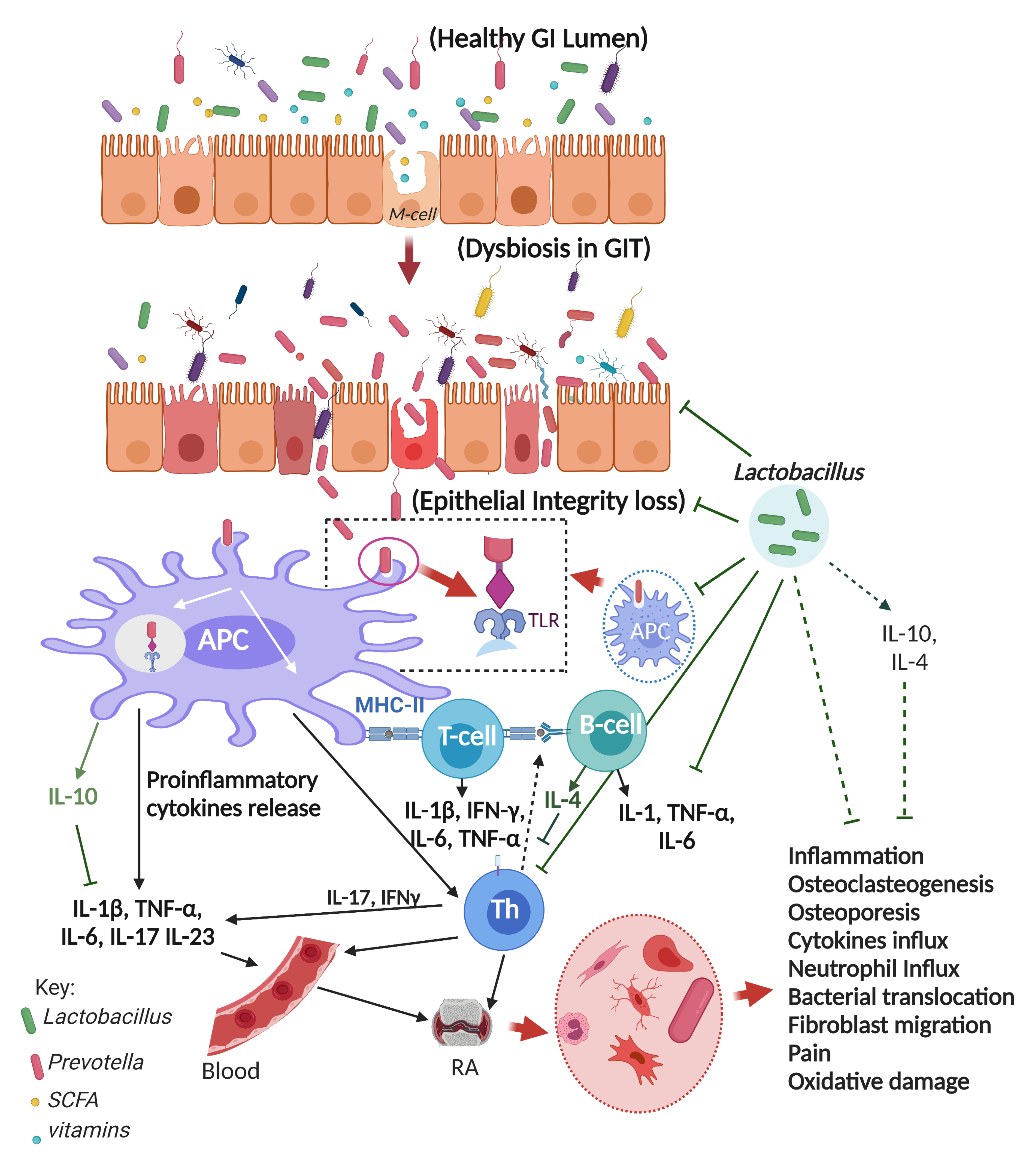
5. Probiotics
6. Kimchi
7. Sauerkraut
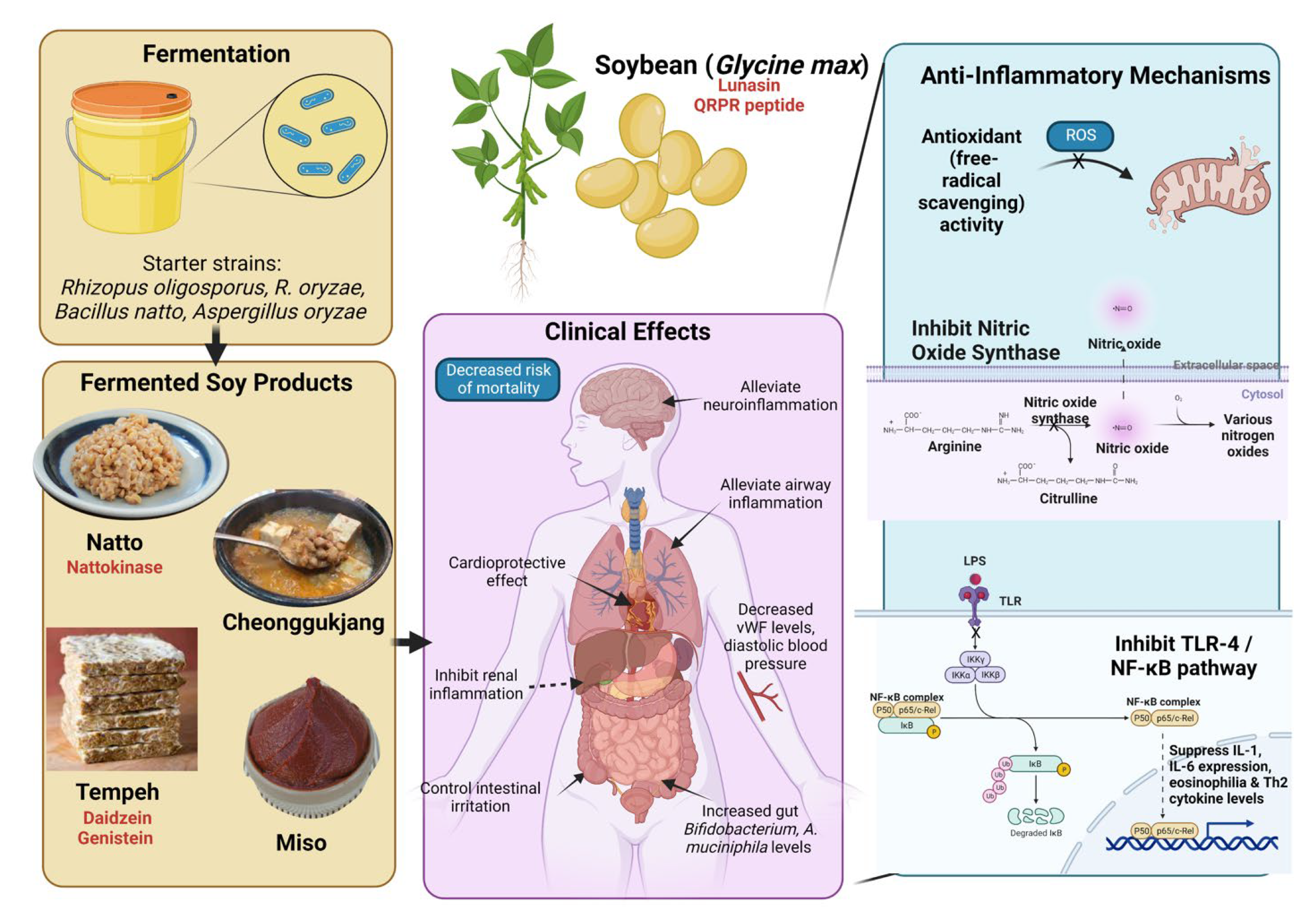
8. Soy-Based Fermented Foods
9. Limitations and Adverse Effects
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hasan, M.N.; Sultan, M.Z.; Mar-E-Um, M. Significance of fermented food in nutrition and food science. J. Sci. Res. 2014, 6, 373–386. [Google Scholar] [CrossRef]
- Moslehi-Jenabian, S.; Lindegaard, L.; Jespersen, L. Beneficial effects of probiotic and food borne yeasts on human health. Nutrients 2010, 2, 449–473. [Google Scholar] [CrossRef]
- Blandino, A.; Al-Aseeri, M.E.; Pandiella, S.S.; Cantero, D.; Webb, C. Cereal-based fermented foods and beverages. Food Res. Int. 2003, 36, 527–543. [Google Scholar] [CrossRef]
- Septembre-Malaterre, A.; Remize, F.; Poucheret, P. Fruits and vegetables, as a source of nutritional compounds and phytochemicals: Changes in bioactive compounds during lactic fermentation. Food Res. Int. 2018, 104, 86–99. [Google Scholar] [CrossRef]
- Varzakas, T.; Zakynthinos, G.; Proestos, C.; Radwanska, M. Fermented vegetables. In Minimally Processed Refrigerated Fruits and Vegetables; Yildiz, F., Wiley, R.C., Eds.; Springer: Boston, MA, USA, 2017; pp. 537–584. [Google Scholar]
- Şanlier, N.; Gökcen, B.B.; Sezgin, A.C. Health benefits of fermented foods. Crit. Rev. Food Sci. Nutr. 2019, 59, 506–527. [Google Scholar] [CrossRef]
- Feng, Y.; Zhang, M.; Mujumdar, A.S.; Gao, Z. Recent research process of fermented plant extract: A review. Trends Food Sci. Technol. 2017, 65, 40–48. [Google Scholar] [CrossRef]
- Shahbazi, R.; Sharifzad, F.; Bagheri, R.; Alsadi, N.; Yasavoli-Sharahi, H.; Matar, C. Anti-inflammatory and immunomodulatory properties of fermented plant foods. Nutrients 2021, 13, 1516. [Google Scholar] [CrossRef] [PubMed]
- Selhub, E.M.; Logan, A.C.; Bested, A.C. Fermented foods, microbiota, and mental health: Ancient practice meets nutritional psychiatry. J. Physiol. Anthropol. 2014, 33, 2. [Google Scholar] [CrossRef]
- Kim, J.; Choi, K.-B.; Park, J.H.; Kim, K.H. Metabolite profile changes and increased antioxidative and anti-inflammatory activities of mixed vegetables after fermentation by Lactobacillus plantarum. PLoS ONE 2019, 14, e0217180. [Google Scholar] [CrossRef]
- Dimidi, E.; Cox, S.R.; Rossi, M.; Whelan, K. Fermented foods: Definitions and characteristics, impact on the gut microbiota and effects on gastrointestinal health and disease. Nutrients 2019, 11, 1806. [Google Scholar] [CrossRef]
- Villarreal-Soto, S.A.; Beaufort, S.; Bouajila, J.; Souchard, J.P.; Taillandier, P. Understanding kombucha tea fermentation: A review. J. Food Sci. 2018, 83, 580–588. [Google Scholar] [CrossRef] [PubMed]
- May, A.; Narayanan, S.; Alcock, J.; Varsani, A.; Maley, C.; Aktipis, A. Kombucha: A novel model system for cooperation and conflict in a complex multi-species microbial ecosystem. PeerJ 2019, 7, e7565. [Google Scholar] [CrossRef] [PubMed]
- Cuamatzin-García, L.; Rodríguez-Rugarcía, P.; El-Kassis, E.G.; Galicia, G.; Meza-Jiménez, M.L.; Baños-Lara, M.D.R.; Zaragoza-Maldonado, D.S.; Pérez-Armendáriz, B. Traditional fermented foods and beverages from around the world and their health benefits. Microorganisms 2022, 10, 1151. [Google Scholar] [CrossRef] [PubMed]
- Diez-Ozaeta, I.; Astiazaran, O.J. Recent advances in kombucha tea: Microbial consortium, chemical parameters, health implications and biocellulose production. Int. J. Food Microbiol. 2022, 377, 109783. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Rutherfurd-Markwick, K.; Zhang, X.X.; Mutukumira, A.N. Isolation and characterisation of dominant acetic acid bacteria and yeast isolated from kombucha samples at point of sale in new zealand. Curr. Res. Food Sci. 2022, 5, 835–844. [Google Scholar] [CrossRef]
- Nyiew, K.Y.; Kwong, P.J.; Yow, Y.Y. An overview of antimicrobial properties of kombucha. Compr. Rev. Food Sci. Food Saf. 2022, 21, 1024–1053. [Google Scholar] [CrossRef]
- Kruk, M.; Trząskowska, M.; Ścibisz, I.; Pokorski, P. Application of the “scoby” and kombucha tea for the production of fermented milk drinks. Microorganisms 2021, 9, 123. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Hashemi, S.A.; Zarei, M.; Gholami, A.; Lai, C.W.; Chiang, W.H.; Omidifar, N.; Bahrani, S.; Mazraedoost, S. Recent progress in chemical composition, production, and pharmaceutical effects of kombucha beverage: A complementary and alternative medicine. Evid.-Based Complement. Altern. Med. 2020, 2020, 4397543. [Google Scholar] [CrossRef]
- Jakubczyk, K.; Gutowska, I.; Antoniewicz, J.; Janda, K. Evaluation of fluoride and selected chemical parameters in kombucha derived from white, green, black and red tea. Biol. Trace Elem. Res. 2021, 199, 3547–3552. [Google Scholar] [CrossRef]
- Pakravan, N.; Mahmoudi, E.; Hashemi, S.A.; Kamali, J.; Hajiaghayi, R.; Rahimzadeh, M.; Mahmoodi, V. Cosmeceutical effect of ethyl acetate fraction of kombucha tea by intradermal administration in the skin of aged mice. J. Cosmet. Derm. 2018, 17, 1216–1224. [Google Scholar] [CrossRef]
- Vázquez-Cabral, B.D.; Larrosa-Pérez, M.; Gallegos-Infante, J.A.; Moreno-Jiménez, M.R.; González-Laredo, R.F.; Rutiaga-Quiñones, J.G.; Gamboa-Gómez, C.I.; Rocha-Guzmán, N.E. Oak kombucha protects against oxidative stress and inflammatory processes. Chem.-Biol. Interact. 2017, 272, 1–9. [Google Scholar] [CrossRef]
- Haghmorad, D.; Yazdanpanah, E.; Sadighimoghaddam, B.; Yousefi, B.; Sahafi, P.; Ghorbani, N.; Rashidy-Pour, A.; Kokhaei, P. Kombucha ameliorates experimental autoimmune encephalomyelitis through activation of treg and th2 cells. Acta Neurol. Belg. 2021, 121, 1685–1692. [Google Scholar] [CrossRef]
- Wang, P.; Feng, Z.; Sang, X.; Chen, W.; Zhang, X.; Xiao, J.; Chen, Y.; Chen, Q.; Yang, M.; Su, J. Kombucha ameliorates lps-induced sepsis in a mouse model. Food Funct. 2021, 12, 10263–10280. [Google Scholar] [CrossRef] [PubMed]
- Yong, C.C.; Yoon, Y.; Yoo, H.S.; Oh, S. Effect of Lactobacillus fermentation on the anti-inflammatory potential of turmeric. J. Microbiol. Biotechnol. 2019, 29, 1561–1569. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Yue, Y.; Zheng, X.; Zhang, K.; Chen, S.; Du, Z. Curcumin, inflammation, and chronic diseases: How are they linked? Molecules 2015, 20, 9183–9213. [Google Scholar] [CrossRef] [PubMed]
- Daily, J.W.; Yang, M.; Park, S. Efficacy of turmeric extracts and curcumin for alleviating the symptoms of joint arthritis: A systematic review and meta-analysis of randomized clinical trials. J. Med. Food 2016, 19, 717–729. [Google Scholar] [CrossRef] [PubMed]
- Paul, A.K.; Jahan, R.; Paul, A.; Mahboob, T.; Bondhon, T.A.; Jannat, K.; Hasan, A.; Nissapatorn, V.; Wilairatana, P.; De Lourdes Pereira, M.; et al. The role of medicinal and aromatic plants against obesity and arthritis: A review. Nutrients 2022, 14, 985. [Google Scholar] [CrossRef] [PubMed]
- Ishrat, T.; Hoda, M.N.; Khan, M.B.; Yousuf, S.; Ahmad, M.; Khan, M.M.; Ahmad, A.; Islam, F. Amelioration of cognitive deficits and neurodegeneration by curcumin in rat model of sporadic dementia of Alzheimer’s type (sdat). Eur. Neuropsychopharmacol. 2009, 19, 636–647. [Google Scholar] [CrossRef]
- Ma, Q.L.; Yang, F.; Rosario, E.R.; Ubeda, O.J.; Beech, W.; Gant, D.J.; Chen, P.P.; Hudspeth, B.; Chen, C.; Zhao, Y.; et al. Beta-amyloid oligomers induce phosphorylation of tau and inactivation of insulin receptor substrate via c-jun n-terminal kinase signaling: Suppression by omega-3 fatty acids and curcumin. J. Neurosci. 2009, 29, 9078–9089. [Google Scholar] [CrossRef]
- Arafa, H.M. Curcumin attenuates diet-induced hypercholesterolemia in rats. Med. Sci. Monit. 2005, 11, Br228–Br234. [Google Scholar]
- Jin, S.; Hong, J.H.; Jung, S.H.; Cho, K.H. Turmeric and laurel aqueous extracts exhibit in vitro anti-atherosclerotic activity and in vivo hypolipidemic effects in a zebrafish model. J. Med. Food 2011, 14, 247–256. [Google Scholar] [CrossRef]
- Polasa, K.; Raghuram, T.C.; Krishna, T.P.; Krishnaswamy, K. Effect of turmeric on urinary mutagens in smokers. Mutagenesis 1992, 7, 107–109. [Google Scholar] [CrossRef] [PubMed]
- Boonhok, R.; Sangkanu, S.; Phumjan, S.; Jongboonjua, R.; Sangnopparat, N.; Kwankaew, P.; Tedasen, A.; Lim, C.L.; Pereira, M.L.; Rahmatullah, M.; et al. Curcumin effect on Acanthamoeba triangularis encystation under nutrient starvation. PeerJ 2022, 10, e13657. [Google Scholar] [CrossRef]
- Marcu, K.B.; Otero, M.; Olivotto, E.; Borzi, R.M.; Goldring, M.B. Nf-kappab signaling: Multiple angles to target oa. Curr. Drug Targets 2010, 11, 599–613. [Google Scholar] [CrossRef] [PubMed]
- Suokas, A.K.; Sagar, D.R.; Mapp, P.I.; Chapman, V.; Walsh, D.A. Design, study quality and evidence of analgesic efficacy in studies of drugs in models of oa pain: A systematic review and a meta-analysis. Osteoarthr. Cartil. 2014, 22, 1207–1223. [Google Scholar] [CrossRef]
- Schnitzer, T.J. Update on guidelines for the treatment of chronic musculoskeletal pain. Clin. Rheumatol. 2006, 25 (Suppl. S1), S22–S29. [Google Scholar] [CrossRef] [PubMed]
- Chan, M.M. Inhibition of tumor necrosis factor by curcumin, a phytochemical. Biochem. Pharm. 1995, 49, 1551–1556. [Google Scholar] [CrossRef] [PubMed]
- Chan, M.M.; Huang, H.I.; Fenton, M.R.; Fong, D. In vivo inhibition of nitric oxide synthase gene expression by curcumin, a cancer preventive natural product with anti-inflammatory properties. Biochem. Pharm. 1998, 55, 1955–1962. [Google Scholar] [CrossRef] [PubMed]
- Rinkunaite, I.; Simoliunas, E.; Alksne, M.; Dapkute, D.; Bukelskiene, V. Anti-inflammatory effect of different curcumin preparations on adjuvant-induced arthritis in rats. BMC Complement. Med. Ther. 2021, 21, 39. [Google Scholar] [CrossRef]
- Atsumi, T.; Fujisawa, S.; Tonosaki, K. Relationship between intracellular ros production and membrane mobility in curcumin- and tetrahydrocurcumin-treated human gingival fibroblasts and human submandibular gland carcinoma cells. Oral Dis. 2005, 11, 236–242. [Google Scholar] [CrossRef]
- McNally, S.J.; Harrison, E.M.; Ross, J.A.; Garden, O.J.; Wigmore, S.J. Curcumin induces heme oxygenase 1 through generation of reactive oxygen species, p38 activation and phosphatase inhibition. Int. J. Mol. Med. 2007, 19, 165–172. [Google Scholar] [CrossRef]
- Aljutaily, T. Evaluating the nutritional and immune potentiating characteristics of unfermented and fermented turmeric camel milk in cyclophosphamide-induced immunosuppression in rats. Antioxid 2022, 11, 792. [Google Scholar] [CrossRef]
- Kim, S.-B.; Kang, B.-H.; Kwon, H.-S.; Kang, J.-H. Anti-inflammatory and antiallergic activity of fermented turmeric by Lactobacillus johnsonii idcc 9203. Microbiol. Biotechnol. Lett. 2011, 39, 266–273. [Google Scholar]
- Kang, J.-K.; Kang, H.-J.; Seo, J.-H.; Kim, S.-O.; Choi, J.-H.; Cho, D.-Y.; Park, C.-G.; Lee, H.-Y. Effects of fermented turmeric (Curcuma longa) by Bacillus natto supplementation on liver function and serum lipid parameters in mice. J. Korean Soc. Food Sci. Nutr. 2009, 38, 430–435. [Google Scholar] [CrossRef]
- Xu, J.; Wang, M.; Zhao, J.; Wang, Y.-H.; Tang, Q.; Khan, I.A. Yellow tea (Camellia sinensis l.), a promising chinese tea: Processing, chemical constituents and health benefits. Food Res. Int. 2018, 107, 567–577. [Google Scholar] [CrossRef]
- Tan, Y.M.; Wang, Y.P.; Ge, Y.Y.; Ren, X.X.; Wang, Y.C.; Liu, Z.Y. Isolation and molecular identification of Aspergillus cristatus in fermented “fuzhuan” brick tea from guizhou province. Mycosystema 2017, 36, 154–163. [Google Scholar] [CrossRef]
- Zhu, M.Z.; Li, N.; Zhou, F.; Ouyang, J.; Lu, D.M.; Xu, W.; Li, J.; Lin, H.Y.; Zhang, Z.; Xiao, J.B.; et al. Microbial bioconversion of the chemical components in dark tea. Food Chem. 2020, 312, 126043. [Google Scholar] [CrossRef]
- Chen, G.; Xie, M.; Wan, P.; Chen, D.; Ye, H.; Chen, L.; Zeng, X.; Liu, Z. Digestion under saliva, simulated gastric and small intestinal conditions and fermentation in vitro by human intestinal microbiota of polysaccharides from fuzhuan brick tea. Food Chem. 2018, 244, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Xie, Q.; Kong, P.; Liu, L.; Sun, S.; Xiong, B.; Huang, B.; Yan, L.; Sheng, J.; Xiang, H. Polyphenol-and caffeine-rich postfermented pu-erh tea improves diet-induced metabolic syndrome by remodeling intestinal homeostasis in mice. Infect. Immun. 2018, 86, e00601–e00617. [Google Scholar] [CrossRef] [PubMed]
- Ling, T.-J.; Wan, X.-C.; Ling, W.-W.; Zhang, Z.-Z.; Xia, T.; Li, D.-X.; Hou, R.-Y. New triterpenoids and other constituents from a special microbial-fermented tea—Fuzhuan brick tea. J. Agric. Food Chem. 2010, 58, 4945–4950. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.-M.; Du, H.-X.; Li, L.-X.; An, M.-Q.; Zhang, Z.-Z.; Wan, X.-C.; Bao, G.-H.; Zhang, L.; Ling, T.-J. Fuzhuanins a and b: The b-ring fission lactones of flavan-3-ols from fuzhuan brick-tea. J. Agric. Food Chem. 2013, 61, 6982–6990. [Google Scholar] [CrossRef]
- Zhu, Y.-F.; Chen, J.-J.; Ji, X.-M.; Hu, X.; Ling, T.-J.; Zhang, Z.-Z.; Bao, G.-H.; Wan, X.-C. Changes of major tea polyphenols and production of four new b-ring fission metabolites of catechins from post-fermented jing-wei fu brick tea. Food Chem. 2015, 170, 110–117. [Google Scholar] [CrossRef]
- Li, Q.; Chai, S.; Li, Y.; Huang, J.; Luo, Y.; Xiao, L.; Liu, Z. Biochemical components associated with microbial community shift during the pile-fermentation of primary dark tea. Front. Microbiol. 2018, 9, 1509. [Google Scholar] [CrossRef]
- Li, Z.; Feng, C.; Luo, X.; Yao, H.; Zhang, D.; Zhang, T. Revealing the influence of microbiota on the quality of pu-erh tea during fermentation process by shotgun metagenomic and metabolomic analysis. Food Microbiol. 2018, 76, 405–415. [Google Scholar] [CrossRef] [PubMed]
- Lyu, C.; Chen, C.; Ge, F.; Liu, D.; Zhao, S.; Chen, D. A preliminary metagenomic study of puer tea during pile fermentation. J. Sci. Food Agric. 2013, 93, 3165–3174. [Google Scholar] [CrossRef] [PubMed]
- Pou, K.R.J. Fermentation: The key step in the processing of black tea. J. Biosyst. Eng. 2016, 41, 85–92. [Google Scholar] [CrossRef]
- Bhuyan, L.P.; Sabhapondit, S.; Baruah, B.D.; Bordoloi, C.; Gogoi, R.; Bhattacharyya, P. Polyphenolic compounds and antioxidant activity of ctc black tea of north-east india. Food Chem. 2013, 141, 3744–3751. [Google Scholar] [CrossRef]
- Łuczaj, W.; Skrzydlewska, E. Antioxidative properties of black tea. Prev. Med. 2005, 40, 910–918. [Google Scholar] [CrossRef]
- Halder, J.; Bhaduri, A.N. Protective role of black tea against oxidative damage of human red blood cells. Biochem. Biophys. Res. Commun. 1998, 244, 903–907. [Google Scholar] [CrossRef]
- Chan, E.W.C.; Lim, Y.Y.; Chew, Y.L. Antioxidant activity of Camellia sinensis leaves and tea from a lowland plantation in malaysia. Food Chem. 2007, 102, 1214–1222. [Google Scholar] [CrossRef]
- Yang, C.S.; Wang, Z.Y. Tea and cancer. J. Natl. Cancer Inst. 1993, 85, 1038–1049. [Google Scholar] [CrossRef] [PubMed]
- Mikuls, T.R.; Cerhan, J.R.; Criswell, L.A.; Merlino, L.; Mudano, A.S.; Burma, M.; Folsom, A.R.; Saag, K.G. Coffee, tea, and caffeine consumption and risk of rheumatoid arthritis: Results from the iowa women’s health study. Arthritis Rheum. 2002, 46, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Comstock, G.W.; Burke, A.E.; Hoffman, S.C.; Helzlsouer, K.J.; Bendich, A.; Masi, A.T.; Norkus, E.P.; Malamet, R.L.; Gershwin, M.E. Serum concentrations of alpha tocopherol, beta carotene, and retinol preceding the diagnosis of rheumatoid arthritis and systemic lupus erythematosus. Ann. Rheum. Dis. 1997, 56, 323–325. [Google Scholar] [CrossRef] [PubMed]
- Heliövaara, M.; Knekt, P.; Aho, K.; Aaran, R.K.; Alfthan, G.; Aromaa, A. Serum antioxidants and risk of rheumatoid arthritis. Ann. Rheum. Dis. 1994, 53, 51–53. [Google Scholar] [CrossRef]
- Holzapfel, W.H.; Haberer, P.; Geisen, R.; Björkroth, J.; Schillinger, U. Taxonomy and important features of probiotic microorganisms in food and nutrition. Am. J. Clin. Nutr. 2001, 73, 365s–373s. [Google Scholar] [CrossRef]
- Mombelli, B.; Gismondo, M.R. The use of probiotics in medical practice. Int. J. Antimicrob. Agents 2000, 16, 531–536. [Google Scholar] [CrossRef]
- Cichońska, P.; Ziarno, M. Legumes and legume-based beverages fermented with lactic acid bacteria as a potential carrier of probiotics and prebiotics. Microorganisms 2022, 10, 91. [Google Scholar] [CrossRef]
- Ranadheera, C.S.; Vidanarachchi, J.K.; Rocha, R.S.; Cruz, A.G.; Ajlouni, S. Probiotic delivery through fermentation: Dairy vs. Non-dairy beverages. Fermentation 2017, 3, 67. [Google Scholar] [CrossRef]
- Ayivi, R.D.; Gyawali, R.; Krastanov, A.; Aljaloud, S.O.; Worku, M.; Tahergorabi, R.; Silva, R.C.D.; Ibrahim, S.A. Lactic acid bacteria: Food safety and human health applications. Dairy 2020, 1, 202–232. [Google Scholar] [CrossRef]
- Pasolli, E.; De Filippis, F.; Mauriello, I.E.; Cumbo, F.; Walsh, A.M.; Leech, J.; Cotter, P.D.; Segata, N.; Ercolini, D. Large-scale genome-wide analysis links lactic acid bacteria from food with the gut microbiome. Nat. Commun. 2020, 11, 2610. [Google Scholar] [CrossRef]
- Möller, B.; Kollert, F.; Sculean, A.; Villiger, P.M. Infectious triggers in periodontitis and the gut in rheumatoid arthritis (ra): A complex story about association and causality. Front Immunol. 2020, 11, 1108. [Google Scholar] [CrossRef] [PubMed]
- Paul, A.K.; Paul, A.; Jahan, R.; Jannat, K.; Bondhon, T.A.; Hasan, A.; Nissapatorn, V.; Pereira, M.L.; Wilairatana, P.; Rahmatullah, M. Probiotics and amelioration of rheumatoid arthritis: Significant roles of Lactobacillus casei and Lactobacillus acidophilus. Microorganisms 2021, 9, 1070. [Google Scholar] [CrossRef] [PubMed]
- Amdekar, S.; Singh, V.; Singh, R.; Sharma, P.; Keshav, P.; Kumar, A. Lactobacillus casei reduces the inflammatory joint damage associated with collagen-induced arthritis (cia) by reducing the pro-inflammatory cytokines: Lactobacillus casei: Cox-2 inhibitor. J. Clin. Immunol. 2011, 31, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Paul, A.K.; Hossain, M.K.; Mahboob, T.; Nissapatorn, V.; Wilairatana, P.; Jahan, R.; Jannat, K.; Bondhon, T.A.; Hasan, A.; De Lourdes Pereira, M.; et al. Does oxidative stress management help alleviation of COVID-19 symptoms in patients experiencing diabetes? Nutrients 2022, 14, 321. [Google Scholar] [CrossRef] [PubMed]
- Paul, A.K.; Paul, A.; Jannat, K.; Afrose, S.; Bondhon, T.A.; Hasan, A.; Jahan, R.; Rahmatullah, R. The role of Lactobacillus probiotics in dysentery. EC Gastroenterol. Dig. Syst. 2021, 8, 78–85. [Google Scholar]
- Van Vollenhoven, R.F. Sex differences in rheumatoid arthritis: More than meets the eye. BMC Med. 2009, 7, 12. [Google Scholar] [CrossRef]
- Sapir-Koren, R.; Livshits, G. Postmenopausal osteoporosis in rheumatoid arthritis: The estrogen deficiency-immune mechanisms link. Bone 2017, 103, 102–115. [Google Scholar] [CrossRef]
- Tit, D.M.; Bungau, S.; Iovan, C.; Nistor Cseppento, D.C.; Endres, L.; Sava, C.; Sabau, A.M.; Furau, G.; Furau, C. Effects of the hormone replacement therapy and of soy isoflavones on bone resorption in postmenopause. J. Clin. Med. 2018, 7, 297. [Google Scholar] [CrossRef]
- Ţiţ, D.M.; Pallag, A.; Iovan, C.; Furău, G.; Furău, C.; Bungău, S. Somatic-vegetative symptoms evolution in postmenopausal women treated with phytoestrogens and hormone replacement therapy. Iran J. Public Health 2017, 46, 1528–1534. [Google Scholar]
- Fert-Bober, J.; Darrah, E.; Andrade, F. Insights into the study and origin of the citrullinome in rheumatoid arthritis. Immunol. Rev. 2020, 294, 133–147. [Google Scholar] [CrossRef] [PubMed]
- Haber, A.L.; Biton, M.; Rogel, N.; Herbst, R.H.; Shekhar, K.; Smillie, C.; Burgin, G.; Delorey, T.M.; Howitt, M.R.; Katz, Y.; et al. A single-cell survey of the small intestinal epithelium. Nature 2017, 551, 333–339. [Google Scholar] [CrossRef]
- Bungau, S.G.; Behl, T.; Singh, A.; Sehgal, A.; Singh, S.; Chigurupati, S.; Vijayabalan, S.; Das, S.; Palanimuthu, V.R. Targeting probiotics in rheumatoid arthritis. Nutrients 2021, 13, 3376. [Google Scholar] [CrossRef]
- Lee, S.H.; Whon, T.W.; Roh, S.W.; Jeon, C.O. Unraveling microbial fermentation features in kimchi: From classical to meta-omics approaches. Appl. Microbiol. Biotechnol. 2020, 104, 7731–7744. [Google Scholar] [CrossRef] [PubMed]
- Park, K.Y.; Kim, H.Y.; Jeong, J.K. Chapter 20-kimchi and its health benefits. In Fermented Foods in Health and Disease Prevention; Frias, J., Martinez-Villaluenga, C., Peñas, E., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 477–502. [Google Scholar]
- Patra, J.K.; Das, G.; Paramithiotis, S.; Shin, H.-S. Kimchi and other widely consumed traditional fermented foods of korea: A review. Front. Microbiol. 2016, 7, 1493. [Google Scholar] [CrossRef]
- Park, K.Y.J.; Jeon, J.K.; Lee, Y.E.; Daily, J.M., III. Health benefits of kimchi (Korean fermented vegetables) as a probiotic food. J. Med. Food 2014, 17, 6–20. [Google Scholar] [CrossRef]
- Nout, M.J.R. Fermented foods and food safety. Food Res. Int. 1994, 27, 291–298. [Google Scholar] [CrossRef]
- Oliviero, T.; Verkerk, R.; Dekker, M. Isothiocyanates from brassica vegetables—Effects of processing, cooking, mastication, and digestion. Mol. Nutr. Food Res. 2018, 62, 1701069. [Google Scholar] [CrossRef]
- Kim, B.K.; Choi, J.M.; Kang, S.A.; Park, K.Y.; Cho, E.J. Antioxidative effects of kimchi under different fermentation stage on radical-induced oxidative stress. Nutr. Res. Pract. 2014, 8, 638–643. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.W.; Choi, I.W.; Jo, G.H.; Kim, G.Y.; Kim, J.; Suh, H.; Ryu, C.H.; Kim, W.J.; Park, K.Y.; Choi, Y.H. Anti-inflammatory effects of 3-(4’-hydroxyl-3’,5’-dimethoxyphenyl)propionic acid, an active component of Korean cabbage kimchi, in lipopolysaccharide-stimulated bv2 microglia. J. Med. Food 2015, 18, 677–684. [Google Scholar] [CrossRef]
- Rahman, M.S.; Alam, M.B.; Kim, Y.K.; Madina, M.H.; Fliss, I.; Lee, S.H.; Yoo, J.C. Activation of nrf2/ho-1 by peptide yd1 attenuates inflammatory symptoms through suppression of tlr4/myyd88/nf-κb signaling cascade. Int. J. Mol. Sci. 2021, 22, 5161. [Google Scholar] [CrossRef]
- Kwon, M.-S.; Lim, S.K.; Jang, J.-Y.; Lee, J.; Park, H.K.; Kim, N.; Yun, M.; Shin, M.-Y.; Jo, H.E.; Oh, Y.J.; et al. Lactobacillus sakei wikim 30 ameliorates atopic dermatitis-like skin lesions by inducing regulatory t cells and altering gut microbiota structure in mice. Front. Immunol. 2018, 9, 1905. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Joe, I.; Rhee, P.D.; Jeong, C.S.; Jeong, G. A lactic acid bacterium isolated from kimchi ameliorates intestinal inflammation in dss-induced colitis. J. Microbiol. 2017, 55, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Youn, H.S.; Kim, J.H.; Lee, J.S.; Yoon, Y.Y.; Choi, S.J.; Lee, J.Y.; Kim, W.; Hwang, K.W. Lactobacillus plantarum reduces low-grade inflammation and glucose levels in a mouse model of chronic stress and diabetes. Infect. Immun. 2021, 89, e0061520. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Sekhon, S.S.; Kim, H.C.; Won, K.; Ahn, J.-Y.; Lee, K.; Kim, Y.-H. Anti-inflammatory effect of lactic acid bacteria isolated from kimchi on acid-induced acute colitis in model mice. Toxicol. Environ. Health Sci. 2017, 9, 279–283. [Google Scholar] [CrossRef]
- Park, S.S.; Lim, S.K.; Lee, J.; Park, H.K.; Kwon, M.S.; Yun, M.; Kim, N.; Oh, Y.J.; Choi, H.J. Latilactobacillus sakei wikim31 decelerates weight gain in high-fat diet-induced obese mice by modulating lipid metabolism and suppressing inflammation. J. Microbiol. Biotechnol. 2021, 31, 1568–1575. [Google Scholar] [CrossRef] [PubMed]
- Seo, H.; Seong, H.; Kim, G.Y.; Jo, Y.M.; Cheon, S.W.; Song, Y.; Ryu, B.H.; Kang, H.; Han, N.S. Development of anti-inflammatory probiotic Limosilactobacillus reuteri efel6901 as kimchi starter: In vitro and in vivo evidence. Front. Microbiol. 2021, 12, 760476. [Google Scholar] [CrossRef] [PubMed]
- Han, K.J.; Lee, J.E.; Lee, N.K.; Paik, H.D. Antioxidant and anti-inflammatory effect of probiotic Lactobacillus plantarum ku15149 derived from Korean homemade diced-radish kimchi. J. Microbiol. Biotechnol. 2020, 30, 591–598. [Google Scholar] [CrossRef]
- Peñas, E.; Martinez-Villaluenga, C.; Frias, J. Chapter 24-sauerkraut: Production, composition, and health benefits. In Fermented Foods in Health and Disease Prevention; Frias, J., Martinez-Villaluenga, C., Peñas, E., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 557–576. [Google Scholar]
- Raak, C.; Ostermann, T.; Boehm, K.; Molsberger, F. Regular consumption of sauerkraut and its effect on human health: A bibliometric analysis. Glob. Adv. Health Med. 2014, 3, 12–18. [Google Scholar] [CrossRef]
- Nielsen, E.S.; Garnås, E.; Jensen, K.J.; Hansen, L.H.; Olsen, P.S.; Ritz, C.; Krych, L.; Nielsen, D.S. Lacto-fermented sauerkraut improves symptoms in ibs patients independent of product pasteurisation–a pilot study. Food Funct. 2018, 9, 5323–5335. [Google Scholar] [CrossRef]
- Xu, X.; Qiao, Y.; Peng, Q.; Shi, B.; Dia, V.P. Antioxidant and immunomodulatory properties of partially purified exopolysaccharide from Lactobacillus casei isolated from Chinese northeast sauerkraut. Immunol. Investig. 2022, 51, 748–765. [Google Scholar] [CrossRef]
- Zhou, Q.; Zang, S.; Zhao, Z.; Li, X. Dynamic changes of bacterial communities and nitrite character during northeastern chinese sauerkraut fermentation. Food Sci. Biotechnol. 2018, 27, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Touret, T.; Oliveira, M.; Semedo-Lemsaddek, T. Putative probiotic lactic acid bacteria isolated from sauerkraut fermentations. PLoS ONE 2018, 13, e0203501. [Google Scholar] [CrossRef] [PubMed]
- Zabat, M.A.; Sano, W.H.; Wurster, J.I.; Cabral, D.J.; Belenky, P. Microbial community analysis of sauerkraut fermentation reveals a stable and rapidly established community. Foods 2018, 7, 77. [Google Scholar] [CrossRef]
- Zubaidah, E.; Susanti, I.; Yuwono, S.S.; Rahayu, A.P.; Srianta, I.; Tewfik, I. The combined impact of sauerkraut with leuconostoc mesenteroides to enhance immunomodulatory activity in Escherichia coli-infected mice. Eur. Food Res. Technol. 2020, 246, 1889–1893. [Google Scholar] [CrossRef]
- Du, R.; Ge, J.; Zhao, D.; Sun, J.; Ping, W.; Song, G. Bacterial diversity and community structure during fermentation of Chinese sauerkraut with Lactobacillus casei 11mz-5-1 by illumina miseq sequencing. Lett. Appl. Microbiol. 2018, 66, 55–62. [Google Scholar] [CrossRef]
- Huang, L.; Cui, K.; Mao, W.; Du, Y.; Yao, N.; Li, Z.; Zhao, H.; Ma, W. Weissella cibaria attenuated lps-induced dysfunction of intestinal epithelial barrier in a caco-2 cell monolayer model. Front. Microbiol. 2020, 11, 2039. [Google Scholar] [CrossRef]
- Gille, D.; Schmid, A.; Walther, B.; Vergères, G. Fermented food and non-communicable chronic diseases: A review. Nutrients 2018, 10, 448. [Google Scholar] [CrossRef]
- Kim, I.S.; Yang, W.S.; Kim, C.H. Beneficial effects of soybean-derived bioactive peptides. Int. J. Mol. Sci. 2021, 22, 8570. [Google Scholar] [CrossRef]
- Hogervorst, E.; Kassam, S.; Kridawati, A.; Soni, M.; Xin, X.; Shifu, X.; Rahardjo, T.B. Nutrition research in cognitive impairment/dementia, with a focus on soya and folate. Proc. Nutr. Soc. 2017, 76, 437–442. [Google Scholar] [CrossRef]
- Pan, F.; Wang, L.; Cai, Z.; Wang, Y.; Wang, Y.; Guo, J.; Xu, X.; Zhang, X. Soybean peptide qrpr activates autophagy and attenuates the inflammatory response in the raw264.7 cell model. Protein Pept. Lett. 2019, 26, 301–312. [Google Scholar] [CrossRef]
- Wang, W.; De Mejia, E.G. A new frontier in soy bioactive peptides that may prevent age-related chronic diseases. Compr. Rev. Food Sci. Food Saf. 2005, 4, 63–78. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Fan, X.; Guo, H.; Yao, Y.; Ren, G.; Lv, X.; Yang, X. Overexpression of the bioactive lunasin peptide in soybean and evaluation of its anti-inflammatory and anti-cancer activities in vitro. J. Biosci. Bioeng. 2020, 129, 395–404. [Google Scholar] [CrossRef] [PubMed]
- El-Ashmawy, N.E.; Khedr, E.G.; Shamloula, M.M.; Kamel, M.M. Evaluation of the antirheumatic effects of isoflavone-free soy protein isolate and etanercept in rats with adjuvant-induced arthritis. Exp. Biol. Med. 2019, 244, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Cavalcante, R.M.S.; Lima, M.M.; Parente, J.M.L.; Moura, M.S.B.; Nogueira, N.D.N. Inflammatory bowel diseases and diet: An integrative review. Rev. Assoc. Med. Bras. 2020, 66, 1449–1454. [Google Scholar] [CrossRef]
- Bajerska, J.; Łagowska, K.; Mori, M.; Reguła, J.; Skoczek-Rubińska, A.; Toda, T.; Mizuno, N.; Yamori, Y. A meta-analysis of randomized controlled trials of the effects of soy intake on inflammatory markers in postmenopausal women. J. Nutr. 2022, 152, 5–15. [Google Scholar] [CrossRef]
- Kim, I.S.; Hwang, C.W.; Yang, W.S.; Kim, C.H. Current perspectives on the physiological activities of fermented soybean-derived cheonggukjang. Int. J. Mol. Sci. 2021, 22, 5746. [Google Scholar] [CrossRef]
- Mani, V.; Ming, L.C. Chapter 19 tempeh and other fermented soybean products rich in isoflavones. In Fermented Foods in Health and Disease Prevention; Academic Press: Cambridge, MA, USA, 2017; pp. 453–474. [Google Scholar]
- Ahmad, A.; Ramasamy, K.; Majeed, A.B.; Mani, V. Enhancement of β-secretase inhibition and antioxidant activities of tempeh, a fermented soybean cake through enrichment of bioactive aglycones. Pharm. Biol. 2015, 53, 758–766. [Google Scholar] [CrossRef]
- Kuligowski, M.; Pawłowska, K.; Jasińska-Kuligowska, I.; Nowak, J. Isoflavone composition, polyphenols content and antioxidative activity of soybean seeds during tempeh fermentation. CyTA-J. Food 2017, 15, 27–33. [Google Scholar] [CrossRef]
- Barus, T.; Titarsole, N.N.; Mulyono, N.; Prasasty, V.D. Tempeh antioxidant activity using dpph method: Effects of fermentation, processing, and microorganisms. J. Food Eng. Technol. 2019, 8, 75–80. [Google Scholar] [CrossRef]
- Watanabe, N.; Fujimoto, K.; Aoki, H. Antioxidant activities of the water-soluble fraction in tempeh-like fermented soybean (gaba-tempeh). Int. J. Food Sci. Nutr. 2007, 58, 577–587. [Google Scholar] [CrossRef]
- Stephanie, T.; Kartawidjajaputra, F.; Silo, W.; Yogiara, Y.; Suwanto, A. Tempeh consumption enhanced beneficial bacteria in the human gut. Food Res. 2019, 3, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Jang, C.H.; Oh, J.; Lim, J.S.; Kim, H.J.; Kim, J.S. Fermented soy products: Beneficial potential in neurodegenerative diseases. Foods 2021, 10, 636. [Google Scholar] [CrossRef]
- Kim, D.C.; Quang, T.H.; Yoon, C.S.; Ngan, N.T.T.; Lim, S.I.; Lee, S.Y.; Kim, Y.C.; Oh, H. Anti-neuroinflammatory activities of indole alkaloids from kanjang (Korean fermented soy source) in lipopolysaccharide-induced bv2 microglial cells. Food Chem. 2016, 213, 69–75. [Google Scholar] [CrossRef]
- Jeong, D.Y.; Ryu, M.S.; Yang, H.J.; Park, S. Γ-pga-rich chungkookjang, short-term fermented soybeans: Prevents memory impairment by modulating brain insulin sensitivity, neuro-inflammation, and the gut-microbiome-brain axis. Foods 2021, 10, 221. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.W.; Chung, Y.-S.; Kwak, C.S.; Kwon, Y.H. Doenjang, a Korean traditional fermented soybean paste, ameliorates neuroinflammation and neurodegeneration in mice fed a high-fat diet. Nutrients 2019, 11, 1702. [Google Scholar] [CrossRef]
- Chen, H.; McGowan, E.M.; Ren, N.; Lal, S.; Nassif, N.; Shad-Kaneez, F.; Qu, X.; Lin, Y. Nattokinase: A promising alternative in prevention and treatment of cardiovascular diseases. Biomark. Insights 2018, 13, 1177271918785130. [Google Scholar] [CrossRef] [PubMed]
- Jensen, G.S.; Lenninger, M.; Ero, M.P.; Benson, K.F. Consumption of nattokinase is associated with reduced blood pressure and von willebrand factor, a cardiovascular risk marker: Results from a randomized, double-blind, placebo-controlled, multicenter north american clinical trial. Integr. Blood Press. Control 2016, 9, 95–104. [Google Scholar] [CrossRef]
- Wu, H.; Wang, Y.; Zhang, Y.; Xu, F.; Chen, J.; Duan, L.; Zhang, T.; Wang, J.; Zhang, F. Breaking the vicious loop between inflammation, oxidative stress and coagulation, a novel anti-thrombus insight of nattokinase by inhibiting lps-induced inflammation and oxidative stress. Redox Biol. 2020, 32, 101500. [Google Scholar] [CrossRef] [PubMed]
- Katagiri, R.; Sawada, N.; Goto, A.; Yamaji, T.; Iwasaki, M.; Noda, M.; Iso, H.; Tsugane, S. Association of soy and fermented soy product intake with total and cause specific mortality: Prospective cohort study. BMJ 2020, 368, m34. [Google Scholar] [CrossRef]
- Kadotani, H.; Asai, K.; Miyamoto, A.; Iwasaki, K.; Kawai, T.; Nishimura, M.; Tohda, M.; Okamoto, A.; Sato, K.; Yamada, K.; et al. The fermented soy product immubalance(tm) suppresses airway inflammation in a murine model of asthma. Nutrients 2021, 13, 3380. [Google Scholar] [CrossRef]
- He, L.X.; Abdolmaleky, H.M.; Yin, S.; Wang, Y.; Zhou, J.R. Dietary fermented soy extract and oligo-lactic acid alleviate chronic kidney disease in mice via inhibition of inflammation and modulation of gut microbiota. Nutrients 2020, 12, 2376. [Google Scholar] [CrossRef] [PubMed]
- McCabe-Sellers, B.J.; Staggs, C.G.; Bogle, M.L. Tyramine in foods and monoamine oxidase inhibitor drugs: A crossroad where medicine, nutrition, pharmacy, and food industry converge. J. Food Compos. Anal. 2006, 19, S58–S65. [Google Scholar] [CrossRef]
- Lee, J.H.; Ha, J.H.; Lee, H.W.; Lee, J.Y.; Hwang, Y.S.; Lee, H.M.; Kim, S.J. Analysis of microbiological contamination in kimchi and its ingredients. J. Food Hyg. Saf. 2018, 33, 94–101. [Google Scholar] [CrossRef]
- Nam, G.W.; Jeong, M.; Heo, E.J.; Chang, O.K.; Kim, M.G.; Kwak, H.S.; Suh, S.H. Quantitative microbial risk assessment of pathogenic Escherichia coli in commercial kimchi in South Korea. Food Sci. Biotechnol. 2021, 30, 1455–1464. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paul, A.K.; Lim, C.L.; Apu, M.A.I.; Dolma, K.G.; Gupta, M.; de Lourdes Pereira, M.; Wilairatana, P.; Rahmatullah, M.; Wiart, C.; Nissapatorn, V. Are Fermented Foods Effective against Inflammatory Diseases? Int. J. Environ. Res. Public Health 2023, 20, 2481. https://doi.org/10.3390/ijerph20032481
Paul AK, Lim CL, Apu MAI, Dolma KG, Gupta M, de Lourdes Pereira M, Wilairatana P, Rahmatullah M, Wiart C, Nissapatorn V. Are Fermented Foods Effective against Inflammatory Diseases? International Journal of Environmental Research and Public Health. 2023; 20(3):2481. https://doi.org/10.3390/ijerph20032481
Chicago/Turabian StylePaul, Alok K., Chooi Ling Lim, Md. Aminul Islam Apu, Karma G. Dolma, Madhu Gupta, Maria de Lourdes Pereira, Polrat Wilairatana, Mohammed Rahmatullah, Christophe Wiart, and Veeranoot Nissapatorn. 2023. "Are Fermented Foods Effective against Inflammatory Diseases?" International Journal of Environmental Research and Public Health 20, no. 3: 2481. https://doi.org/10.3390/ijerph20032481
APA StylePaul, A. K., Lim, C. L., Apu, M. A. I., Dolma, K. G., Gupta, M., de Lourdes Pereira, M., Wilairatana, P., Rahmatullah, M., Wiart, C., & Nissapatorn, V. (2023). Are Fermented Foods Effective against Inflammatory Diseases? International Journal of Environmental Research and Public Health, 20(3), 2481. https://doi.org/10.3390/ijerph20032481









