Remediation of Acid Mine Drainage (AMD) Using Steel Slag: Mechanism of the Alkalinity Decayed Process
Abstract
:1. Introduction
2. Experiments
2.1. Materials
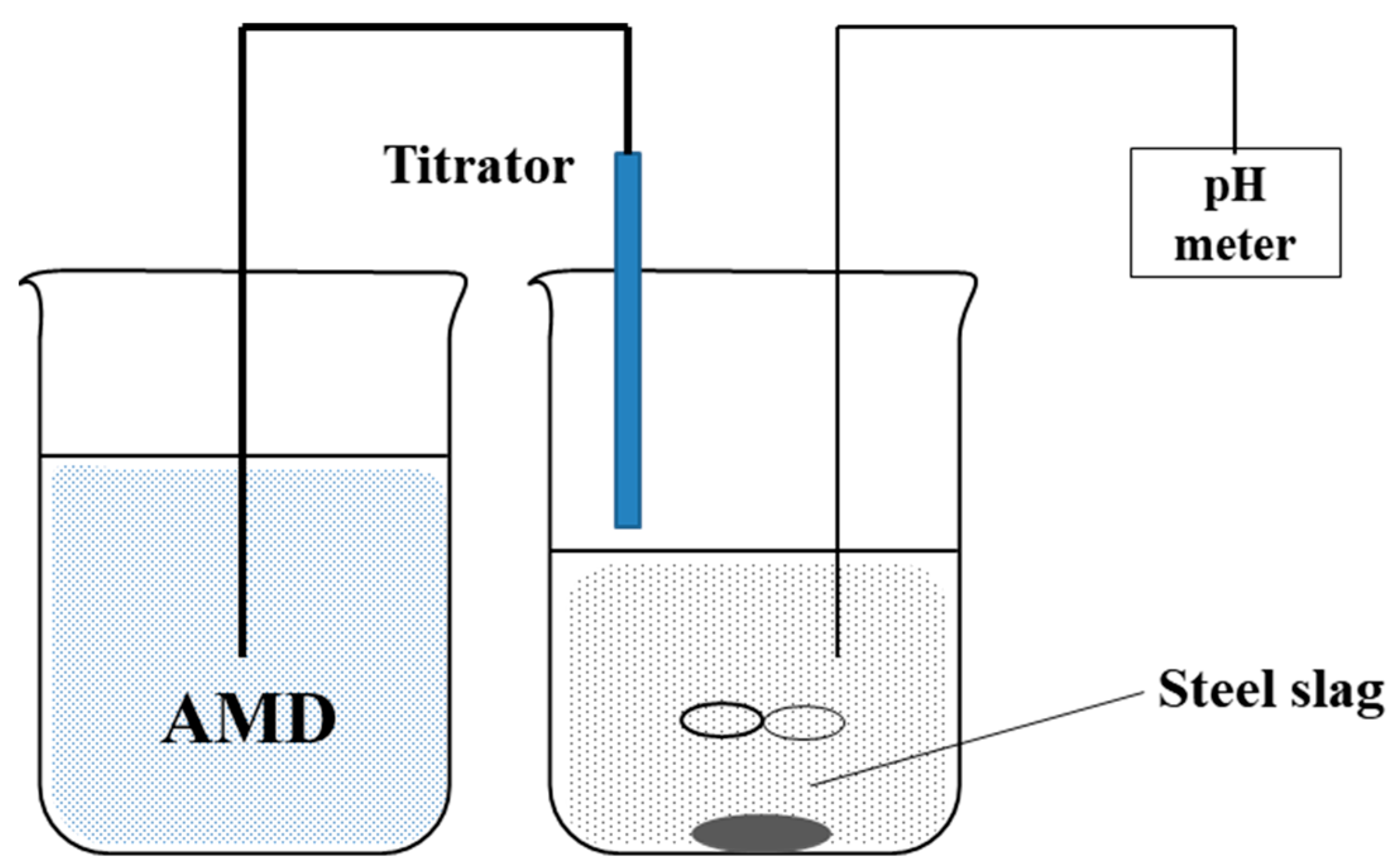
2.2. Experimental Procedure
2.2.1. Alkalinity Production Determination
2.2.2. ANC Determination
2.2.3. Leaching Ratio
2.2.4. Characterization Techniques
3. Results and Discussion
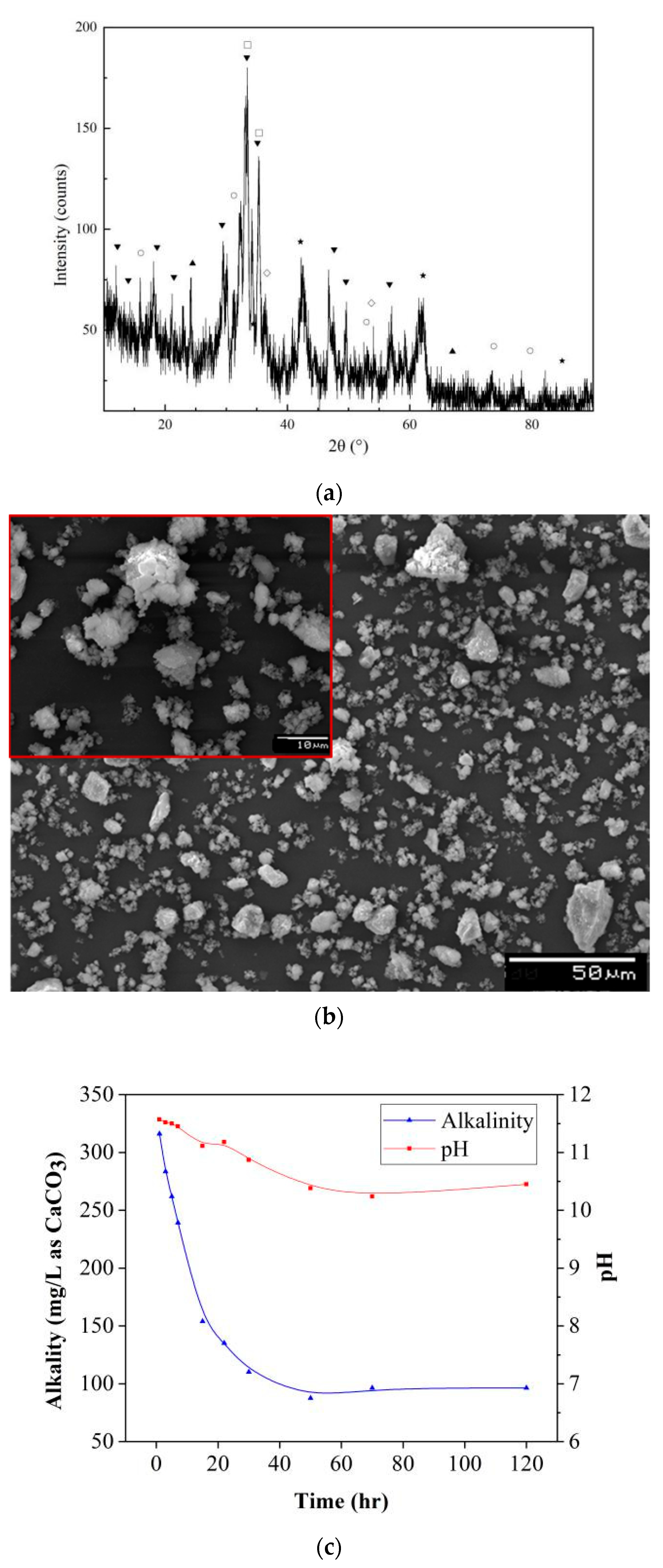
3.1. Characterization and Alkalinity Production
3.2. Acid Neutralization Capacity Tests
3.3. Slag Characteristics Variation during Alkalinity Consumption Process
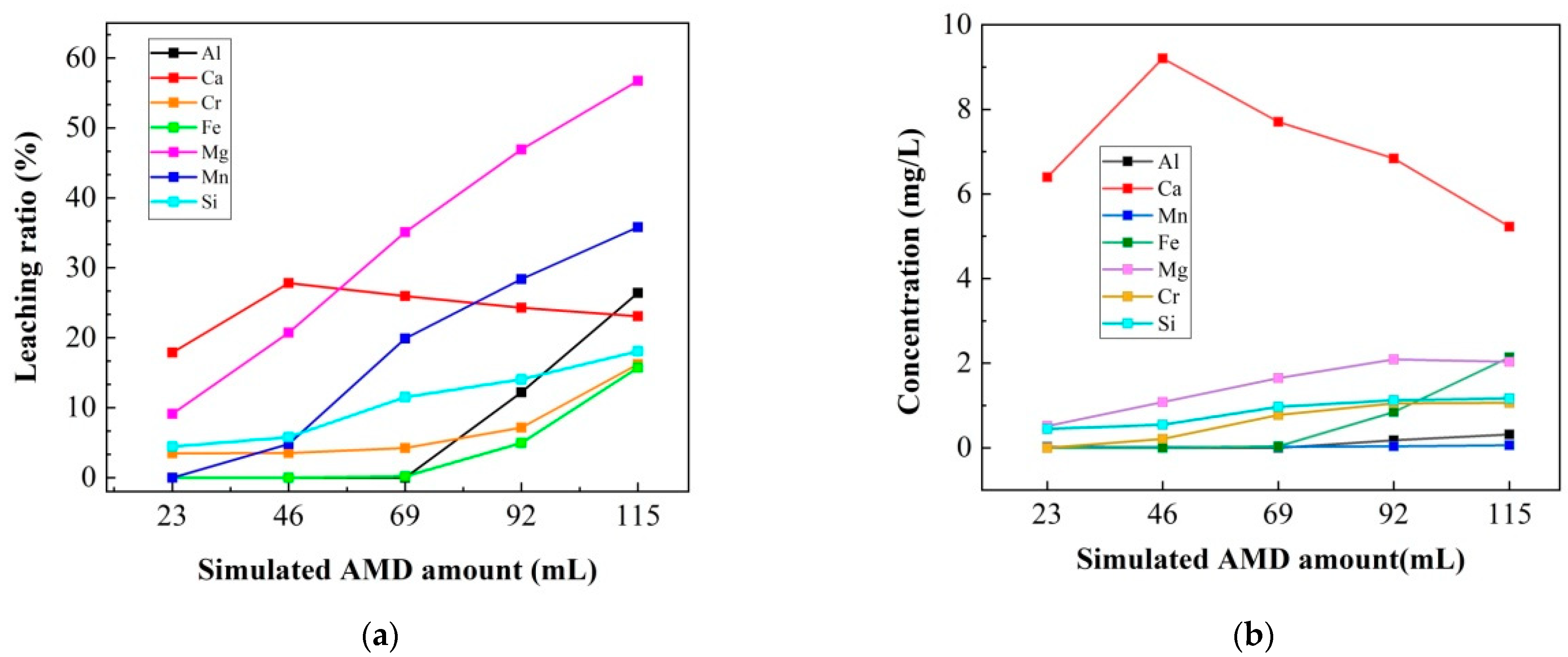
3.3.1. Leaching Characteristics
3.3.2. Phase Characteristics
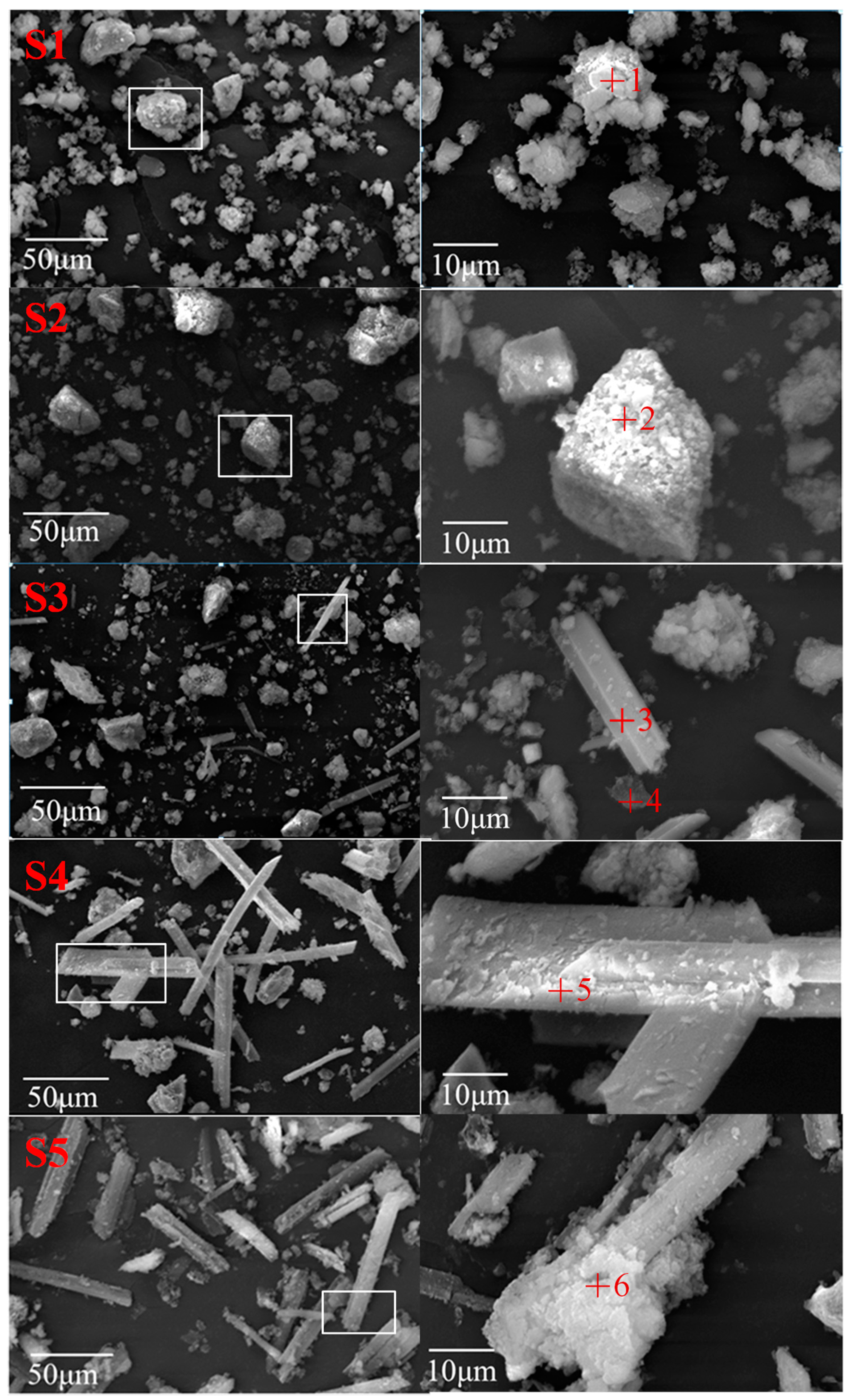
3.3.3. Morphology Characteristics
3.4. ANC Test by Real AMD
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alakangas, L.; Andersson, E.; Mueller, S. Neutralization/prevention of acid rock drainage using mixtures of alkaline by-products and sulfidic mine wastes. Environ. Sci. Pollut. Res. Int. 2013, 20, 7907–7916. [Google Scholar] [CrossRef]
- Yang, M.; Lu, C.; Quan, X.; Cao, D. Mechanism of acid mine drainage remediation with steel slag: A review. ACS Omega 2021, 6, 30205–30213. [Google Scholar] [CrossRef]
- Mohammed, N.H.B.; Yaacob, W.Z. Remediation of AMD using industrial waste adsorbents. AIP Conf. 2016, 1784, 060043. [Google Scholar] [CrossRef]
- Feng, D.; Deventer, J.; Aldrich, C. Removal of pollutants from acid mine wastewater using metallurgical by-product slags. Sep. Purif. Technol. 2004, 40, 61–67. [Google Scholar] [CrossRef]
- Zhang, X.L.; Chen, J.X.; Jiang, J.J.; Li, J.; Tyagi, R.D.; Surampalli, R.Y. The potential utilization of slag generated from iron- and steelmaking industries: A review. Environ. Geochem. Health 2020, 42, 1321–1334. [Google Scholar] [CrossRef]
- Li, B.; Zhang, H.; Long, J.; Fan, J.; Wu, P.; Chen, M.; Liu, P.; Li, T. Migration mechanism of pollutants in karst groundwater system of tailings impoundment and management control effect analysis: Gold mine tailing impoundment case. J. Clean. Prod. 2022, 350, 131434. [Google Scholar] [CrossRef]
- Li, B.; Zhang, H.L.; Luo, Y.L.; Liu, L.; Li, T. Mine inflow prediction model based on unbiased Grey-Markov theory and its application. Earth Sci. Inform. 2022, 15, 855–862. [Google Scholar] [CrossRef]
- Wu, Y.D.; Peng, B.; Wu, L.; Lv, W.; Zhang, G.H. Review on global development of treatment and utilization of steel slag. Environ. Eng. 2021, 39, 161–165. [Google Scholar] [CrossRef]
- Saha, S.; Sarkar, S.; Sinha, A. Use of Basic Oxygen Furnace (BOF) steel slag for acid mine drainage treatment: A laboratory study. Mine Water Environ. 2019, 38, 517–527. [Google Scholar] [CrossRef]
- Zvimba, J.N.; Siyakatshana, N.; Mathye, M. Passive neutralization of acid mine drainage using basic oxygen furnace slag as neutralization material: Experimental and modelling. Water Sci. Technol. 2017, 75, 1014–1024. [Google Scholar] [CrossRef]
- Yu, J.; Liang, W.; Wang, L.; Li, F.; Zou, Y.; Wang, H. Phosphate removal from domestic wastewater using thermally modified steel slag. J. Environ. Sci. 2015, 31, 81–88. [Google Scholar] [CrossRef]
- Liang, Z.; Li, J.; Li, P.; Yan, B.J.; Guo, H.W. Selective leaching behaviors of calcium and magnesium from steel slag. China Metall. 2020, 30, 51–57. [Google Scholar] [CrossRef]
- Gao, Y.; Jiang, J.; Tian, S.; Li, K.; Yan, F.; Liu, N.; Yang, M.; Chen, X. BOF steel slag as a low-cost sorbent for vanadium (V) removal from soil washing effluent. Sci. Rep. 2017, 7, 11177. [Google Scholar] [CrossRef] [PubMed]
- Tabelin, C.B.; Corpuz, R.D.; Igarashi, T.; Villacorte-Tabelin, M.; Alorro, R.D.; Yoo, K.; Raval, S.; Ito, M.; Hiroyoshi, N. Acid mine drainage formation and arsenic mobility under strongly acidic conditions: Importance of soluble phases, iron oxyhydroxides/oxides and nature of oxidation layer on pyrite. J. Hazard. Mater. 2020, 399, 122844. [Google Scholar] [CrossRef] [PubMed]
- Sithole, N.T.; Ntuli, F.; Okonta, F. Fixed bed column studies for decontamination of acidic mineral effluent using porous fly ash-basic oxygen furnace slag based geopolymers. Miner. Eng. 2020, 154, 106397. [Google Scholar] [CrossRef]
- Zhan, X.H.; Xiao, L.P.; Liang, B. Removal of Pb(II) from Acid Mine Drainage with Bentonite-Steel Slag Composite Particles. Sustainability 2019, 11, 4476. [Google Scholar] [CrossRef]
- Asere, T.; Stevens, C.V.; Du, L.G. Use of (modified) natural adsorbents for arsenic remediation: A review. Sci. Total Environ. 2019, 676, 706–720. [Google Scholar] [CrossRef]
- Molahid, V.L.M.; Kusin, F.M.; Madzin, Z. Role of multiple substrates (spent mushroom compost, ochre, steel slag, and limestone) in passive remediation of metal-containing acid mine drainage. Environ. Technol. 2019, 40, 1323–1336. [Google Scholar] [CrossRef]
- Riley, A.L.; Mayes, W.M. Long-term evolution of highly alkaline steel slag drainage waters. Environ. Monit. Assess. 2015, 187, 463. [Google Scholar] [CrossRef]
- Sukati, B.H.; De Jager, P.C.; Annandale, J.G.; Tanner, P.D. The hazardous status of high density sludge from acid mine drainage neutralization. Sustainability 2018, 10, 4185. [Google Scholar] [CrossRef] [Green Version]
- Gomes, H.I.; Mayes, W.M.; Baxter, H.A.; Jarvis, A.P.; Burke, I.T.; Stewart, D.I.; Rogerson, M. Options for managing alkaline steel slag leachate: A life cycle assessment. J. Clean. Prod. 2018, 202, 401–412. [Google Scholar] [CrossRef]
- Kruse, N.A.; Mackey, A.L.; Bowman, J.R.; Brewster, K.; Riefler, R.G. Alkalinity production as an indicator of failure in steel slag leach beds treating acid mine drainage. Environ. Earth Sci. 2012, 67, 1389–1395. [Google Scholar] [CrossRef]
- Goetz, E.; Riefler, G. Geochemistry of CO2 in Steel Slag Leach Beds. Mine Water Environ. 2014, 34, 42–49. [Google Scholar] [CrossRef]
- Wang, Q.; Yan, P.Y. Hydration properties of basic oxygen furnace steel slag. Constr. Build. Mater. 2010, 24, 1134–1140. [Google Scholar] [CrossRef]
- Manchisi, J.; Matinde, E.; Rowson, N.A.; Simmons, M.J.H.; Simate, G.S.; Ndlovu, S.; Mwewa, B. Ironmaking and steelmaking Slags as sustainable adsorbents for industrial effluents and wastewater treatment: A Critical Review of Properties, Performance, Challenges and Opportunities. Sustainability 2020, 12, 2118. [Google Scholar] [CrossRef]
- Name, T.; Sheridan, C. Remediation of acid mine drainage using metallurgical slags. Miner. Eng. 2014, 64, 15–22. [Google Scholar] [CrossRef]
- Alizadeh, A.; Naseri, P.S.Z. An Investigation on the Influence of EAF slag particle size on AMD neutralization behavior in static and dynamic slag leaching systems. Mine Water Environ. 2014, 34, 204–212. [Google Scholar] [CrossRef]
- Yan, J.Y.; Luis, M.; Ivars, N. The long-term acid neutralizing capacity of steel slag. Waste Manag. 2000, 20, 217–223. [Google Scholar] [CrossRef]
- Iakovleva, E.; Mäkilä, E.; Salonen, J.; Sitarz, M.; Wang, S.; Sillanpää, M. Acid mine drainage (AMD) vs unmodified and modified limestone. Ecol. Eng. 2015, 81, 30–40. [Google Scholar] [CrossRef]
- Hall, C.L.; Large, D.J.; Adderley, B.; West, H.M. Calcium leaching from waste steelmaking slag: Significance of leachate chemistry and effects on slag grain mineralogy. Miner. Eng. 2014, 65, 156–162. [Google Scholar] [CrossRef]
- Masindi, V.; Osman, M.S.; Abu-Mahfouz, A.M. Integrated treatment of acid mine drainage using BOF slag, lime/soda ash and reverse osmosis (RO): Implication for the production of drinking water. Desalination 2017, 424, 45–52. [Google Scholar] [CrossRef]
- Bodurtha, P.; Brassard, P. Neutralization of Acid by Steel-Making Slags. Environ. Technol. 2000, 21, 1271–1281. [Google Scholar] [CrossRef]
- Xue, Y.; Wu, S.; Zhou, M. Adsorption characterization of Cu(II) from aqueous solution onto basic oxygen furnace slag. Chem. Eng. J. 2013, 231, 355–364. [Google Scholar] [CrossRef]
- Masindi, V.; Osman, M.S.; Mbhele, R.N.; Rikhotso, R. Fate of pollutants post treatment of acid mine drainage with basic oxygen furnace slag: Validation of experimental results with a geochemical model. J. Clean. Prod. 2018, 172, 2899–2909. [Google Scholar] [CrossRef]
- Sephton, M.G.; Webb, J.A. The role of secondary minerals in remediation of acid mine drainage by Portland cement. J. Hazard. Mater. 2019, 367, 267–276. [Google Scholar] [CrossRef]
- Ntuli, F.; Magwa, N. Sulphate removal from acid rock drainage using steel slag. IOP Conf. Ser. Earth Environ. Sci. 2018, 191, 012116. [Google Scholar] [CrossRef]
- Skousen, J.G.; Ziemkiewicz, P.F.; Mcdonald, L.M. Acid mine drainage formation, control and treatment: Approaches and strategies. Extr. Ind. Soc. 2019, 6, 241–249. [Google Scholar] [CrossRef]





| Composition | CaO | Fe2O3 | SiO2 | MgO | MnO | Al2O3 | P2O5 | V2O5 | Cr2O3 |
|---|---|---|---|---|---|---|---|---|---|
| % | 41.82 | 25.05 | 11.89 | 6.61 | 5.48 | 2.18 | 1.59 | 0.73 | 0.69 |
| Component | Reactions |
| Site | Ca | S | O | Mg | Si | Al | Fe |
|---|---|---|---|---|---|---|---|
| 1 | 16.72 | 12.12 | 47.64 | 0.02 | 22.7 | 0.26 | 0.54 |
| 2 | 24.36 | 19.74 | 53.94 | 0.03 | 1.88 | 0.05 | - |
| 3 | 48.05 | 34.12 | 17.7 | - | 0.07 | - | - |
| 4 | 5.8 | 3.96 | 40.62 | 0.02 | 48.37 | 0.58 | 0.58 |
| 5 | 42.12 | 31.62 | 26.09 | 0.04 | - | 0.04 | - |
| 6 | 57.06 | 34.4 | 6.63 | - | 0.54 | - | 1.30 |
| Element | Na | Mg | Al | K | Ca | Mn | Fe | Co | Ni | Zn | SO42− |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Content (mg/L) | 67.34 | 123.13 | 43.16 | 9.80 | 85.35 | 1.56 | 495.82 | 0.32 | 0.40 | 1.17 | 2689.96 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, L.; Tang, Y.; Cao, D.; Yang, M. Remediation of Acid Mine Drainage (AMD) Using Steel Slag: Mechanism of the Alkalinity Decayed Process. Int. J. Environ. Res. Public Health 2023, 20, 2805. https://doi.org/10.3390/ijerph20042805
Yang L, Tang Y, Cao D, Yang M. Remediation of Acid Mine Drainage (AMD) Using Steel Slag: Mechanism of the Alkalinity Decayed Process. International Journal of Environmental Research and Public Health. 2023; 20(4):2805. https://doi.org/10.3390/ijerph20042805
Chicago/Turabian StyleYang, Lei, Yuegang Tang, Duanning Cao, and Mingyuan Yang. 2023. "Remediation of Acid Mine Drainage (AMD) Using Steel Slag: Mechanism of the Alkalinity Decayed Process" International Journal of Environmental Research and Public Health 20, no. 4: 2805. https://doi.org/10.3390/ijerph20042805
APA StyleYang, L., Tang, Y., Cao, D., & Yang, M. (2023). Remediation of Acid Mine Drainage (AMD) Using Steel Slag: Mechanism of the Alkalinity Decayed Process. International Journal of Environmental Research and Public Health, 20(4), 2805. https://doi.org/10.3390/ijerph20042805






