Modulatory Effect of Lifestyle-Related, Environmental and Genetic Factors on Paraoxonase-1 Activity: A Review
Abstract
:1. Introduction
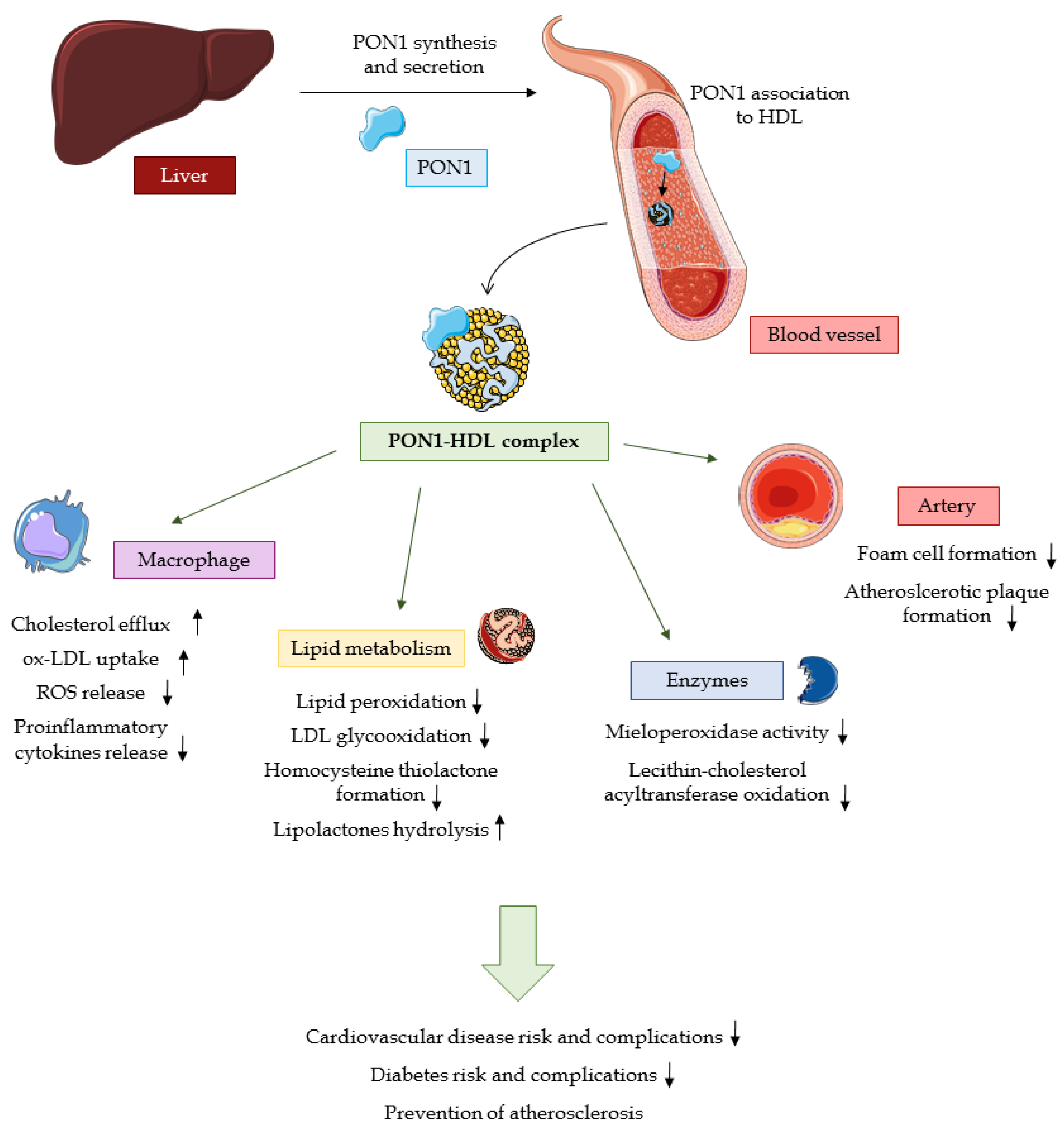
2. PON1 Structure and Physiological Functions
2.1. Structure and Function of Human PON1
2.2. Physiological activity
2.2.1. Substrate Hydrolysis
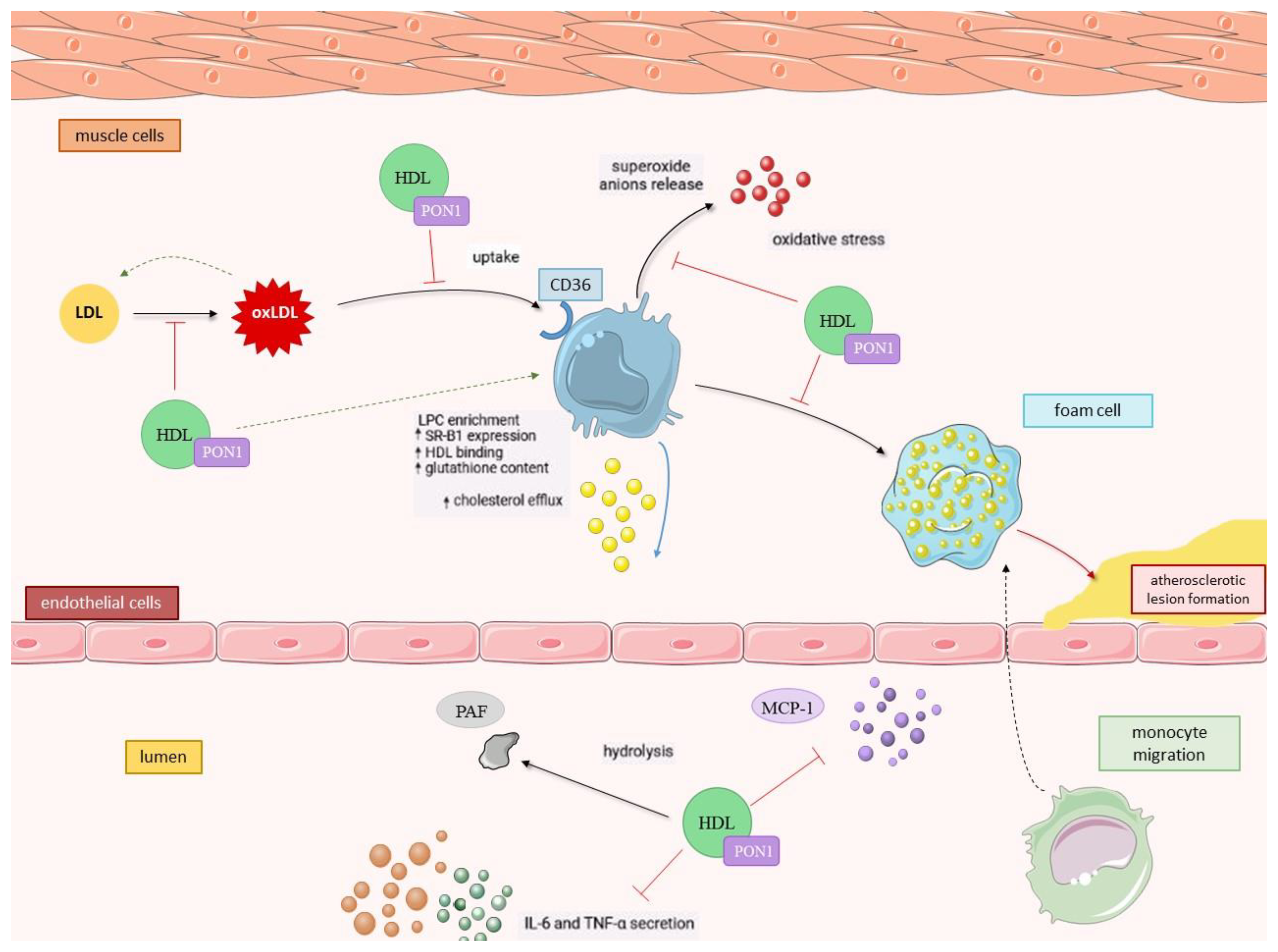
2.2.2. PON1 as an Antioxidant
2.2.3. Anti-inflammatory Activity of PON1
2.3. PON1 Status in Disease Conditions
3. Modifiable Factors Impacting Human PON1 Activity
3.1. Environmental Factors Influencing PON1 Activity
3.1.1. Organophosphates (OPs)
3.1.2. Heavy Metals
3.2. Influence of Diet and Nutritional Habits on PON1 Activity
3.2.1. Dietary Lipids
3.2.2. Glucose Intake
3.2.3. Plant-derived Compounds
3.2.4. Vitamins and Trace Elements
3.3. Alcohol Consumption
3.4. Cigarette Smoking
3.5. Pharmaceuticals
3.5.1. Cardiovascular Drugs
3.5.2. Pharmaceuticals Applied in the Treatment of Mood Disorders and Antiepileptic Drugs
3.5.3. Antidiabetic Drugs
3.5.4. Drugs Regulating Lipid Metabolism
3.5.5. Oral Contraceptives
3.5.6. Pharmaceuticals Used in Chemotherapy
4. Non-Modifiable Factors Modulating PON1 Activity
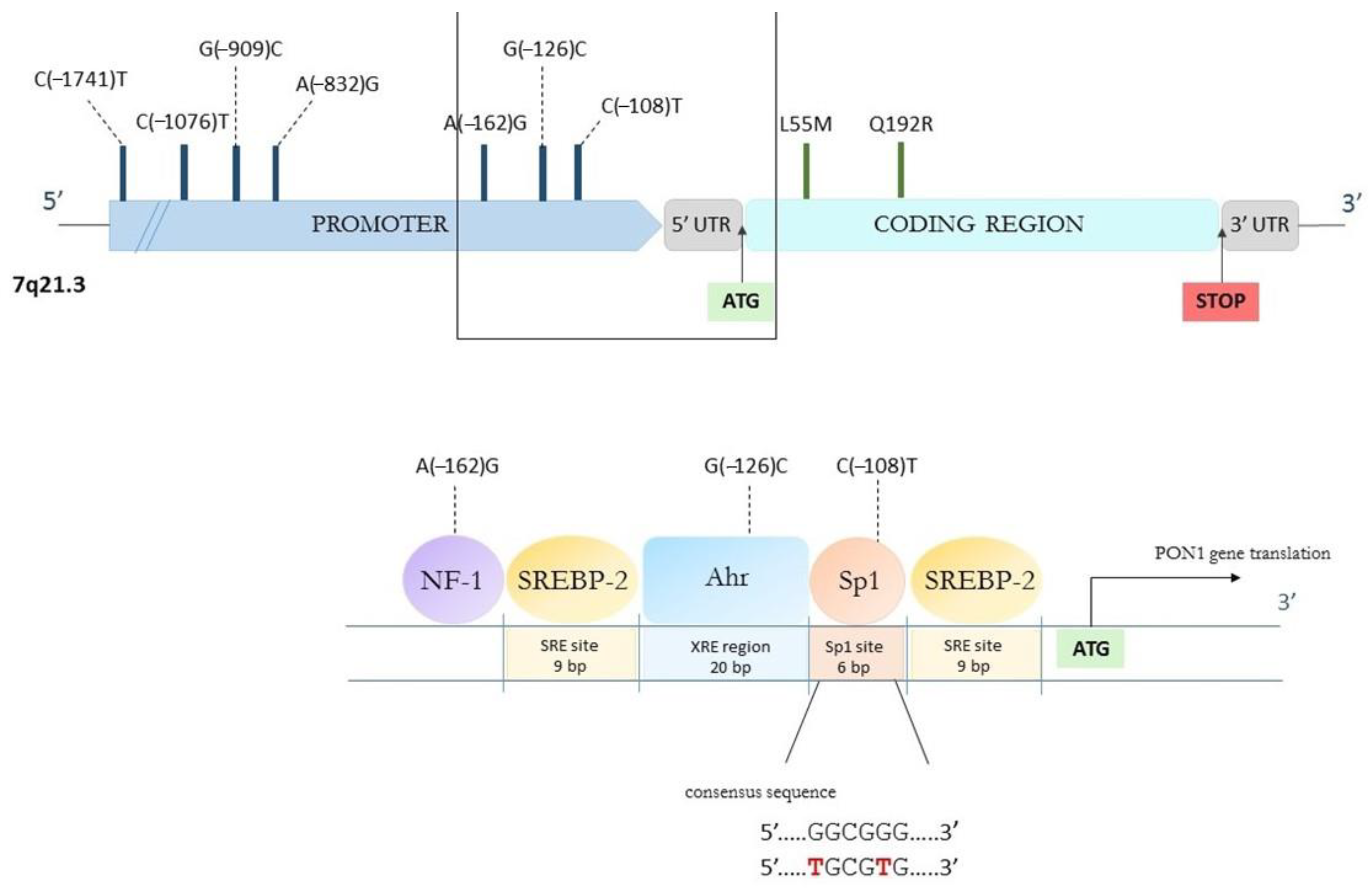
4.1. Genetic Polymorphisms
4.2. Age
4.3. Gender
5. Conclusions and Future Perspectives
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Primo-Parmo, S.L.; Sorenson, R.C.; Teiber, J.; La Du, B.N. The Human Serum Paraoxonase/Arylesterase Gene (PON1) Is One Member of a Multigene Family. Genomics 1996, 33, 498–507. [Google Scholar] [CrossRef] [PubMed]
- Mackness, B.; Durrington, P.N.; Mackness, M.I. Human serum paraoxonase. Gen. Pharmac. 1998, 31, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Kulka, M. A review of paraoxonase 1 properties and diagnostic applications. Pol. J. Veter-Sci. 2016, 19, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Aldridge, W.N. Serum esterases. I. Two types of esterase (A and B) hydrolysing p-nitrophenyl acetate, propionate and butyrate, and a method for their determination. Biochem. J. 1953, 53, 110–117. [Google Scholar] [CrossRef]
- Richter, R.J.; Furlong, C.E. Determination of paraoxonase (PON1) status requires more than genotyping. Pharmacogenetics 1999, 9, 745–753. [Google Scholar] [CrossRef]
- Rojas-García, A.E.; Solís-Heredia, M.J.; Piña-Guzmán, B.; Vega, L.; López-Carrillo, L.; Quintanilla-Vega, B. Genetic polymor-phisms and activity of PON1 in a Mexican population. Toxicol. Appl. Pharm. 2005, 205, 282–289. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0041008X04004843 (accessed on 2 February 2023). [CrossRef]
- Ortiz, P.; Torres-Sánchez, A.; López-Moreno, A.; Cerk, K.; Ruiz-Moreno, Á.; Monteoliva-Sánchez, M.; Ampatzoglou, A.; Aguilera, M.; Gruszecka-Kosowska, A. Impact of Cu-mulative Environmental and Dietary Xenobiotics on Human Microbiota: Risk Assessment for One Health. J. Xenobiotics 2022, 12, 56–63. [Google Scholar] [CrossRef]
- Sozmen, E.Y.; Sagin, F.G.; Kayikcioglu, M.; Sozmen, B. Oxidative Stress & Antioxidants and PON1 in Health and Dis-ease. In The Paraoxonases: Their Role in Disease Development and Xenobiotic Metabolism; Springer: Dordrecht, The Netherlands, 2007; pp. 61–73. [Google Scholar]
- Toma, L.; Barbălată, T.; Sanda, G.M.; Niculescu, L.S.; Sima, A.V.; Stancu, C.S. CRISPR/dCas9 Transcriptional Activation of Endogenous Apolipoprotein AI and Paraoxonase 1 in Enterocytes Alleviates Endothelial Cell Dysfunction. Biomolecules 2021, 11, 1769. [Google Scholar] [CrossRef]
- Harel, M.; Aharoni, A.; Gaidukov, L.; Brumshtein, B.; Khersonsky, O.; Meged, R.; Dvir, H.; Ravelli, R.; McCarthy, A.; Toker, L.; et al. Structure and evolution of the serum paraoxonase family of detoxifying and anti-atherosclerotic enzymes. Nat. Struct. Mol. Biol. 2004, 11, 412–419. [Google Scholar] [CrossRef]
- Kuo, C.L.; La Du, B.N. Calcium binding by human and rabbit serum paraoxonases. Structural stability and enzymatic activity. Drug Metab. Dispos. 1998, 26, 653–660. [Google Scholar]
- Taler-Verčič, A.; Goličnik, M.; Bavec, A. The Structure and Function of Paraoxonase-1 and Its Comparison to Paraoxonase-2 and -3. Molecules 2020, 25, 5980. [Google Scholar] [CrossRef] [PubMed]
- Blaha-Nelson, D.; Krüger, D.M.; Szeler, K.; Ben-David, M.; Kamerlin, S.C.L. Active Site Hydrophobicity and the Convergent Evolution of Paraoxonase Activity in Structurally Divergent Enzymes: The Case of Serum Paraoxonase 1. J. Am. Chem. Soc. 2017, 139, 1155–1167. [Google Scholar] [CrossRef] [PubMed]
- Mahrooz, A.; Mackness, M.; Bagheri, A.; Ghaffari-Cherati, M.; Masoumi, P. The epigenetic regulation of paraoxonase 1 (PON1) as an important enzyme in HDL function: The missing link between environmental and genetic regulation. Clin. Biochem. 2019, 73, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Khersonsky, O.; Tawfik, D.S. The Histidine 115-Histidine 134 Dyad Mediates the Lactonase Activity of Mammalian Serum Paraoxonases. J. Biol. Chem. 2006, 281, 7649–7656. [Google Scholar] [CrossRef] [PubMed]
- Grunkemeyer, T.J.; Mata, D.G.; Doddapaneni, K.; Murali, S.; Magliery, T.J. Insights into the mechanism of paraoxonase-1: Comparing the reactivity of the six-bladed β-propeller hydrolases. Biochemistry 2018. [Google Scholar] [CrossRef]
- Rajkovic, M.G.; Rumora, L.; Barisic, K. The paraoxonase 1, 2 and 3 in humans. Biochem. Med. 2011, 21, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Gugliucci, A.; Menini, T. Paraoxonase 1 and HDL maturation. Clin. Chim. Acta 2015, 439, 5–13. [Google Scholar] [CrossRef]
- Mackness, M.; Mackness, B. Human paraoxonase-1 (PON1): Gene structure and expression, promiscuous activities and mul-tiple physiological roles. Gene 2015, 567, 12–21. [Google Scholar] [CrossRef]
- Gaidukov, L.; Rosenblat, M.; Aviram, M.; Tawfik, D.S. The 192R/Q polymorphs of serum paraoxonase PON1 differ in HDL binding, lipolactonase stimulation, and cholesterol efflux. J. Lipid Res. 2006, 47, 2492–2502. [Google Scholar] [CrossRef]
- Deakin, S.; Moren, X.; James, R.W. Very low density lipoproteins provide a vector for secretion of paraoxonase-1 from cells. Atherosclerosis 2005, 179, 17–25. [Google Scholar] [CrossRef]
- Fuhrman, B.; Volkova, N.; Aviram, M. Paraoxonase 1 (PON1) is present in postprandial chylomicrons. Atherosclerosis 2005, 180, 55–61. [Google Scholar] [CrossRef]
- Deakin, S.P.; Bioletto, S.; Bochaton-Piallat, M.L.; James, R.W. HDL-associated paraoxonase-1 can redistribute to cell mem-branes and influence sensitivity to oxidative stress. Free Radic. Biol. Med. 2011, 50, 102–109. [Google Scholar] [CrossRef]
- Aviram, M.; Vaya, J. Paraoxonase 1 activities, regulation, and interactions with atherosclerotic lesion. Curr. Opin. Lipidol. 2013, 24, 339–344. [Google Scholar] [CrossRef]
- Furlong, C.E.; Marsillach, J.; Jarvik, G.P.; Costa, L.G. Paraoxonases-1, -2 and -3: What are their functions? Chem. Biol. Interact. 2016, 259, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Tomàs, E.; Acosta, J.C.; Torres-Royo, L.; De Febrer, G.; Baiges-Gaya, G.; Castañé, H.; Jiménez, A.; Vasco, C.; Araguas, P.; Gómez, J.; et al. Effect of Low-Dose Radi-otherapy on the Circulating Levels of Paraoxonase-1-Related Variables and Markers of Inflammation in Patients with COVID-19 Pneumonia. Antioxidants 2022, 11, 1184. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Gao, X.; Yang, S.; Meng, M.; Yang, X.; Ge, B. The role of endoplasmic reticulum stress in endothelial dysfunction induced by homocysteine thiolactone. Fundam. Clin. Pharmacol. 2015, 29, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Jakubowski, X.H. Homocysteine modification in protein structure/function and human disease. Physiol. Rev. 2019, 99, 555–604. Available online: www.prv.org (accessed on 2 February 2023). [CrossRef] [PubMed]
- Borowczyk, K.; Shih, D.M.; Jakubowski, H. Metabolism and Neurotoxicity of Homocysteine Thiolactone in Mice: Evidence for a Protective Role of Paraoxonase 1. J. Alzheimer’s Dis. 2012, 30, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Marsillach, J.; Suzuki, S.M.; Richter, R.J.; McDonald, M.G.; Rademacher, P.M.; MacCoss, M.J.; Hsieh, E.J.; Rettie, A.E.; Furlong, A.E. Human valacyclovir hydrolase/biphenyl hydrolase-like protein is a highly efficient homocysteine thiolactonase. PLoS ONE 2014, 9, e110054. [Google Scholar] [CrossRef]
- Eryanni-Levin, S.; Khatib, S.; Levy-Rosenzvig, R.; Tamir, S.; Szuchman-Sapir, A. 5,6-δ-DHTL, a stable metabolite of arachidonic acid, is a potential substrate for paraoxonase 1. Biochim. Biophys. Acta Mol. Cell Biol. Lipids. 2015, 1851, 1118–1122. [Google Scholar] [CrossRef]
- Teiber, J.F.; Xiao, J.; Kramer, G.L.; Ogawa, S.; Ebner, C.; Wolleb, H.; Carreira, E.; Shih, D.; Haley, R. Identification of biologically active δ-lactone eicosanoids as paraoxonase substrates. Biochem. Biophys. Res. Commun. 2018, 505, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Meijide, S.; Pérez-Ruiz, I.; Hernández, M.L.; Navarro, R.; Ferrando, M.; Larreategui, Z.; Ruiz-Sanz, J.-I.; Ruiz-Larrea, M.B. Paraoxonase activities in human follicular fluid: Role in follicular maturation. Reprod. Biomed. Online 2017, 35, 351–362. [Google Scholar] [CrossRef] [PubMed]
- Aviram, M.; Rosenblat, M.; Bisgaier, C.L.; Newton, R.S.; Primo-Parmo, S.L.; Du, B.N.L. Paraoxonase Inhibits High-density Lipoprotein Oxidation and Preserves its Functions A Possible Peroxidative Role for paraoxonase. J. Clin. Investig. 1998, 101, 1581–1590. Available online: http://www.jci.org (accessed on 2 February 2023). [CrossRef] [PubMed]
- Yang, Y.-H.; Aloysius, H.; Inoyama, D.; Chen, Y.; Hu, L.-Q. Enzyme-mediated hydrolytic activation of prodrugs. Acta Pharm. Sin. B 2011, 1, 143–159. [Google Scholar] [CrossRef]
- Tougou, K.; Nakamura, A.; Watanabe, S.; Okuyama, Y.; Morino, A. Paraoxonase has a major role in the hydrolysis of prulifloxacin (NM441), a prodrug of a new antibacterial agent. Drug Metab. Dispos. 1998, 26, 355–359. [Google Scholar]
- Ishizuka, T.; Fujimori, I.; Nishida, A.; Sakurai, H.; Yoshigae, Y.; Nakahara, K.; Kurihara, A.; Ikeda, T.; Izumi, T. Paraoxonase 1 as a major bioactivating hydrolase for olmesartan medoxomil in human blood circulation: Molecular identification and contribution to plasma metabolism. Drug Metab. Dispos. 2012, 40, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Biggadike, K.; Angell, R.M.; Burgess, C.M.; Farrell, R.M.; Hancock, A.P.; Harker, A.J.; Irving, W.R.; Ioannou, C.; Procopiou, P.A.; Shaw, R.E.; et al. Selective Plasma Hydrolysis of Glucocorticoid γ-Lactones and Cyclic Carbonates by the Enzyme Paraoxonase: An Ideal Plasma Inactivation Mechanism. J. Med. Chem. 1999, 43, 19–21. [Google Scholar] [CrossRef]
- Aviram, M.; Fuhrman, B. Wine Flavonoids Protect against LDL Oxidation and Atherosclerosis. Ann. N. Y. Acad. Sci. 2002, 957, 146–161. [Google Scholar] [CrossRef]
- Meneses, M.J.; Silvestre, R.; Sousa-Lima, I.; Macedo, M.P. Paraoxonase-1 as a regulator of glucose and lipid homeostasis: Impact on the onset and progression of metabolic disorders. Int. J. Mol. Sci. 2019, 20, 4049. [Google Scholar] [CrossRef]
- Poznyak, A.V.; Nikiforov, N.G.; Markin, A.M.; Kashirskikh, D.A.; Myasoedova, V.A.; Gerasimova, E.V.; Orekhov, A.N. Overview of OxLDL and Its Impact on Cardiovascular Health: Focus on Atherosclerosis. Front. Pharmacol. 2021, 11, 613780. [Google Scholar] [CrossRef]
- Shih, D.M.; Gu, L.; Xia, Y.R.; Navab, M.; Li, W.F.; Hama, S.; Castellani, L.W.; Furlong, C.E.; Costa, L.G.; Fogelman, A.M.; et al. Mice lacking serum paraoxonase are susceptible to organo-phosphate toxicity and atherosclerosis. Nature 1998, 394, 284–287. [Google Scholar] [CrossRef]
- Liu, Y.; Mackness, B.; Mackness, M. Comparison of the ability of paraoxonases 1 and 3 to attenuate the in vitro oxidation of low-density lipoprotein and reduce macrophage oxidative stress. Free. Radic. Biol. Med. 2008, 45, 743–748. [Google Scholar] [CrossRef] [PubMed]
- Rosenblat, M.; Vaya, J.; Shih, D.; Aviram, M. Paraoxonase 1 (PON1) enhances HDL-mediated macrophage cholesterol efflux via the ABCA1 transporter in association with increased HDL binding to the cells: A possible role for lysophosphatidylcholine. Atherosclerosis 2005, 179, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Rozenberg, O.; Shih, D.M.; Aviram, M. Human serum paraoxonase 1 decreases macrophage cholesterol biosynthesis: Possible role for its phospholipase-A2-like activity and lysophosphatidylcholine formation. Arter. Thromb. Vasc. Biol. 2003, 23, 461–467. [Google Scholar] [CrossRef]
- White, C.R.; Anantharamaiah, G.M. Cholesterol reduction and macrophage function: Role of paraoxonases. Curr. Opin. Lipidol. 2017, 28, 397–402. [Google Scholar] [CrossRef]
- Fuhrman, B.; Volkova, N.; Aviram, M. Oxidative stress increases the expression of the CD36 scavenger receptor and the cellular uptake of oxidized low-density lipoprotein in macrophages from atherosclerotic mice: Protective role of antioxidants and of paraoxonase. Atherosclerosis 2002, 161, 307–316. Available online: www.elsevier.com/locate/atherosclerosis (accessed on 2 February 2023). [CrossRef] [PubMed]
- Rozenberg, O.; Shih, D.M.; Aviram, M. Paraoxonase 1 (PON1) attenuates macrophage oxidative status: Studies in PON1 transfected cells and in PON1 transgenic mice. Atherosclerosis 2005, 181, 9–18. [Google Scholar] [CrossRef]
- Efrat, M.; Aviram, M. Macrophage paraoxonase 1 (PON1) binding sites. Biochem. Biophys. Res. Commun. 2008, 376, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Camps, J.; Castañé, H.; Rodríguez-Tomàs, E.; Baiges-Gaya, G.; Hernández-Aguilera, A.; Arenas, M.; Iftimie, S.; Joven, J. On the Role of Paraoxonase-1 and Chemokine Ligand 2 (C-C motif) in Metabolic Alterations Linked to Inflammation and Disease. A 2021 Update. Biomolecules 2021, 11, 971. Available online: https://www.mdpi.com/2218-273X/11/7/971 (accessed on 2 February 2023). [CrossRef]
- Camps, J.; Hernandez-Aguilera, A.; Garcia-Heredia, A.; Cabre, N.; Luciano-Mateo, F.; Arenas, M.; Joven, J. Relationships Between Metformin, Paraoxonase-1 and the Chemokine (C-C Motif) Ligand 2. Curr. Clin. Pharm. 2016, 11, 250–258. Available online: http://www.eurekaselect.com/openurl/content.php?genre=article&issn=1574-8847&volume=11&issue=4&spage=250 (accessed on 2 February 2023). [CrossRef]
- Loued, S.; Isabelle, M.; Berrougui, H.; Khalil, A. The anti-inflammatory effect of paraoxonase 1 against oxidized lipids depends on its association with high density lipoproteins. Life Sci. 2012, 90, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Mackness, M.; Mackness, B. Paraoxonase 1 and atherosclerosis: Is the gene or the protein more important? Free. Radic. Biol. Med. 2004, 37, 1317–1323. [Google Scholar] [CrossRef] [PubMed]
- Aharoni, S.; Aviram, M.; Fuhrman, B. Paraoxonase 1 (PON1) reduces macrophage inflammatory responses. Atherosclerosis 2013, 228, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Rosenblat, M.; Volkova, N.; Ward, J.; Aviram, M. Paraoxonase 1 (PON1) inhibits monocyte-to-macrophage differentiation. Atherosclerosis 2011, 219, 49–56. [Google Scholar] [CrossRef]
- Cho, K.-H.; Kim, J.-R.; Lee, I.-C.; Kwon, H.-J. Native High-Density Lipoproteins (HDL) with Higher Paraoxonase Exerts a Potent Antiviral Effect against SARS-CoV-2 (COVID-19), While Glycated HDL Lost the Antiviral Activity. Antioxidants 2021, 10, 209. [Google Scholar] [CrossRef]
- Tang, W.W.; Hartiala, J.; Fan, Y.; Wu, Y.; Stewart, A.F.; Erdmann, J.; Kathiresan, S.; Roberts, R.; McPherson, R.; Allayee, H.; et al. Clinical and Genetic Association of Serum Paraoxonase and Arylesterase Activities With Cardiovascular Risk. Arter. Thromb. Vasc. Biol. 2012, 32, 2803–2812. [Google Scholar] [CrossRef]
- Shokri, Y.; Variji, A.; Nosrati, M.; Khonakdar-Tarsi, A.; Kianmehr, A.; Kashi, Z.; Bahar, A.; Bagheri, A.; Mahrooz, A. Importance of paraoxonase 1 (PON1) as an antioxidant and antiatherogenic enzyme in the cardiovascular complications of type 2 diabetes: Genotypic and phenotypic evaluation. Diabetes Res. Clin. Pract. 2020, 161, 108067. [Google Scholar] [CrossRef]
- Medina-Díaz, I.M.; Ponce-Ruíz, N.; Rojas-García, A.E.; Zambrano-Zargoza, J.F.; Bernal-Hernández, Y.Y.; González-Arias, C.A.; Barrón-Vivanco, B.S.; Herrera-Moreno, J.F. The Relationship between Cancer and Paraoxonase 1. Antioxidants 2022, 11, 697. [Google Scholar] [CrossRef]
- Zhou, M.; Zhao, G.; Zeng, Y.; Zhu, J.; Cheng, F.; Liang, W. Aging and Cardiovascular Disease: Current Status and Challenges. Rev. Cardiovasc. Med. 2022, 23, 135. [Google Scholar] [CrossRef]
- Ashiq, S.; Ashiq, K. The Role of Paraoxonase 1 (PON1) Gene Polymorphisms in Coronary Artery Disease: A Systematic Review and Meta-Analysis. Biochem. Genet. 2021, 59, 919–939. [Google Scholar] [CrossRef]
- Kowalska, K.; Socha, E.; Milnerowicz, H. Review: The role of paraoxonase in cardiovascular diseases. Ann. Clin. Lab. Sci. 2015, 45, 226–233. [Google Scholar]
- Kotur-Stevuljević, J.; Vekić, J.; Stefanović, A.; Zeljković, A.; Ninić, A.; Ivanišević, J.; Miljković, M.; Sopić, M.; Munjas, J.; Mihajlović, M.; et al. Paraoxonase 1 and atherosclerosis-related diseases. Biofactors 2020, 46, 193–205. [Google Scholar] [CrossRef] [PubMed]
- Cervellati, C.; Valacchi, G.; Tisato, V.; Zuliani, G.; Marsillach, J. Evaluating the link between Paraoxonase-1 levels and Alzheimer’s disease development. Minerva Med. 2019, 110, 238–250. [Google Scholar] [CrossRef] [PubMed]
- Reichert, C.O.; Levy, D.; Bydlowski, S.P. Paraoxonase Role in Human Neurodegenerative Diseases. Antioxidants 2020, 10, 11. [Google Scholar] [CrossRef]
- Salari, N.; Rasoulpoor, S.; Hosseinian-Far, A.; Razazian, N.; Mansouri, K.; Mohammadi, M.; Vaisi-Raygani, A.; Jalali, R.; Shabani, S. Association between serum paraoxonase 1 activity and its polymorphisms with multiple sclerosis: A systematic review. Neurol. Sci. 2020, 42, 491–500. [Google Scholar] [CrossRef] [PubMed]
- Kotani, K.; Watanabe, J.; Miura, K.; Gugliucci, A. Paraoxonase 1 and Non-Alcoholic Fatty Liver Disease: A Meta-Analysis. Molecules 2021, 26, 2323. [Google Scholar] [CrossRef]
- Bassu, S.; Mangoni, A.A.; Satta, R.; Argiolas, D.; Carru, C.; Zinellu, A. Paraoxonase and arylesterase activity of serum PON-1 enzyme in psoriatic patients: A systematic review and meta-analysis. Clin. Exp. Med. 2022, 1–11. [Google Scholar] [CrossRef]
- Khalaf, F.K.; Mohammed, C.J.; Dube, P.; Connolly, J.A.; Lad, A.; Ashraf, U.M.; Breidenbach, J.D.; Su, R.C.; Kleinhenz, A.L.; Malhotra, D.; et al. Paraoxonase-1 Regulation of Renal In-flammation and Fibrosis in Chronic Kidney Disease. Antioxidants 2022, 11, 900. [Google Scholar] [CrossRef]
- Watanabe, J.; Kotani, K.; Gugliucci, A. Paraoxonase 1 and Chronic Obstructive Pulmonary Disease: A Meta-Analysis. Antioxidants 2021, 10, 1891. [Google Scholar] [CrossRef]
- Samra, Z.Q.; Pervaiz, S.; Shaheen, S.; Dar, N.; Athar, M.A. Determination of oxygen derived free radicals producer (xanthine oxidase) and scavenger (paraoxonase1) enzymes and lipid parameters in different cancer patients. Clin. Lab. 2011, 57, 741–747. [Google Scholar]
- Bacchetti, T.; Ferretti, G.; Sahebkar, A. The role of paraoxonase in cancer. Semin. Cancer Biol. 2019, 56, 72–86. [Google Scholar] [CrossRef] [PubMed]
- Borris, M.; Aldonza, D.; Son, Y.S.; Sung, H.-J.; Mo Ahn, J.; Choi, Y.-J.; Cho, S.; Cho, J.-Y. Paraoxonase-1 (PON1) induces metastatic potential and apoptosis escape via its antioxidative function in lung cancer cells. Available online: www.impactjournals.com/oncotarget (accessed on 2 February 2023).
- Li, X.; Yu, Q. PON1 hypermethylation is associated with progression of renal cell carcinoma. J. Cell. Mol. Med. 2019, 23, 6646–6657. [Google Scholar] [CrossRef] [PubMed]
- El-Tokhy, A.K.; Zin, E.-D.D.H.; Foda, A.A.M.; Moussa, G.I.; Abo, E.-N.S.E.-D.A.E.-F. The interplay between paraoxonase-1 and epigenetic changes in colorectal carcinoma. Afr. J. Biochem. Res. 2018, 12, 63–72. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Liu, S.; Ding, G.; Liu, W.; Zhou, J.; Kuang, M.; Ji, Y.; Kondo, T.; Fan, J. Quantitative Proteomic Analysis Identified Paraoxonase 1 as a Novel Serum Biomarker for Microvascular Invasion in Hepatocellular Carcinoma. J. Proteome Res. 2013, 12, 1838–1846. [Google Scholar] [CrossRef]
- Ding, G.-Y.; Zhu, X.-D.; Ji, Y.; Shi, G.-M.; Shen, Y.-H.; Zhou, J.; Fan, J.; Sun, H.-C.; Lv, Y. Serum PON1 as a biomarker for the estimation of microvascular invasion in hepatocellular carcinoma. Ann. Transl. Med. 2020, 8, 204. [Google Scholar] [CrossRef]
- Jasinski, M.; Olszewska-Slonina, D. Serum Paraoxonase-1 Activity and the Risk of Prostate Cancer Recurrence in Patients Treated with Radiotherapy. Antioxidants 2022, 11, 346. [Google Scholar] [CrossRef]
- Begue, F.; Tanaka, S.; Mouktadi, Z.; Rondeau, P.; Veeren, B.; Diotel, N.; Tran-Dinh, A.; Robert, T.; Vélia, E.; Mavingui, P.; et al. Altered high-density lipoprotein composition and functions during severe COVID-19. Sci. Rep. 2021, 11, 1–16. [Google Scholar] [CrossRef]
- Cho, K.-H. Importance of Apolipoprotein A-I and A-II Composition in HDL and Its Potential for Studying COVID-19 and SARS-CoV-2. Medicines 2021, 8, 38. [Google Scholar] [CrossRef]
- Rodríguez-Tomàs, E.; Iftimie, S.; Castañé, H.; Baiges-Gaya, G.; Hernández-Aguilera, A.; González-Viñas, M.; Castro, A.; Camps, J.; Joven, J. Clinical Perfor-mance of Paraoxonase-1-Related Variables and Novel Markers of Inflammation in Coronavirus Disease-19. A Machine Learning Approach. Antioxidants 2021, 10, 991. [Google Scholar] [CrossRef]
- Gabaldó, X.; Juanpere, M.; Castañé, H.; Rodríguez-Tomàs, E.; López-Azcona, A.F.; Baiges-Gaya, G.; Castro, L.; Valverde-Díaz, E.; Muñoz-Blázquez, A.; Giménez-Cuenca, L.; et al. Usefulness of the Measurement of Serum Paraoxonase-1 Arylesterase Activity in the Diagnoses of COVID-19. Biomolecules. 2022, 12, 879. [Google Scholar] [CrossRef]
- Tsai, Y.-H.; Lein, P.J. Mechanisms of organophosphate neurotoxicity. Curr. Opin. Toxicol. 2021, 26, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Costa, L.G.; Richter, R.J.; Li, W.-F.; Cole, T.; Guizzetti, M.; Furlong, C.E. Paraoxonase (PON 1) as a biomarker of susceptibility for organophosphate toxicity. Biomarkers 2003, 8, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ivanović, S.R.; Dimitrijević, B.; Ćupić, V.; Jezdimirović, M.; Borozan, S.; Savić, M.; Savić, D. Downregulation of nicotinic and muscarinic receptor function in rats after subchronic exposure to diazinon. Toxicol. Reports 2016, 3, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Lukaszewicz-Hussain, A. Role of oxidative stress in organophosphate insecticide toxicity—Short review. Pestic. Biochem. Physiol. 2010, 98, 145–150. [Google Scholar] [CrossRef]
- Vanova, N.; Pejchal, J.; Herman, D.; Dlabkova, A.; Jun, D. Oxidative stress in organophosphate poisoning: Role of standard an-tidotal therapy. J. Appl. Toxicol. 2018, 38, 1058–1070. [Google Scholar] [CrossRef]
- Farkhondeh, T.; Mehrpour, O.; Forouzanfar, F.; Roshanravan, B.; Samarghandian, S. Oxidative stress and mitochondrial dys-function in organophosphate pesticide-induced neurotoxicity and its amelioration: A review. Environ. Sci. Pollut. Res. 2020, 27, 24799–24814. [Google Scholar] [CrossRef]
- Costa, L.G.; Cole, T.B.; Furlong, C.E. Polymorphisms of Paraoxonase (PON1) and Their Significance in Clinical Toxicology of Organophosphates. J. Toxicol. Clin. Toxicol. 2003, 41, 37–45. [Google Scholar] [CrossRef]
- Furlong, C.; Suzuki, S.; Stevens, R.; Marsillach, J.; Richter, R.; Jarvik, G.; Checkoway, H.; Samii, A.; Costa, L.; Griffith, A.; et al. Human PON1, a biomarker of risk of disease and exposure. Chem. Biol. Interact. 2010, 187, 355–361. [Google Scholar] [CrossRef]
- Osaki, F.; Ikeda, Y.; Suehiro, T.; Ota, K.; Tsuzura, S.; Arii, K.; Kumon, Y.; Hashimoto, K. Roles of Sp1 and protein kinase C in regulation of human serum paraoxonase 1 (PON1) gene transcription in HepG2 cells. Atherosclerosis 2004, 176, 279–287. [Google Scholar] [CrossRef]
- Medina-Díaz, I.M.; Ponce-Ruiz, N.; Ramírez-Chávez, B.; Rojas-García, A.E.; Barrón-Vivanco, B.S.; Elizondo, G.; Bernal-Hernández, Y.Y. Downregulation of human paraoxonase 1 (PON1) by organophosphate pesticides in HepG2 cells. Environ. Toxicol. 2016, 32, 490–500. [Google Scholar] [CrossRef]
- Camps, J.; García-Heredia, A.; Rull, A.; Alonso-Villaverde, C.; Aragonès, G.; Beltrán-Debón, R.; Rodríguez-Gallego, E.; Joven, J. PPARs in Regulation of Paraoxonases: Control of Oxidative Stress and Inflammation Pathways. PPAR Res. 2012, 2012, 616371. [Google Scholar] [CrossRef]
- Solenkova, N.V.; Newman, J.D.; Berger, J.S.; Thurston, G.; Hochman, J.S.; Lamas, G.A. Metal pollutants and cardiovascular disease: Mechanisms and consequences of exposure. Am. Heart J. 2014, 168, 812–822. [Google Scholar] [CrossRef] [PubMed]
- Costa, L.G.; Cole, T.B.; Garrick, J.M.; Marsillach, J.; Furlong, C.E. Metals and Paraoxonases. In Advances in Neurobiology; Springer: Berlin/Heidelberg, Germany, 2017; pp. 85–111. [Google Scholar]
- Erdös, E.G.; Boggs, L.E. Hydrolysis of Paraoxon in Mammalian Blood. Nature 1961, 190, 716–717. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Fan, Y.; Sheng, J.; Gu, L.; Tao, Q.; Huang, R.; Liu, K.; Yang, L.; Chen, G.; Cao, H.; et al. Association Between Blood Heavy Metal Concentrations and Dyslipidemia in the Elderly. Biol. Trace Element Res. 2020, 199, 1280–1290. [Google Scholar] [CrossRef]
- Josse, D.; Lockridge, O.; Xie, W.; Bartels, C.F.; Schopfer, L.M.; Masson, P. The active site of human paraoxonase (PON1). J. Appl. Toxicol. 2001, 21, S7–S11. [Google Scholar] [CrossRef]
- Kamal, M.; Fathy, M.M.; Taher, E.; Hasan, M.; Tolba, M. Assessment of the role of paraoxonase gene polymorphism (Q192R) and paraoxonase activity in the susceptibility to atherosclerosis among lead-exposed workers. Ann. Saudi Med. 2011, 31, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Laird, B.D.; Goncharov, A.B.; Ayotte, P.; Chan, H.M. Relationship between the esterase paraoxonase-1 (PON1) and metal con-centrations in the whole blood of Inuit in Canada. Chemosphere 2015, 120, 479–485. [Google Scholar] [CrossRef]
- Drescher, O.; Dewailly, E.; Diorio, C.; Ouellet, N.; Sidi, E.A.L.; Abdous, B.; Valera, B.; Ayotte, P. Methylmercury exposure, PON1 gene variants and serum paraoxonase activity in Eastern James Bay Cree adults. J. Expo. Sci. Environ. Epidemiol. 2014, 24, 608–614. [Google Scholar] [CrossRef]
- Ferretti, G.; Bacchetti, T. Effect of dietary lipids on paraoxonase-1 activity and gene expression. Nutr. Metab. Cardiovasc. Dis. 2012, 22, 88–94. [Google Scholar] [CrossRef]
- De Roos, N.M.; Schouten, E.G.; Scheek, L.M.; Van Tol, A.; Katan, M.B. Replacement of dietary saturated fat with trans fat reduces serum paraoxonase activity in healthy men and women. Metabolism 2002, 51, 1534–1537. [Google Scholar] [CrossRef]
- Cherki, M.; Derouiche, A.; Drissi, A.; El Messal, M.; Bamou, Y.; Idrissi-Ouadghiri, A.; Khalil, A.; Adlouni, A. Consumption of argan oil may have an antiatherogenic effect by improving paraoxonase activities and antioxidant status: Intervention study in healthy men. Nutr. Metab. Cardiovasc. Dis. 2005, 15, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, S.D.; Sok, D.-E. Beneficial effect of oleoylated lipids on paraoxonase 1: Protection against oxidative inactivation and stabilization. Biochem. J. 2003, 375, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Tomás, M.; Sentí, M.; Elosua, R.; Vila, J.; Sala, J.; Masià, R.; Marrugat, J. Interaction between the Gln-Arg 192 Variants of the Paraoxonase Gene and Oleic acid Intake as a Determinant of High-Density Lipoprotein Cholesterol and Paraoxonase Activity. Available online: www.elsevier.com/locate/ejphar (accessed on 2 February 2023).
- Efrat, M.; Rosenblat, M.; Mahmood, S.; Vaya, J.; Aviram, M. Di-oleoyl phosphatidylcholine (PC-18:1) stimulates paraoxonase 1 (PON1) enzymatic and biological activities: In vitro and in vivo studies. Atherosclerosis 2009, 202, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Fedor, D.; Kelley, D.S. Prevention of insulin resistance by n-3 polyunsaturated fatty acids. Curr. Opin. Clin. Nutr. Metab. Care 2009, 12, 138–146. [Google Scholar] [CrossRef]
- Varatharajalu, R.; Garige, M.; Leckey, L.C.; Gong, M.; Lakshman, M.R. Betaine protects chronic alcohol and ω-3 PUFA-mediated down-regulations of PON1 gene, serum PON1 and homocysteine thiolactonase activities with restoration of liver GSH. Alcohol. Clin. Exp. Res. 2010, 34, 424–431. [Google Scholar] [CrossRef]
- Calabresi, L.; Villa, B.; Canavesi, M.; Sirtori, C.R.; James, R.W.; Bernini, F.; Franceschini, G. An ω-3 Polyunsaturated Fatty Acid Concentrate Increases Plasma High-Density Lipoprotein 2 Cholesterol and Paraoxonase Levels in Patients with Familial Combined Hy-perlipidemia. Metabolism 2004, 53, 153–158. [Google Scholar] [CrossRef]
- Boemi, M.; Leviev, I.; Sirolla, C.; Pieri, C.; Marra, M.; James, R.W. Serum paraoxonase is reduced in type 1 diabetic patients com-pared to non-diabetic, first degree relatives; influence on the ability of HDL to protect LDL from oxidation. Atherosclerosis 2001, 155, 229–235. Available online: www.elsevier.com/locate/atherosclerosis (accessed on 2 February 2023). [CrossRef]
- Ikeda, Y.; Suehiro, T.; Inoue, M.; Nakauchi, Y.; Morita, T.; Arii, K.; Ito, H.; Kumon, Y.; Hashimoto, K. Serum paraoxonase activity and its relationship to diabetic complications in patients with non—Insulin-dependent diabetes mellitus. Metabolism 1998, 47, 598–602. [Google Scholar] [CrossRef]
- Karabina, S.A.P.; Lehner, A.N.; Frank, E.; Parthasarathy, S.; Santanam, N. Oxidative inactivation of paraoxonase—Implications in diabetes mellitus and atherosclerosis. Biochim. Biophys. Acta Gen. Subj. 2005, 1725, 213–221. [Google Scholar] [CrossRef]
- Hedrick, C.C.; Thorpe, S.R.; Fu, M.-X.; Harper, C.M.; Yoo, J.; Kim, S.-M.; Wong, H.; Peters, A.L. Glycation impairs high-density lipoprotein function. Diabetologia 2000, 43, 312–320. [Google Scholar] [CrossRef]
- Mastorikou, M.; Mackness, B.; Liu, Y. Glycation of paraoxonase-1 inhibits its activity and impairs the ability of high-density lipoprotein to metabolize membrane lipid hydroperoxides. Diabet. Med. 2008, 25, 1049–1055. [Google Scholar] [CrossRef] [PubMed]
- Rosenblat, M.; Sapir, O.; Aviram, M. Glucose Inactivates Paraoxonase 1 (PON1) and Displaces it from High Density Lipopro-tein (HDL) to a Free PON1 Form. In The Paraoxonases: Their Role in Disease Development and Xenobiotic Metabolism; Springer: Berlin/Heidelberg, Germany, 2008; pp. 35–49. [Google Scholar]
- Rosenblat, M.; Karry, R.; Aviram, M. Paraoxonase 1 (PON1) is a more potent antioxidant and stimulant of macrophage cho-lesterol efflux, when present in HDL than in lipoprotein-deficient serum: Relevance to diabetes. Atherosclerosis 2006, 187, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, Y.; Suehiro, T.; Arii, K.; Kumon, Y.; Hashimoto, K. High glucose induces transactivation of the human paraoxonase 1 gene in hepatocytes. Metabolism 2008, 57, 1725–1732. [Google Scholar] [CrossRef]
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.E.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (poly)phenolics in human health: Structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid. Redox Signal. 2013, 18, 1818–1892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rock, W.; Rosenblat, M.; Miller-Lotan, R.; Levy, A.P.; Elias, M.; Aviram, M. Consumption of Wonderful Variety Pomegranate Juice and Extract by Diabetic Patients Increases Paraoxonase 1 Association with High-Density Lipoprotein and Stimulates Its Catalytic Activities. J. Agric. Food Chem. 2008, 56, 8704–8713. [Google Scholar] [CrossRef] [PubMed]
- Aviram, M.; Rosenblat, M.; Gaitini, D.; Nitecki, S.; Hoffman, A.; Dornfeld, L.; Volkova, N.; Presser, D.; Attias, J.; Liker, H.; et al. Pomegranate juice consumption for 3 years by patients with carotid artery stenosis reduces common carotid intima-media thickness, blood pressure and LDL oxidation. Clin. Nutr. 2004, 23, 423–433. [Google Scholar] [CrossRef]
- Rosenblat, M.; Hayek, T.; Aviram, M. Anti-oxidative effects of pomegranate juice (PJ) consumption by diabetic patients on serum and on macrophages. Atherosclerosis 2006, 187, 363–371. [Google Scholar] [CrossRef]
- Rosenblat, M.; Volkova, N.; Attias, J.; Mahamid, R.; Aviram, M. Consumption of polyphenolic-rich beverages (mostly pome-granate and black currant juices) by healthy subjects for a short term increased serum antioxidant status, and the serum’s ability to attenuate macrophage cholesterol accumulation. Food Funct. 2010, 1, 99–109. [Google Scholar] [CrossRef]
- Parsaeyan, N.; Mozaffari–Khosravi, H.; Mozayan, M.R. Effect of pomegranate juice on paraoxonase enzyme activity in patients with type 2 diabetes. J. Diabetes Metab. Disord. 2012, 11, 11. [Google Scholar] [CrossRef]
- Wu, P.-T.; Fitschen, P.J.; Kistler, B.M.; Jeong, J.H.; Chung, H.R.; Aviram, M.; Phillips, S.A.; Fernhall, B.; Wilund, K.R. Effects of Pomegranate Extract Supplementation on Cardiovascular Risk Factors and Physical Function in Hemodialysis Patients. J. Med. Food 2015, 18, 941–949. [Google Scholar] [CrossRef]
- Fuhrman, B.; Volkova, N.; Aviram, M. Pomegranate juice polyphenols increase recombinant paraoxonase-1 binding to high-density lipoprotein: Studies in vitro and in diabetic patients. Nutrition 2010, 26, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Gouédard, C.; Barouki, R.; Morel, Y. Dietary Polyphenols Increase Paraoxonase 1 Gene Expression by an Aryl Hydrocarbon Receptor-Dependent Mechanism. Mol. Cell Biol. 2004, 24, 5209–5222. [Google Scholar] [CrossRef] [PubMed]
- Khateeb, J.; Gantman, A.; Kreitenberg, A.J.; Aviram, M.; Fuhrman, B. Paraoxonase 1 (PON1) expression in hepatocytes is upreg-ulated by pomegranate polyphenols: A role for PPAR-γ pathway. Atherosclerosis 2010, 208, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Martini, D.; Del Bo’, C.; Porrini, M.; Ciappellano, S.; Riso, P. Role of polyphenols and polyphenol-rich foods in the modulation of PON1 activity and expression. J. Nutr. Biochem. 2017, 48, 1–8. [Google Scholar] [CrossRef]
- Arab, Z.N.; Khayatan, D.; Razavi, S.M.; Zare, K.; Kheradkhah, E.; Momtaz, S.; Ferretti, G.; Bacchetti, T.; Sathyapalan, T.; Emami, S.A.; et al. Phytochemicals as Modulators of Paraoxonase-1 in Health and Diseases. Antioxidants 2022, 11, 1273. [Google Scholar] [CrossRef] [PubMed]
- Garige, M.; Gong, M.; Varatharajalu, R.; Lakshman, M.R. Quercetin up-regulates paraoxonase 1 gene expression via sterol reg-ulatory element binding protein 2 that translocates from the endoplasmic reticulum to the nucleus where it specifically in-teracts with sterol responsive element-like sequence in paraoxonase 1 promoter in HuH7 liver cells. Metabolism 2010, 59, 1372–1378. [Google Scholar]
- Cheng, C.-C.; Hsueh, C.-M.; Liang, K.-W.; Ting, C.-T.; Wen, C.-L.; Hsu, S.-L. Role of JNK and c-Jun signaling pathway in regulation of human serum paraoxonase 1 gene transcription by berberine in human HepG2 cells. Eur. J. Pharmacol. 2011, 650, 519–525. [Google Scholar] [CrossRef]
- Atrahimovich, D.; Vaya, J.; Tavori, H.; Khatib, S. Glabridin Protects Paraoxonase 1 from Linoleic Acid Hydroperoxide Inhibition via Specific Interaction: A Fluorescence-Quenching Study. J. Agric. Food Chem. 2012, 60, 3679–3685. [Google Scholar] [CrossRef]
- Schrader, C.; Ernst, I.M.A.; Sinnecker, H.; TSoukup, S.; Kulling, S.E.; Rimbach, G. Genistein as a potential inducer of the an-ti-atherogenic enzyme paraoxonase-1: Studies in cultured hepatocytes in vitro and in rat liver in vivo. J. Cell Mol. Med. 2012, 16, 2331–2341. [Google Scholar] [CrossRef]
- Oh, H.Y.; Kim, S.S.; Chung, H.-Y.; Yoon, S. Isoflavone Supplements Exert Hormonal and Antioxidant Effects in Postmenopausal Korean Women with Diabetic Retinopathy. J. Med. Food 2005, 8, 1–7. [Google Scholar] [CrossRef]
- Zhu, Y.; Huang, X.; Zhang, Y.; Wang, Y.; Liu, Y.; Sun, R.; Xia, M. Anthocyanin Supplementation Improves HDL-Associated Paraoxonase 1 Activity and Enhances Cholesterol Efflux Capacity in Subjects With Hypercholesterolemia. J. Clin. Endocrinol. Metab. 2014, 99, 561–569. [Google Scholar] [CrossRef]
- Fuhrman, B.; Aviram, M. Preservation of Paraoxonase Activity by Wine Flavonoids. Ann. N. Y. Acad. Sci. 2022, 957, 321–324. [Google Scholar] [CrossRef]
- Kaufman, P.B.; Duke, J.A.; Brielmann, H.; Boik, J.; Hoyt, J.E. A Comparative Survey of Leguminous Plants as Sources of the Isoflavones, Genistein and Daidzein: Implications for Human Nutrition and Health. J. Altern. Complement. Med. 1997, 3, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Schrader, C.; Schiborr, C.; Frank, J.; Rimbach, G. Curcumin induces paraoxonase 1 in cultured hepatocytes in vitro but not in mouse liver in vivo. Br. J. Nutr. 2010, 105, 167–170. [Google Scholar] [CrossRef] [PubMed]
- Roxo, D.F.; Arcaro, C.A.; Gutierres, V.O.; Costa, M.C.; Oliveira, J.O.; de Lima, T.F.O.; Assis, R.P.; Brunetti, I.L.; Baviera, A.M. Curcumin combined with metformin decreases glycemia and dyslipidemia, and increases paraoxonase activity in diabetic rats. Diabetol. Metab. Syndr. 2019, 11, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ganjali, S.; Blesso, C.N.; Banach, M.; Pirro, M.; Majeed, M.; Sahebkar, A. Effects of curcumin on HDL functionality. Pharmacol. Res. 2017, 119, 208–218. [Google Scholar] [CrossRef] [PubMed]
- Navarro-García, F.; Ponce-Ruíz, N.; Rojas-García, A.E.; Ávila-Villarreal, G.; Herrera-Moreno, J.F.; Barrón-Vivanco, B.S.; Ber-nal-Hernández, Y.Y.; González-Arias, C.A.; Medina-Díaz, I.M. The Role of Nutritional Habits and Moderate Red Wine Con-sumption in PON1 Status in Healthy Population. Appl. Sci. 2021, 11, 9503. [Google Scholar] [CrossRef]
- Wade, L.; Nadeem, N.; Young, I.S.; Woodside, J.V.; McGinty, A.; McMaster, C.; McEneny, J. α-Tocopherol induces proatherogenic changes to HDL2 & HDL3: An in vitro and ex vivo investigation. Atherosclerosis 2013, 226, 392–397. [Google Scholar] [CrossRef]
- Tsakiris, S.; Karikas, G.A.; Parthimos, T.; Tsakiris, T.; Bakogiannis, C.; Schulpis, K.H. Alpha-tocopherol supplementation prevents the exercise-induced reduction of serum paraoxonase 1/arylesterase activities in healthy individuals. Eur. J. Clin. Nutr. 2009, 63, 215–221. [Google Scholar] [CrossRef]
- Kim, D.S.; Marsillach, J.; E Furlong, C.; Jarvik, G.P. Pharmacogenetics of paraoxonase activity: Elucidating the role of high-density lipoprotein in disease. Pharmacogenomics 2013, 14, 1495–1515. [Google Scholar] [CrossRef]
- Kunes, J.P.; Cordero-Koning, K.S.; Lee, L.H.; Lynch, S.M. Vitamin C attenuates hypochlorite-mediated loss of paraoxonase-1 ac-tivity from human plasma. Nutr. Res. 2009, 29, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Taş, S.; Sarandöl, E.; Dirican, M. Vitamin B6 Supplementation Improves Oxidative Stress and Enhances Serum Paraoxonase/Arylesterase Activities in Streptozotocin-Induced Diabetic Rats. Sci. World J. 2014, 2014, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Manolescu, B.N.; Berteanu, M.; Cintezã, D. Effect of the nutritional supplement ALAnerv® on the serum PON1 activity in post-acute stroke patients. Pharmacol. Rep. 2013, 65, 743–750. [Google Scholar] [CrossRef] [PubMed]
- Salmonowicz, B.; Krzystek-Korpacka, M.; Noczyńska, A. Trace Elements, Magnesium, and the Efficacy of Antioxidant Systems in Children with Type 1 Diabetes Mellitus and in Their Siblings. Adv. Clin. Exp. Med. 2014, 23, 259–268. [Google Scholar] [CrossRef]
- Rahimi-Ardabili, B.; Argani, H.; Ghorbanihaghjo, A.; Rashtchizadeh, N.; Naghavi-Behzad, M.; Ghorashi, S.; Nezami, N. Paraoxonase Enzyme Activity Is Enhanced by Zinc Supplementation in Hemodialysis Patients. Ren. Fail. 2012, 34, 1123–1128. [Google Scholar] [CrossRef] [Green Version]
- Rovira, J.; Hernández-Aguilera, A.; Luciano-Mateo, F.; Cabré, N.; Baiges-Gaya, G.; Nadal, M.; Martín-Paredero, V.; Camps, J.; Joven, J.; Domingo, J.L. Trace Elements and Paraoxonase-1 Activity in Lower Extremity Artery Disease. Biol. Trace Elem. Res. 2018, 186, 74–84. [Google Scholar] [CrossRef]
- Luciano-Mateo, F.; Cabré, N.; Nadal, M.; García-Heredia, A.; Baiges-Gaya, G.; Hernández-Aguilera, A.; Camps, J.; Joven, J.; Domingo, J.L. Serum concentrations of trace elements and their relationships with paraoxonase-1 in morbidly obese women. J. Trace Elem. Med. Biol. 2018, 48, 8–15. [Google Scholar] [CrossRef]
- Begcevic, I.; Simundic, A.-M.; Nikolac, N.; Dobrijevic, S.; Rajkovic, M.G.; Tesija-Kuna, A. Can cranberry extract and vitamin C + Zn supplements affect the in vivo activity of paraoxonase 1, antioxidant potential, and lipid status? Clin. Lab. 2013, 59, 1053–1060. [Google Scholar] [CrossRef]
- Van Der Gaag, M.S.; Van Tol, A.; Scheek, L.M.; James, R.W.; Urgert, R.; Schaafsma, G.; Hendriks, H.F. Daily moderate alcohol consumption increases serum paraoxonase activity; a diet-controlled, randomised intervention study in middle-aged men. Atherosclerosis 1999, 147, 405–410. Available online: www.elsevier.com/locate/atherosclerosis (accessed on 2 February 2023). [CrossRef]
- Rao, M.N.; Marmillot, P.; Gong, M.; A Palmer, D.; Seeff, L.B.; Strader, D.B.; Lakshman, M. Light, but not heavy alcohol drinking, stimulates paraoxonase by upregulating liver mRNA in rats and humans. Metabolism 2003, 52, 1287–1294. [Google Scholar] [CrossRef]
- Marsillach, J.; Ferré, N.; Vila, M.C.; Lligoña, A.; Mackness, B.; Mackness, M.; Deulofeu, R.; Solá, R.; Parés, A.; Pedro-Botet, J.; et al. Serum paraoxonase-1 in chronic alcoholics: Re-lationship with liver disease. Clin. Biochem. 2007, 40, 645–650. [Google Scholar] [CrossRef] [PubMed]
- Markoski, M.M.; Garavaglia, J.; Oliveira, A.; Olivaes, J.; Marcadenti, A. Molecular properties of red wine compounds and cardi-ometabolic benefits. Nutr. Metab. Insights 2016, 9, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Lakshman, R.; Garige, M.; Gong, M.; Leckey, L.; Varatharajalu, R.; Zakhari, S. Is alcohol beneficial or harmful for cardioprotection? Genes Nutr. 2010, 5, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Dorajoo, R.; Ke, T.; Ayala, B.; Chang, X.; Khor, C.-C.; van Dam, R.M.; Yuan, J.-M.; Koh, W.-P.; Liu, J.; et al. Interaction effects between Paraoxonase 1 variants and cigarette smoking on risk of coronary heart disease in a Singaporean Chinese population. Atherosclerosis 2015, 240, 40–45. [Google Scholar] [CrossRef]
- Haj Mouhamed, D.; Ezzaher, A.; Mechri, A.; Neffati, F.; Omezzine, A.; Bouslama, A.; Gaha, L.; Douki, W.; Najjar, M.F. Effect of cigarette smoking on paraoxonase 1 activity according to PON1 L55M and PON1 Q192R gene polymorphisms. Environ. Health Prev. Med. 2012, 17, 316–321. [Google Scholar] [CrossRef] [Green Version]
- Marek, G.; Ściskalska, M.; Grzebieniak, Z.; Milnerowicz, H. Decreases in Paraoxonase-1 Activities Promote a Pro-inflammatory Effect of Lipids Peroxidation Products in Non-smoking and Smoking Patients with Acute Pancreatitis. Int. J. Med. Sci. 2018, 15, 1619–1630. [Google Scholar] [CrossRef]
- Bizoń, A.; Kepinska, M.; Snacki, K.; Milnerowicz, H. The impact of environmental and biological factors on paraoxonase 1 and γ-glutamyltranspeptydase activities in the blood of smelters. Int. J. Environ. Healthc. Res. 2015, 26, 222–238. [Google Scholar] [CrossRef]
- James, R.W.; Leviev, I.; Righetti, A. Smoking Is Associated With Reduced Serum Paraoxonase Activity and Concentration in Patients With Coronary Artery Disease. Circulation 2000, 101, 2252–2257. Available online: http://www.circulationaha.org (accessed on 2 February 2023). [CrossRef]
- lia Ferré, N.; Camps, J.; Ferná ndez-Ballart, J.; Arija, V.; Murphy, M.M.; Ceruelo, S.; Biarnés, E.; Vilella, E.; Tous, M.; Joven, J. Regulation of Serum Paraoxonase Activity by Genetic, Nutritional, and Lifestyle Factors in the General Population. Clin. Chem. 2003, 49, 1491–1497. Available online: https://academic.oup.com/clinchem/article/49/9/1491/5641995 (accessed on 2 February 2023). [CrossRef]
- Costa, L.G.; Vitalone, A.; Cole, T.B.; Furlong, C.E. Modulation of paraoxonase (PON1) activity. Biochem. Pharmacol. 2005, 69, 541–550. [Google Scholar] [CrossRef]
- Solak, Z.A.; Kabaroglu, C.; Cok, G.; Parıldar, Z.; Bayındır, U.; Özmen, D.; Bayındır, O.; Parildar, Z.; Bayindir, U.; Bayindir, O. Effect of different levels of cigarette smoking on lipid peroxidation, glutathione enzymes and paraoxonase 1 activity in healthy people. Clin. Exp. Med. 2005, 5, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Demir, Y. The behaviour of some antihypertension drugs on human serum paraoxonase-1: An important protector enzyme against atherosclerosis. J. Pharm. Pharmacol. 2019, 71, 1576–1583. [Google Scholar] [CrossRef] [PubMed]
- Türkeş, C.; Beydemir, Ş. Inhibition of Human Serum Paraoxonase-I with Antimycotic Drugs: In Vitro and In Silico Studies. Appl. Biochem. Biotechnol. 2020, 190, 252–269. [Google Scholar] [CrossRef] [PubMed]
- Entürk, M.; Ekinci, D.; Alici, H.A.; Beydemir, Ş. Paraoxonase-1, an organophosphate detoxifier and cardioprotective enzyme, is inhibited by anesthetics: An in vitro and in vivo insight. Pestic. Biochem. Physiol. 2011, 101, 206–211. [Google Scholar] [CrossRef]
- Türkeş, C. A potential risk factor for paraoxonase 1: In silico and in-vitro analysis of the biological activity of proton-pump inhibitors. J. Pharm. Pharmacol. 2019, 71, 1553–1564. [Google Scholar] [CrossRef]
- Beydemir, Ş.; Demir, Y. Antiepileptic drugs: Impacts on human serum paraoxonase-1. J. Biochem. Mol. Toxicol. 2017, 31, e21889. [Google Scholar] [CrossRef]
- Alici, H.A.; Ekinci, D.; Beydemir, S. Intravenous anesthetics inhibit human paraoxonase-1 (PON1) activity in vitro and in vivo. Clin. Biochem. 2008, 41, 1384–1390. [Google Scholar] [CrossRef]
- Alim, Z.; Beydemir, Ş. Some Anticancer Agents Act on Human Serum Paraoxonase-1 to Reduce Its Activity. Chem. Biol. Drug Des. 2016, 88, 188–196. [Google Scholar] [CrossRef]
- Ekinci, D.; Beydemir, Ş. Evaluation of the impacts of antibiotic drugs on PON 1; a major bioscavenger against cardiovascular diseases. Eur. J. Pharmacol. 2009, 617, 84–89. [Google Scholar] [CrossRef]
- Demir, Y.; Balcı, N.; Gürbüz, M. Differential effects of selective serotonin reuptake inhibitors on paraoxonase-1 enzyme ac-tivity: An in vitro study. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2019, 226, 108608. [Google Scholar] [CrossRef]
- Türkeş, C.; Söyüt, H.; Beydemir, S. Effect of calcium channel blockers on paraoxonase-1 (PON1) activity and oxidative stress. Pharmacol. Rep. 2014, 66, 74–80. [Google Scholar] [CrossRef]
- Işgör, M.M.; Beydemir, S. Some cardiovascular therapeutics inhibit paraoxonase 1 (PON1) from human serum. Eur. J. Pharmacol. 2010, 645, 135–142. [Google Scholar] [CrossRef]
- Alışkan, B.; Demir, Y.; Türkeş, C. Ophthalmic drugs: In vitro paraoxonase 1 inhibition and molecular docking studies. Bio-technol. Appl. Biochem. 2021, 69, 2273–2283. [Google Scholar] [CrossRef]
- Catella-Lawson, F.; Reilly, M.P.; Kapoor, S.C.; Cucchiara, A.J.; DeMarco, S.; Tournier, B.; Vyas, S.N.; FitzGerald, G.A. Cyclooxygenase Inhibitors and the Antiplatelet Effects of Aspirin. N. Engl. J. Med. 2001, 345, 1809–1817. [Google Scholar] [CrossRef] [PubMed]
- Blatter-Garin, M.C.; Kalix, B.; De Pree, S.; James, R.W. Aspirin use is associated with higher serum concentrations of the an-ti-oxidant enzyme, paraoxonase-1. Diabetologia 2003, 46, 593–594. [Google Scholar] [CrossRef] [PubMed]
- Mouhamed, D.H.; Ezzaher, A.; Gaha, L.; Douki, W.; Najjar, M.F. In vitro Effects of Salicylic Acid on Plasma Paraoxonase 1 Activity. J. Drug Metab. Toxicol. 2013, 4, 1000148. [Google Scholar] [CrossRef]
- Jaichander, P.; Selvarajano, K.; Garelnabi, M.; Parthasarathy, S. Induction of paraoxonase 1 and apolipoprotein A-I gene ex-pression by aspirin. J. Lipid Res. 2008, 49, 2142–2148. [Google Scholar] [CrossRef] [PubMed]
- Santanam, N.; Parthasarathy, S. Aspirin is a substrate for paraoxonase-like activity: Implications in atherosclerosis. Atherosclerosis 2007, 191, 272–275. [Google Scholar] [CrossRef] [PubMed]
- Bradley, S.M.; Rumsfeld, J.S. Depression and cardiovascular disease. Trends Cardiovasc. Med. 2015, 25, 614–622. [Google Scholar] [CrossRef] [PubMed]
- Moreira, E.G.; Correia, D.G.; Bonifácio, K.L.; de Moraes, J.B.; Cavicchioli, F.L.; Nunes, C.S.; Nunes, S.O.V.; Vargas, H.O.; Barbosa, D.S.; Maes, M. Lowered PON1 activities are strongly associated with depression and bipolar disorder, recurrence of (hypo)mania and depression, increased disability and lowered quality of life. World J. Biol. Psychiatry 2017, 20, 368–380. [Google Scholar] [CrossRef]
- Ceron, J.J.; Tecles, F.; Tvarijonaviciute, A. Serum paraoxonase 1 (PON1) measurement: An update. BMC Veter-Res. 2014, 10, 74. [Google Scholar] [CrossRef] [PubMed]
- Katı, C.; Karadas, S.; Aslan, M.; Gonullu, H.; Duran, L.; Demir, H. Serum Paraoxonase and Arylesterase Activities and Oxidative Stress Levels in Patients with SSRI Intoxication. J. Membr. Biol. 2013, 247, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Avcikurt, A.S.; Sinan, S.; Kockar, F. Antidepressant and antipsychotic drugs differentially affect PON1 enzyme activity. J. Enzym. Inhib. Med. Chem. 2014, 30, 245–249. [Google Scholar] [CrossRef] [PubMed]
- Saadaoui, M.H.; Hellara, I.; Neffati, F.; Mechri, A.; Douki, W.; Gaha, L.; Najjar, M.F. In vitro effects of three antidepressant drugs on plasma paraoxonase activity. Drug Metab. Drug Interact. 2012, 27, 209–215. [Google Scholar] [CrossRef]
- Işık, M.; Demir, Y.; Kırıcı, M.; Demir, R.; Şimşek, F.; Beydemir, S. Changes in the anti-oxidant system in adult epilepsy patients receiving anti-epileptic drugs. Arch. Physiol. Biochem. 2015, 121, 97–102. [Google Scholar] [CrossRef]
- Tutanc, M.; Aras, M.; Dokuyucu, R.; Altas, M.; Zeren, C.; Arica, V.; Ozturk, O.H.; Motor, S.; Yilmaz, C. Oxidative Status in Epileptic Children Using Carbamazepine. Iran. J. Pediatr. 2015, 25, e3885. [Google Scholar] [CrossRef]
- Lehrke, M.; Lazar, M.A. The many faces of PPARγ. Cell 2005, 123, 993–999. [Google Scholar] [CrossRef]
- Carreón-Torres, E.; Rendón-Sauer, K.; Monter-Garrido, M.; Toledo-Ibelles, P.; Gamboa, R.; Menjivar, M.; López-Marure, R.; Luc, G.; Fievet, C.; Cruz, D.; et al. Rosiglitazone modifies HDL structure and increases HDL-apo AI synthesis and catabolic rates. Clin. Chim. Acta 2009, 401, 37–41. [Google Scholar] [CrossRef]
- Atamer, Y.; Atamer, A.; Can, A.S.; Hekimoglu, A.; Ilhan, N.; Yenice, N.; Kocyigit, Y. Effects of rosiglitazone on serum paraoxonase activity and metabolic parameters in patients with type 2 diabetes mellitus. Braz. J. Med. Biol. Res. 2013, 46, 528–532. [Google Scholar] [CrossRef]
- Van Wijk, J.; Coll, B.; Cabezas, M.C.; Koning, E.; Camps, J.; Mackness, B.; Joven, J. Rosiglitazone modulates fasting and post-prandial paraoxonase 1 activity in type 2 diabetic patients. Clin. Exp. Pharmacol. Physiol. 2006, 33, 1134–1137. [Google Scholar] [CrossRef]
- Esteghamati, A.; Eskandari, D.; Mirmiranpour, H.; Noshad, S.; Mousavizadeh, M.; Hedayati, M.; Nakhjavani, M. Effects of metformin on markers of oxidative stress and antioxidant reserve in patients with newly diagnosed type 2 diabetes: A randomized clinical trial. Clin. Nutr. 2013, 32, 179–185. [Google Scholar] [CrossRef]
- Adeshara, K.A.; Bangar, N.S.; Doshi, P.R.; Diwan, A.; Tupe, R.S. Action of metformin therapy against advanced glycation, oxidative stress and inflammation in type 2 diabetes patients: 3 months follow-up study. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 1449–1458. [Google Scholar] [CrossRef] [PubMed]
- Meaney, A.I.; Paraoxonase, M. Available online: www.ctsjournal.com (accessed on 2 February 2023).
- Wójcicka, G.; Jamroz-Wiśniewska, A.; Czechowska, G.; Korolczuk, A.; Marciniak, S.; Bełtowski, J. The paraoxonase 1 (PON1), platelet-activating factor acetylohydrolase (PAF-AH) and dimethylarginine dimethylaminohydrolase (DDAH) activity in the metformin treated normal and diabetic rats. Eur. J. Pharmacol. 2016, 789, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Ferretti, G.; Bacchetti, T.; Moroni, C.; Savino, S.; Liuzzi, A.; Balzola, F.; Bicchiega, V. Paraoxonase Activity in High-Density Lipoproteins: A Comparison between Healthy and Obese Females. J. Clin. Endocrinol. Metab. 2005, 90, 1728–1733. [Google Scholar] [CrossRef]
- Mackness, B.; Durrington, P.; McElduff, P.; Yarnell, J.; Azam, N.; Watt, M.; Mackness, M. Low Paraoxonase Activity Predicts Coronary Events in the Caerphilly Prospective Study. Circulation 2003, 107, 2775–2779. [Google Scholar] [CrossRef] [Green Version]
- Cervellati, C.; Bonaccorsi, G.; Trentini, A.; Valacchi, G.; Sanz, J.M.; Squerzanti, M.; Spagnolo, M.; Massari, L.; Crivellari, I.; Greco, P.; et al. Paraoxonase, arylesterase and lactonase activities of paraoxonase-1 (PON1) in obese and severely obese women. Scand. J. Clin. Lab. Investig. 2018, 78, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Bełtowski, J.; Wójcicka, G.; Jamroz, A. Leptin decreases plasma paraoxonase 1 (PON1) activity and induces oxidative stress: The possible novel mechanism for proatherogenic effect of chronic hyperleptinemia. Atherosclerosis 2003, 170, 21–29. [Google Scholar] [CrossRef]
- Muls, E.; Kolanowski, J.; Scheen, A.; Gaal, L.V. The effects of orlistat on weight and on serum lipids in obese patients with hypercholesterolemia: A randomized, double-blind, placebo-controlled, multicentre study. Int. J. Obes. 2001, 25, 1713–1721. Available online: www.nature.com/ijo (accessed on 2 February 2023). [CrossRef]
- Audikovszky, M.; Pados, G.; Seres, I.; Harangi, M.; Fülöp, P.; Katona, E.; Illyés, L.; Winkler, G.; Katona, E.M.; Paragh, G. Orlistat increases serum paraoxonase activity in obese patients. Nutr. Metab. Cardiovasc. Dis. 2007, 17, 268–273. [Google Scholar] [CrossRef]
- Deakin, S.; Leviev, I.; Guernier, S.; James, R.W. Simvastatin Modulates Expression of the PON1 Gene and Increases Serum Paraoxonase: A Role for Sterol Regulatory Element-Binding Protein-2. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 2083–2089. [Google Scholar] [CrossRef]
- Ota, K.; Suehiro, T.; Arii, K.; Ikeda, Y.; Kumon, Y.; Osaki, F.; Hashimoto, K. Effect of pitavastatin on transactivation of human serum paraoxonase 1 gene. Metabolism 2005, 54, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Gouédard, C.; Koum-Besson, N.; Barouki, R.; Morel, Y. Opposite regulation of the human paraoxonase-1 gene PON-1 by feno-fibrate and statins. Mol. Pharmacol. 2003, 63, 945–956. [Google Scholar] [CrossRef] [PubMed]
- Arii, K.; Suehiro, T.; Ota, K.; Ikeda, Y.; Kumon, Y.; Osaki, F.; Inoue, M.; Inada, S.; Ogami, N.; Takata, H.; et al. Pitavastatin induces PON1 expression through p44/42 mito-gen-activated protein kinase signaling cascade in Huh7 cells. Atherosclerosis 2009, 202, 439–445. [Google Scholar] [CrossRef]
- Deakin, S.; Guernier, S.; James, R.W. Pharmacogenetics D: Pharmacogenetic interaction between paraoxonase-1 gene promoter polymorphism C-107T and statin. Pharm. Genomics. 2007, 17, 451–457. Available online: http://journals.lww.com/jpharmacogenetics (accessed on 2 February 2023). [CrossRef] [PubMed]
- Fuhrman, B.; Koren, L.; Volkova, N.; Keidar, S.; Hayek, T.; Aviram, M. Atorvastatin Therapy in Hypercholesterolemic Patients Suppresses Cellular Uptake of Oxidized-LDL by Differentiating Monocytes. Available online: www.elsevier.com/locate/atherosclerosis (accessed on 2 February 2023).
- Kassai, A.; Illyés, L.; Mirdamadi, H.Z.; Seres, I.; Kalmár, T.; Audikovszky, M.; Paragh, G. The effect of atorvastatin therapy on leci-thin:cholesterol acyltransferase, cholesteryl ester transfer protein and the antioxidant paraoxonase. Clin. Biochem. 2007, 40, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Mirdamadi, H.Z.; Sztanek, F.; Derdak, Z.; Seres, I.; Harangi, M.; Paragh, G. The human paraoxonase-1 phenotype modifies the effect of statins on paraoxonase activity and lipid parameters. Br. J. Clin. Pharmacol. 2008, 66, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Turfaner, N.; Uzun, H.; Balci, H.; Ercan, M.A.; Karter, Y.H.; Caner, M.; Sipahioglu, F.; Genc, H. Ezetimibe Therapy and Its Influence on Oxidative Stress and Fibrinolytic Activity. South Med. J. 2010, 103, 428–433. [Google Scholar] [CrossRef]
- Nakou, E.S.; Filippatos, T.D.; Kiortsis, D.N.; Derdemezis, C.S.; Tselepis, A.D.; Mikhailidis, D.P.; Elisaf, M.; Filippatos, T.; Elisaf, M. The effects of ezetimibe and orlistat, alone or in combination, on high-density lipoprotein (HDL) subclasses and, H.D.L.-associated enzyme activities in overweight and obese patients with hyperlipidaemia. Expert Opin. Pharm. 2008, 9, 3151–3158. [Google Scholar] [CrossRef]
- Guedes, J.V.M.; Nunes, N.R.; Ferreira, L.G.R.; Vilar, T.G.; Pinheiro, M.B.; Domingueti, C.P. Evaluation of lipid profile, high-sensitivity C-reactive protein and D-dimer in users of oral contraceptives of different types. J. Bras. De Patol. E Med. Lab. 2018, 54, 14–20. [Google Scholar] [CrossRef]
- Kowalska, K.; Ściskalska, M.; Bizoń, A.; Śliwińska-Mossoń, M.; Milnerowicz, H. Influence of oral contraceptives on lipid profile and paraoxonase and commonly hepatic enzymes activities. J. Clin. Lab. Anal. 2017, 32, e22194. [Google Scholar] [CrossRef]
- Vincent-Viry, M.; Sass, C.; Bastien, S.; Aguillon, D.; Siest, G.; Visvikis, S. PON1-192 Phenotype and Genotype Assessments in 918 Subjects of the Stanislas Cohort Study. Clin. Chem. Lab. Med. 2003, 41, 535–540. [Google Scholar] [CrossRef] [PubMed]
- van den Berg, E.H.; Gruppen, E.G.; James, R.W.; Bakker, S.J.L.; Dullaart, R.P.F. Serum paraoxonase 1 activity is paradoxically maintained in nonalcoholic fatty liver disease despite low HDL cholesterol. J. Lipid Res. 2019, 60, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Demir, Y. Naphthoquinones, benzoquinones, and anthraquinones: Molecular docking, ADME and inhibition studies on human serum paraoxonase-1 associated with cardiovascular diseases. Drug Dev. Res. 2020, 81, 628–636. [Google Scholar] [CrossRef] [PubMed]
- Thompson, E.W.; Demissei, B.G.; Smith, A.M.; Brahmbhatt, P.; Wang, J.; Clark, A.; DeMichele, A.; Narayan, V.; Shah, P.; Sun, L.; et al. Paraoxonase-1 Activity in Breast Cancer Patients Treated With Doxorubicin With or Without Trastuzumab. JACC: Basic Transl. Sci. 2021, 7, 1–10. [Google Scholar] [CrossRef]
- Luu, A.Z.; Chowdhury, B.; Al-Omran, M.; Teoh, H.; Hess, D.A.; Verma, S. Role of Endothelium in Doxorubicin-Induced Cardio-myopathy. JACC Basic Transl. Sci. 2018, 3, 861–870. [Google Scholar] [CrossRef] [PubMed]
- Deakin, S.P.; James, R.W. Genetic and environmental factors modulating serum concentrations and activities of the antioxidant enzyme paraoxonase-1. Clin. Sci. 2004, 107, 435–447. [Google Scholar] [CrossRef] [PubMed]
- Shunmoogam, N.; Naidoo, P.; Chilton, R. Paraoxonase (PON)-1: A brief overview on genetics, structure, polymorphisms and clinical relevance. Vasc. Healthc. Risk Manag. 2018, 14, 137–143. [Google Scholar] [CrossRef]
- Smolen, A.; Eckerson, H.W.; Gan, K.N.; Hailat, N.; La Du, B.N. Characteristics of the genetically determined allozymic forms of human serum paraoxonase/arylesterase. Drug Metab. Dispos. 1991, 19, 107–112. [Google Scholar]
- Costa, L.G.; Cole, T.B.; Jarvik, G.P.; Furlong, C.E. Functional Genomics of the Paraoxonase (PON1) Polymorphisms: Effects on Pesticide Sensitivity, Cardiovascular Disease, and Drug Metabolism. Annu. Rev. Med. 2003, 54, 371–392. [Google Scholar] [CrossRef]
- Brophy, V.H.; Jampsa, R.L.; Clendenning, J.B.; Mckinstry, L.A.; Jarvik, G.P.; Furlong, C.E. Effects of 5 Regulatory-Region Polymor-phisms on Paraoxonase-Gene (PON1) Expression. Am. J. Hum. Genet. 2001, 68, 1428–1436. [Google Scholar] [CrossRef]
- Mahrooz, A.; Hashemi-Soteh, M.B.; Heydari, M.; Boorank, R.; Ramazani, F.; Mahmoudi, A.; Kianmehr, A.; Alizadeh, A. Paraoxonase 1 (PON1)-L55M among common variants in the coding region of the paraoxonase gene family may contribute to the glycemic control in type 2 diabetes. Clin. Chim. Acta 2018, 484, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Chiu, K.C.; Chuang, L.-M.; Chu, A.; Lu, J.; Hu, J.; Fernando, S. Association of Paraoxonase 1 Polymorphism With Beta-Cell Function A Case of Molecular Heterosis. Pancreasjournal 2004, 28, e96–e103. Available online: http://journals.lww.com/pancreasjournal (accessed on 2 February 2023). [CrossRef] [PubMed]
- Barbieri, M.; Bonafe`, M.; Bonafe`, B.; Marfella, R.; Ragno, E.; Giugliano, D.; Franceschi, C.; Paolisso, G. LL-Paraoxonase Genotype Is Associated with a More Severe Degree of Homeostasis Model Assessment IR in Healthy Subjects. Available online: https://academic.oup.com/jcem/article/87/1/222/2847023 (accessed on 2 February 2023).
- Wang, J.; Yang, M.M.; Rong, S.S.; Ng, T.K.; Li, Y.B.; Liu, X.M. Association of paraoxonase gene polymorphisms with diabetic nephropathy and retinopathy. Mol. Med. Rep. 2013, 8, 1845–1851. [Google Scholar] [CrossRef] [PubMed]
- Saadat, M. Prevalence and mortality of COVID-19 are associated with the L55M functional polymorphism of Paraoxonase 1. Proc. Singap. Healthc. 2021, 31, 20101058211040582. [Google Scholar] [CrossRef]
- Draganov, D.I.; La Du, B.N. Pharmacogenetics of paraoxonases: A brief review. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2004, 369, 78–88. [Google Scholar] [CrossRef]
- Ponce-Ruiz, N.; Murillo-González, F.E.; Rojas-García, A.E.; Mackness, M.; Bernal-Hernández, Y.Y.; Barrón-Vivanco, B.S.; González-Arias, C.A.; Medina-Díaz, I.M. Tran-scriptional regulation of human Paraoxonase 1 by nuclear receptors. Chem. Biol. Interact. 2017, 268, 77–84. [Google Scholar] [CrossRef]
- Marchesani, M.; Hakkarainen, A.; Tuomainen, T.-P.; Kaikkonen, J.; Pukkala, E.; Uimari, P.; Seppälä, E.; Matikainen, M.; Kallioniemi, O.-P.; Schleutker, J.; et al. New Paraoxonase 1 Polymorphism I102V and the Risk of Prostate Cancer in Finnish Men. Gynecol. Oncol. 2003, 95, 812–818. [Google Scholar] [CrossRef]
- Lescai, F.; Marchegiani, F.; Franceschi, C. PON1 is a longevity gene: Results of a meta-analysis. Ageing Res. Rev. 2009, 8, 277–284. [Google Scholar] [CrossRef]
- Caliebe, A.; Kleindorp, R.; Blanché, H.; Christiansen, L.; Puca, A.A.; Rea, I.M.; Slagboom, E.; Flachsbart, F.; Christensen, K.; Rimbach, G.; et al. No or only population-specific effect of PON1 on human longevity: A comprehensive meta-analysis. Ageing Res. Rev. 2010, 9, 238–244. [Google Scholar] [CrossRef]
- Cole, T.B.; Jampsa, R.L.; Walter, B.J.; Arndt, T.L.; Richter, R.J.; Shih, D.M.; Tward, A.; Lusis, A.J.; Jack, R.M.; Costa, L.G.; et al. Expression of human paraoxonase (PON1) during development. Pharmacogenetics 2003, 13, 357–364. [Google Scholar] [CrossRef]
- Huen, K.; Harley, K.; Brooks, J.; Hubbard, A.; Bradman, A.; Eskenazi, B.; Holland, N. Developmental Changes in PON1 Enzyme Activity in Young Children and Effects of PON1 Polymorphisms. Environ. Healthc. Perspect. 2009, 117, 1632–1638. [Google Scholar] [CrossRef] [PubMed]
- El Assar, M.; Angulo, J.; Rodríguez-Mañas, L. Oxidative stress and vascular inflammation in aging. Free Radic. Biol. Med. 2013, 65, 380–401. [Google Scholar] [CrossRef] [PubMed]
- Ademowo, O.S.; Dias, H.K.I.; Burton, D.G.A.; Griffiths, H.R. Lipid (per) oxidation in mitochondria: An emerging target in the ageing process? Biogerontology 2017, 18, 859–879. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Park, C.O.; Noh, J.Y.; Jin, S.; Lee, N.R.; Noh, S.; Lee, J.H.; Lee, K.H. Knockdown of paraoxonase 1 expression influences the ageing of human dermal microvascular endothelial cells. Exp. Dermatol. 2012, 21, 682–687. [Google Scholar] [CrossRef]
- Jaouad, L.; de Guise, C.; Berrougui, H.; Cloutier, M.; Isabelle, M.; Fulop, T.; Payette, H.; Khalil, A. Age-related decrease in high-density lipoproteins antioxidant activity is due to an alteration in the PON1’s free sulfhydyl groups. Atherosclerosis 2006, 185, 191–200. [Google Scholar] [CrossRef]
- Khalil, A.; Wagner, J.R.; Lacombe, G.; Dangoisse, V.; Fülöp, T. Increased susceptibility of low-density lipoprotein (LDL) to oxidation by 7-radiolysis with age. FEBS Lett. 1996, 392, 45–48. [Google Scholar] [CrossRef] [Green Version]
- Jaouad, L.; Milochevitch, C.; Khalil, A. PON1 Paraoxonase Activity is Reduced During HDL Oxidation and is an Indicator of HDL Antioxidant Capacity. Free Radic. Res. 2003, 37, 77–83. [Google Scholar] [CrossRef]
- Park, K.H.; Shin, D.G.; Kim, J.R.; Cho, K.H. Senescence-Related Truncation and Multimerization of Apolipoprotein A-I in High-Density Lipoprotein With an Elevated Level of Advanced Glycated End Products and Cholesteryl Ester Transfer Ac-tivity. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2010, 65, 600–610. [Google Scholar] [CrossRef]
- Milochevitch, C.; Khalil, A. Study of the paraoxonase and platelet-activating factor acetylhydrolase activities with aging. Prostaglandins Leukot. Essent. Fat. Acids 2001, 65, 241–246. Available online: http://www.idealibrary.com (accessed on 2 February 2023). [CrossRef]
- Seres, I.; Paragh, G.; Deschene, E.; Fulop, T.; Khalil, A. Study of factors influencing the decreased HDL associated PON1 activity with aging. Exp. Gerontol. 2003, 39, 59–66. [Google Scholar] [CrossRef]
- Mehdi, M.M.; Rizvi, S.I. Human Plasma Paraoxonase 1 (PON1) Arylesterase Activity During Aging: Correlation with Suscepti-bility of LDL Oxidation. Arch. Med. Res. 2012, 43, 438–443. [Google Scholar] [CrossRef] [PubMed]
- Loued, S.; Berrougui, H.; Componova, P.; Ikhlef, S.; Helal, O.; Khalil, A. Extra-virgin olive oil consumption reduces the age-related decrease in HDL and paraoxonase 1 anti-inflammatory activities. Br. J. Nutr. 2013, 110, 1272–1284. [Google Scholar] [CrossRef] [PubMed]
- Sentí, M.; Tomás, M.; Vila, J.; Marrugat, J.; Elosua, R.; Sala, J.; Masiá, R. Relationship of age-related myocardial infarction risk and Gln/Arg 192 variants of the human paraoxonase1 gene: The REGICOR study. Atherosclerosis 2001, 156, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.S.; Burt, A.A.; Ranchalis, J.E.; Richter, R.J.; Marshall, J.K.; Nakayama, K.S.; Jarvik, E.R.; Eintracht, J.F.; Rosenthal, E.A.; Furlong, C.E.; et al. Dietary cholesterol increases paraoxonase 1 enzyme activity. J. Lipid Res. 2012, 53, 2450–2458. [Google Scholar] [CrossRef] [PubMed]
- Fenkci, I.V.; Serteser, M.; Fenkci, S.; Akyol, A.M. Effects of intranasal estradiol treatment on serum paraoxonase and lipids in healthy, postmenopausal women. Gynecol. Obstet. Investig. 2006, 61, 203–207. [Google Scholar] [CrossRef]
- Sutherland, W.H.F.; Manning, P.J.; De Jong, S.A.; Allum, A.R.; Jones, S.D.; Williams, S.M. Hormone-replacement therapy increases serum paraoxonase arylesterase activity in diabetic postmenopausal women. Metabolism 2001, 50, 319–324. [Google Scholar] [CrossRef]
- Ahmad, S.; Scott, J.E. Estradiol enhances cell-associated paraoxonase 1 (PON1) activity in vitro without altering PON1 ex-pression. Biochem. Biophys. Res. Commun. 2010, 397, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Klaassen, C.D. Hormonal and chemical regulation of paraoxonases in mice. J. Pharmacol. Exp. Ther. 2012, 342, 688–695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Class | Drug | Type | IC50 (mM) | Ki (mM) | Reference |
|---|---|---|---|---|---|
| Antihypertensives | atenolol | NC | 0.136 ± 0.003 | 0.092 ± 0.013 | [167] |
| nadolol | C | 0.214 ± 0.008 | 0.075 ± 0.016 | ||
| pindolol | NC | 0.370 ± 0.008 | 0.287 ± 0.028 | ||
| midodrine | C | 0.131 ± 0.007 | 0.056 ± 0.007 | ||
| Antimycotics | amphotericin B | NC | 0.266 ± 0.002 | 0.320 ± 0.020 | [168] |
| fluconazole | C | 5.728 ± 0.043 | 2.546 ± 0.166 | ||
| caspofungin | C | 0.037 ± 0.001 | 0.011 ± 0.002 | ||
| Proton pump inhibitors | pantoprazole | C | 54.78 ± 0.524 | 39.90 ± 0.005 | [169] |
| omeprazole | C | 86.47 ± 0.818 | 70.11 ± 0.010 | ||
| esomeprazole | C | 93.39 ± 0.885 | 78.87 ± 0.008 | ||
| Sedatives | midazolam | NC | 0.085 | 0.057 ± 0.006 | [170] |
| diazepam | NC | 0.104 | 0.181 ± 0.019 | ||
| Antiepileptics | gabapentin | NC | 0.350 | 0.261 ± 0.027 | [171] |
| phenytoin | NC | 6.300 | 10.30 ± 0.001 | ||
| valproic acid | NC | 0.670 | 0.338 ± 0.313 | ||
| primidone | NC | 0.870 | 0.410 ± 0.184 | ||
| levetiracetam | NC | 53.30 | 43.01 ± 0.003 | ||
| Anesthetics | etomidate | NC | 0.021 | 0.059 ± 0.014 | [172] |
| propofol | C | 0.328 | 0.322 ± 0.111 | ||
| ketamine | UC | 3.800 | 6.480 ± 0.963 | ||
| Antineoplastics | cetuximab | NC | 0.011 | 0.019 ± 0.004 | [173] |
| paclitaxel | C | 0.042 | 0.017 ± 0.011 | ||
| docetaxel | NC | 0.665 | 0.291 ± 0.108 | ||
| etoposide | NC | 0.226 | 0.131 ± 0.071 | ||
| Antibiotics | cefuroxime | C | 13.78 | 6.642 ± 1.541 | [174] |
| cetriaxone | UC | 13.37 | 6.427 ± 0.912 | ||
| ceftazidime | NC | 15.06 | 12.79 ± 1.124 | ||
| teicoplanin | NC | 0.077 | 0.090 ± 0.007 | ||
| rifamycin | C | 0.306 | 0.716 ± 0.076 | ||
| tobramycin | UC | 5.170 | 3.381 ± 0.406 | ||
| amikacin | NC | 40.76 | 55.44 ± 7.783 | ||
| Selective serotonin reuptake inhibitors | mirtazapine | NC | 0.231 | 0.276 ± 0.035 | [175] |
| aripiprazole | NC | 0.139 | 0.202 ± 0.031 | ||
| escitalopram | C | 0.173 | 0.047 ± 0.004 | ||
| risperidone | C | 0.116 | 0.042 ± 0.004 | ||
| Calcium channel blockers | nifedipine | C | 0.121 | 0.222 ± 0.049 | [176] |
| nitrendipine | C | 0.130 | 0.151 ± 0.067 | ||
| israpidine | C | 0.255 | 0.286 ± 0.137 | ||
| amlodipine | NC | 0.304 | 0.321 ± 0.002 | ||
| Cardiovascular drugs | verapamil | UC | 0.672 * | 1.188 ± 0.115 * | [177] |
| metoprolol | NC | 0.621 | 1.115 ± 0.270 | ||
| digoxin | NC | 0.012 | 0.035 ± 0.0127 | ||
| diltiazem | NC | 1.462 | 3.104 ± 1.005 | ||
| amiodarone | C | 3.255 | 5.427 ± 1.341 | ||
| dobutamine | UC | 4.495 | 10.7 ± 3.146 | ||
| Ophthalmic drugs | travoprost | C | 14.95 * | 9.71 ± 2.63 * | [178] |
| latanoprost | NC | 17.03 * | 31.69 ± 3.38 * | ||
| olopatadine | C | 299.6 * | 261.5 ± 59.98 * | ||
| Other | ketotifen | NC | 87.29 * | 239.9 ± 33.25 * | [178] |
| methylprednisolone | C | 47.80 | 109 ± 17.479 | [177] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kunachowicz, D.; Ściskalska, M.; Kepinska, M. Modulatory Effect of Lifestyle-Related, Environmental and Genetic Factors on Paraoxonase-1 Activity: A Review. Int. J. Environ. Res. Public Health 2023, 20, 2813. https://doi.org/10.3390/ijerph20042813
Kunachowicz D, Ściskalska M, Kepinska M. Modulatory Effect of Lifestyle-Related, Environmental and Genetic Factors on Paraoxonase-1 Activity: A Review. International Journal of Environmental Research and Public Health. 2023; 20(4):2813. https://doi.org/10.3390/ijerph20042813
Chicago/Turabian StyleKunachowicz, Dominika, Milena Ściskalska, and Marta Kepinska. 2023. "Modulatory Effect of Lifestyle-Related, Environmental and Genetic Factors on Paraoxonase-1 Activity: A Review" International Journal of Environmental Research and Public Health 20, no. 4: 2813. https://doi.org/10.3390/ijerph20042813







