Application of Near-Infrared Spectroscopy to Detect Modification of the Cation Exchange Properties of Soils from European Beech and Silver Fir Forest Stands in Poland
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Laboratory Analysis
2.3. NIR Spectroscopy Analysis
3. Results and Discussion
3.1. General Characteristic of Investigated Soils
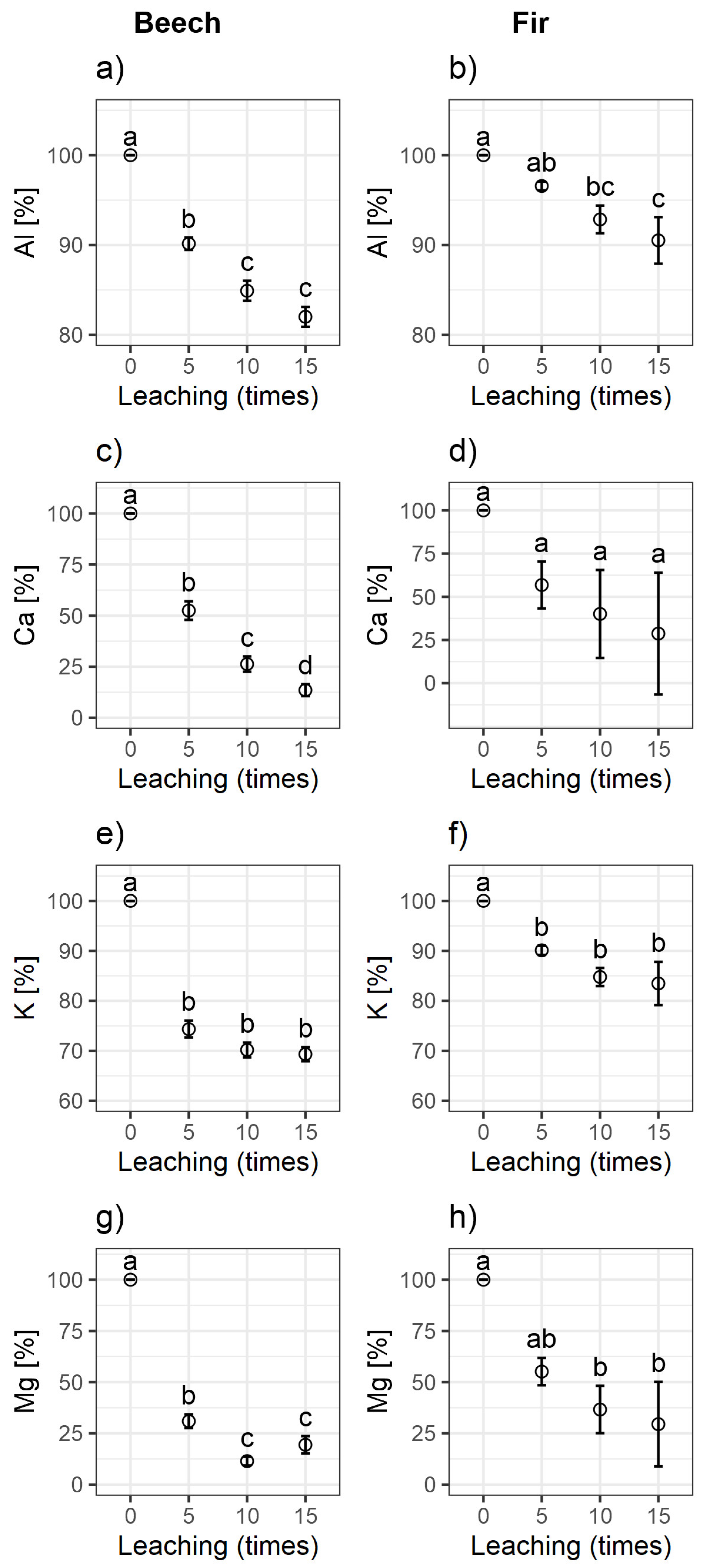
3.2. Results of Soil Leaching with Dilute HCl Solution
3.3. NIR Analysis
3.3.1. General Characteristic of Soil Spectra
3.3.2. Calibration Results
3.3.3. Validation Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Galloway, J.N. Acidifaction of the world: Natural and anthropogenic. Water. Air. Soil Pollut. 2001, 130, 17–24. [Google Scholar] [CrossRef]
- Galloway, J.N. Global nitrogen cycle: Changes and consequences. Environ. Pollut. 1998, 102, 15–24. [Google Scholar] [CrossRef]
- Koćmit, A.; Raczkowski, B. Influence of beech stand and atmospheric pollutants on the variation of soil chemical properties under West Pomeranian conditions (in Polish). In Systems Monitoring; Kostrzewski, A., Ed.; Environmental Monitoring Library: Warsaw, Poland, 1993; pp. 128–139. [Google Scholar]
- Gruba, P.; Mulder, J.; Pacanowski, P. Combined effects of soil disturbances and tree positions on spatial variability of soil pHCaCl2 under oak and pine stands. Geoderma 2020, 376, 114537. [Google Scholar] [CrossRef]
- Jóźwiak, M.; Kozłowski, R.; Sykała, E. Spatial distribution carbon and nitrogen organic in the mineral horizon soil (0–10 cm) in the part central of the Świętokrzyskie Mountains (in Polish). Rocz. Świętokrzyski 2009, 30, 29–37. [Google Scholar]
- Walna, B.; Polkowska, Z.; Małek, S.; Mȩdrzycka, K.; Namieśnik, J.; Siepak, J. Tendencies of change in the chemistry of precipitation at three monitoring stations 1996–1999. Polish J. Environ. Stud. 2003, 12, 467–472. [Google Scholar]
- Moldan, B.; Cerny, J. Biogeochemistry of small Catchments. A Tool for Environmental Research; John Wiley & Sons: Toronto, ON, Canada, 1994. [Google Scholar]
- Berggren, D.; Mulder, J.; Westerhof, R. Prolonged leaching of mineral forest soils with dilute HCl solutions: The solubility of Al and soil organic matter. Eur. J. Soil Sci. 1998, 49, 305–316. [Google Scholar] [CrossRef]
- Mulder, J.; De Wit, H.A.; Boonen, H.W.J.; Bakken, L.R. Increased levels of aluminum in forest soils: Effects on the stores of soil organic carbon. Water. Air. Soil Pollut. 2001, 130, 989–994. [Google Scholar] [CrossRef]
- Hobbie, S.E.; Ogdahl, M.; Chorover, J.; Chadwick, O.A.; Oleksyn, J.; Zytkowiak, R.; Reich, P.B. Tree species effects on soil organic matter dynamics: The role of soil cation composition. Ecosystems 2007, 10, 999–1018. [Google Scholar] [CrossRef]
- Atkins, P. Physical Chemistry; Polish Scientific Publishers PWN: Warsaw, Poland, 2001. [Google Scholar]
- Steffens, M.; Buddenbaum, H. Laboratory imaging spectroscopy of a stagnic Luvisol profile—High resolution soil characterisation, classification and mapping of elemental concentrations. Geoderma 2013, 195–196, 122–132. [Google Scholar] [CrossRef]
- Viscarra Rossel, R.A.; McGlynn, R.N.; McBratney, A.B. Determining the composition of mineral-organic mixes using UV-vis-NIR diffuse reflectance spectroscopy. Geoderma 2006, 137, 70–82. [Google Scholar] [CrossRef]
- Chang, C.; Laird, D.; Mausbach, M.J. Near-Infrared Reflectance Spectroscopy-Principal Components Regression Analysis of Soil Properties. Soil Sci. Soc. Am. J. 2001, 65, 480–490. [Google Scholar] [CrossRef]
- Stenberg, B.; Viscarra Rossel, R.A.; Mouazen, A.M.; Wetterlind, J. Visible and Near Infrared Spectroscopy in Soil Science. Adv. Agron. 2010, 107, 163–215. [Google Scholar] [CrossRef] [Green Version]
- Ben-Dor, E.; Banin, A. Near-Infrared Analysis as a Rapid Method to Simultaneously Evaluate Several Soil Properties. Soil Sci. Soc. Am. J. 1995, 59, 364–372. [Google Scholar] [CrossRef]
- Islam, K.; Singh, B.; McBratney, A. Simultaneous estimation of several soil properties by ultra-violet, visible, and near-infrared reflectance spectroscopy. Aust. J. Soil Res. 2003, 41, 1101–1114. [Google Scholar] [CrossRef]
- Seema; Ghosh, A.K.; Das, B.S.; Reddy, N. Application of VIS-NIR spectroscopy for estimation of soil organic carbon using different spectral preprocessing techniques and multivariate methods in the middle Indo-Gangetic plains of India. Geoderma Reg. 2020, 23, e00349. [Google Scholar] [CrossRef]
- Kania, M.; Gruba, P. Estimation of selected properties of forest soils using near-infrared spectroscopy (NIR). Soil Sci. Annu. 2016, 67, 32–36. [Google Scholar] [CrossRef]
- Kania, M.; Gruba, P.; Wiecheć, M. Zastosowanie techniki bliskiej podczerwieni do obliczania siedliskowego indeksu glebowego. Sylwan 2017, 161, 935–939. [Google Scholar]
- Chodak, M.; Niklińska, M.; Beese, F. Near-infrared spectroscopy for analysis of chemical and microbiological properties of forest soil organic horizons in a heavy-metal-polluted area. Biol. Fertil. Soils 2007, 44, 171–180. [Google Scholar] [CrossRef]
- Augusto, L.; Ranger, J.; Binkley, D.; Rothe, A. Impact of several common tree species of European temperate forests on soil fertility. Ann. For. Sci. 2002, 59, 233–253. [Google Scholar] [CrossRef]
- Gruba, P.; Mulder, J. Tree species affect cation exchange capacity (CEC) and cation binding properties of organic matter in acid forest soils. Sci. Total Environ. 2015, 511, 655–662. [Google Scholar] [CrossRef]
- Madari, B.E.; Reeves, J.B.; Machado, P.L.O.A.; Guimarães, C.M.; Torres, E.; McCarty, G.W. Mid- and near-infrared spectroscopic assessment of soil compositional parameters and structural indices in two Ferralsols. Geoderma 2006, 136, 245–259. [Google Scholar] [CrossRef]
- Ng, W.; Husnain; Anggria, L.; Siregar, A.F.; Hartatik, W.; Sulaeman, Y.; Jones, E.; Minasny, B. Developing a soil spectral library using a low-cost NIR spectrometer for precision fertilization in Indonesia. Geoderma Reg. 2020, 22, e00319. [Google Scholar] [CrossRef]
- Krajewski, R. Detailed Geological Map of Poland; Polish Geological Institute: Warsaw, Poland, 1955. [Google Scholar]
- IUSS Working Group. WRB World Reference Base for Soil Resources 2014, Update 2015. International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; World Soil Resources Reports No. 106; FAO: Rome, Italy, 2015; ISBN 978-92-5-108369-7. [Google Scholar]
- Buurman, P.; van Lagen, B.; Velthorst, E.J. Manual for Soil and Water Analysis; Backhuys Publishers: Leiden, The Netherlands, 1996. [Google Scholar]
- Jou, A.S.R.; Kamprath, E.J. Copper chloride as an extractant for estimating the potentially reactive aluminium pool in acid soils. Soil Sci. Soc. Am. J. 1979, 43, 35–68. [Google Scholar]
- Ostrowska, A.; Gawliński, S.; Szczubiałka, Z. Methods for Analyzing and Evaluating the Properties of Soils and Plants (in Polish); Institute of Environmental Protection: Warsaw, Poland, 1991. [Google Scholar]
- Demattê, J.A.M.; Campos, R.C.; Alves, M.C.; Fiorio, P.R.; Nanni, M.R. Visible-NIR reflectance: A new approach on soil evaluation. Geoderma 2004, 121, 95–112. [Google Scholar] [CrossRef]
- Pinheiro, É.F.M.; Ceddia, M.B.; Clingensmith, C.M.; Grunwald, S.; Vasques, G.M. Prediction of soil physical and chemical properties by visible and near-infrared diffuse reflectance spectroscopy in the Central Amazon. Remote Sens. 2017, 9, 1–22. [Google Scholar] [CrossRef]
- dos Santos, U.J.; de Demattê, J.A.M.; Menezes, R.S.C.; Dotto, A.C.; Guimarães, C.C.B.; Alves, B.J.R.; Primo, D.C.; de Sampaio, E.V.S.B. Predicting carbon and nitrogen by visible near-infrared (Vis-NIR) and mid-infrared (MIR) spectroscopy in soils of Northeast Brazil. Geoderma Reg. 2020, 23, e00333. [Google Scholar] [CrossRef]
- Pallant, E.; Riha, S.J. Surface Soil Acidification under Red Pine and Norway Spruce. Soil Sci. Soc. Am. J. 1990, 54, 1124–1130. [Google Scholar] [CrossRef]




| Al3+ | Ca2+ | K+ | Mg2+ | C [g∙kg−1] | pH | |
|---|---|---|---|---|---|---|
| [cmol(+)∙kg−1] | ||||||
| Beech | 15.62 ± 6.69 | 2.94 ± 1.52 | 0.91 ± 0.40 | 0.64 ± 0.24 | 24.2 ± 9.26 | 4.35 ± 0.13 |
| Fir | 61.62 ± 19.09 | 6.48 ± 3.20 | 2.26 ± 0.83 | 1.70 ± 0.67 | 46.3 ± 8.14 | 4.28 ± 0.25 |
| Soil Properties | Al3+ | Ca2+ | K+ | Mg2+ | C | pH |
|---|---|---|---|---|---|---|
| Fagus sylvatica L. | ||||||
| R2C a | 0.99 | 0.97 | 0.89 | 0.97 | 0.99 | 0.98 |
| R2V b | 0.87 | 0.33 | 0.47 | 0.72 | 0.88 | 0.70 |
| Abies alba Mill. | ||||||
| R2C | 0.98 | 0.92 | 0.98 | 0.98 | 0.98 | 0.97 |
| R2C | 0.83 | 0.38 | 0.47 | 0.56 | 0.74 | 0.84 |
| Soil Properties a | Number of Leaching Treatments | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 (n b = 3) | 5 (n = 3) | 10 (n = 3) | 15 (n = 4) | ||||||
| Mean Measured | Mean Calculated | Mean Measured | Mean Calculated | Mean Measured | Mean Calculated | Mean Measured | Mean Calculated | p c | |
| Al3+ | 14.99 | 15.32 | 14.47 | 12.74 | 12.92 | 12.83 | 13.05 | 12.35 | NS |
| Ca2+ | 2.56 | 3.38 | 1.34 | 1.47 | 0.61 | 0.38 | 0.59 | 2.02 | NS |
| K+ | 0.81 | 0.79 | 0.80 | 0.58 | 0.58 | 0.37 | 0.61 | 0.77 | NS |
| Mg2+ | 0.55 | 0.55 | 0.18 | 0.25 | 0.05 | 0.10 | 0.10 | 0.18 | NS |
| C | 17.31 | 18.89 | 24.53 | 20.61 | 16.71 | 17.54 | 27.45 | 29.91 | NS |
| pH | 4.40 | 4.17 | 3.23 | 3.30 | 3.10 | 2.95 | 3.06 | 3.21 | NS |
| Soil Properties a | Number of Leaching Treatments | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 (n b = 3) | 5 (n = 3) | 10 (n = 3) | 15 (n = 3) | ||||||
| Mean Measured | Mean Calculated | Mean Measured | Mean Calculated | Mean Measured | Mean Calculated | Mean Measured | Mean Calculated | p c | |
| Al3+ | 52.33 | 52.69 | 59.46 | 58.65 | 46.06 | 52.93 | 62.59 | 60.99 | NS |
| Ca2+ | 5.37 | 5.70 | 5.06 | 3.98 | 2.97 | 3.71 | 4.02 | 4.20 | NS |
| K+ | 1.72 | 2.01 | 2.49 | 1.76 | 1.38 | 1.85 | 1.94 | 2.16 | NS |
| Mg2+ | 1.43 | 1.31 | 1.18 | 0.92 | 0.29 | 0.56 | 0.83 | 0.82 | NS |
| C | 42.17 | 44.62 | 51.26 | 49.39 | 40.83 | 43.90 | 43.33 | 44.54 | NS |
| pH | 4.48 | 4.14 | 3.36 | 3.50 | 3.57 | 3.33 | 3.20 | 3.40 | NS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kania, M.; Kupka, D.; Gruba, P. Application of Near-Infrared Spectroscopy to Detect Modification of the Cation Exchange Properties of Soils from European Beech and Silver Fir Forest Stands in Poland. Int. J. Environ. Res. Public Health 2023, 20, 2654. https://doi.org/10.3390/ijerph20032654
Kania M, Kupka D, Gruba P. Application of Near-Infrared Spectroscopy to Detect Modification of the Cation Exchange Properties of Soils from European Beech and Silver Fir Forest Stands in Poland. International Journal of Environmental Research and Public Health. 2023; 20(3):2654. https://doi.org/10.3390/ijerph20032654
Chicago/Turabian StyleKania, Mateusz, Dawid Kupka, and Piotr Gruba. 2023. "Application of Near-Infrared Spectroscopy to Detect Modification of the Cation Exchange Properties of Soils from European Beech and Silver Fir Forest Stands in Poland" International Journal of Environmental Research and Public Health 20, no. 3: 2654. https://doi.org/10.3390/ijerph20032654





