Locomotor–Respiratory Entrainment upon Phonated Compared to Spontaneous Breathing during Submaximal Exercise
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Experimental Procedure
2.3. Data Acquisition
2.4. Statistical Analysis
3. Results
3.1. PEF Measurements
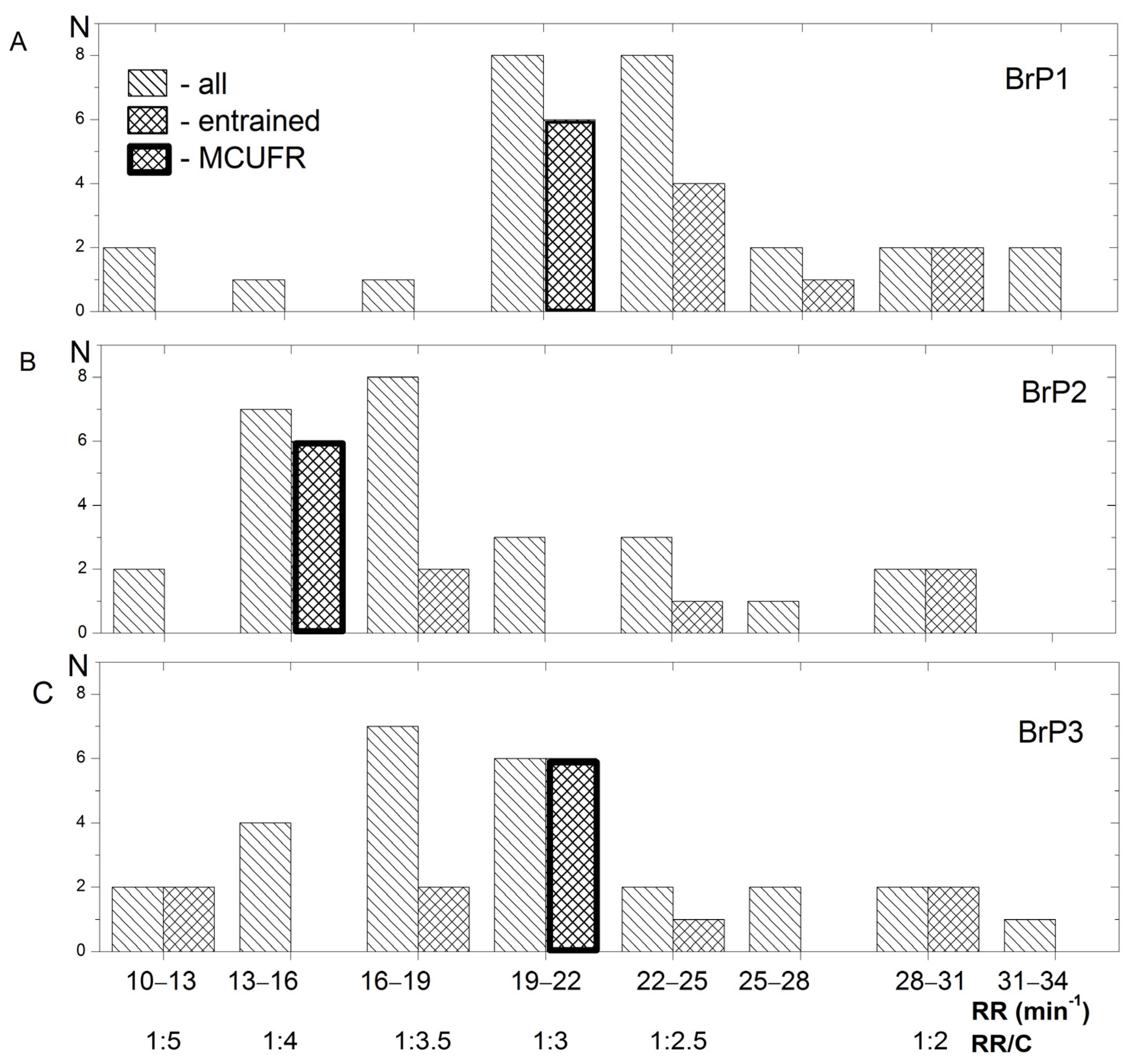
3.2. RR and Entrainment during Exercise at Different BrPs
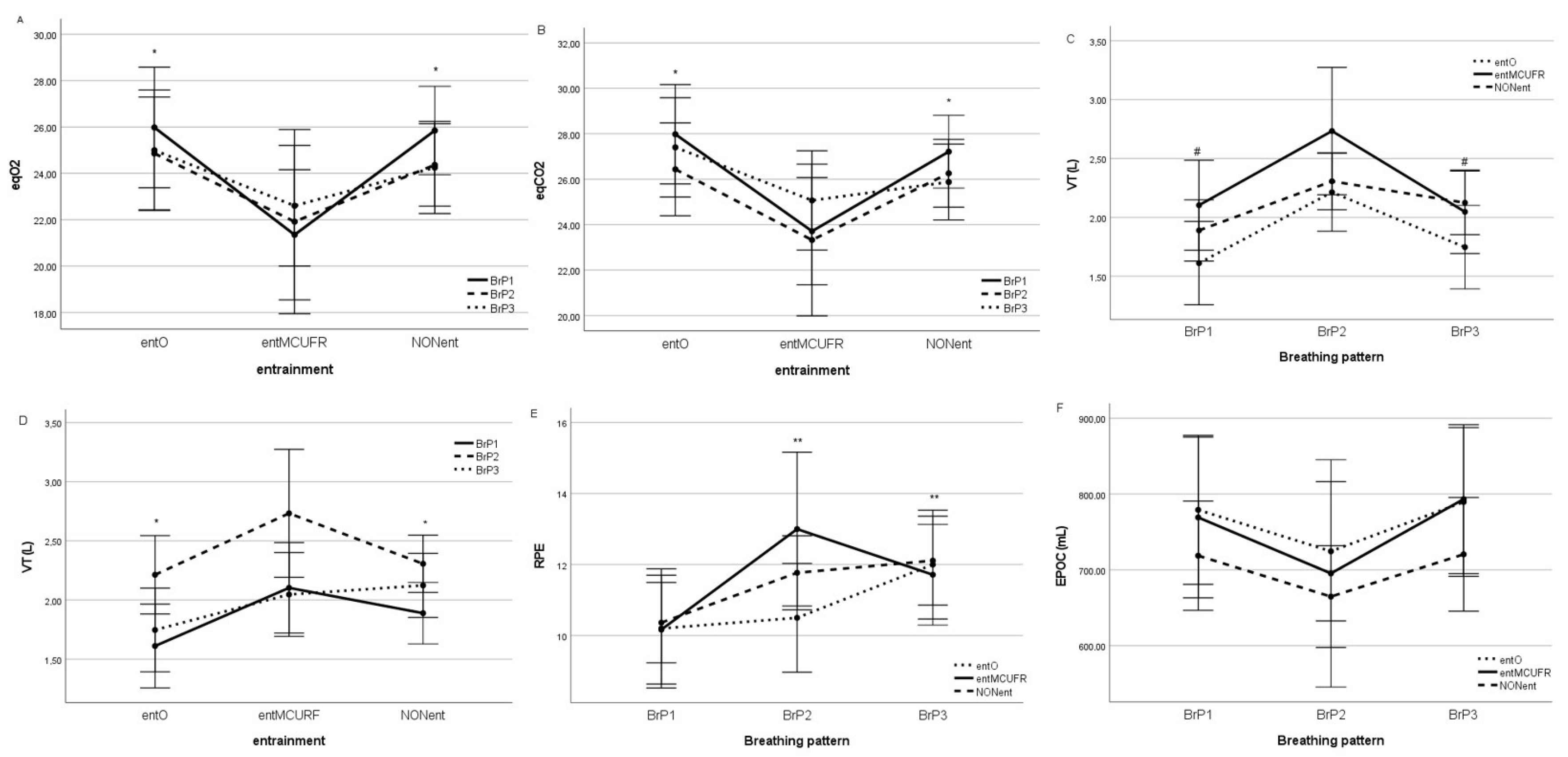
3.3. Other Respiratory and Metabolic Variables
3.4. HR, MAP and RPE
| BrP1 | BrP2 | BrP3 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| ent- MCURF | entO | NONent | entMCURF | entO | NONent | ent- MCURF | entO | NONent | |
| HR (bpm) | 137 ± 12 | 145 ± 21 | 144 ± 18 | 142 ± 11 | 136 ± 20 | 141 ± 24 | 132 ± 17 | 150 ± 19 | 136 ± 21 |
| RR (min−1) * | 20.2 ± 0.7 | 26.6 ± 1.0 | 21.4 ± 8.5 | 14.6 ± 0.7 | 20.9 ± 0.7 | 18.3 ± 6.1 | 20.1 ± 0.7 | 25.3 ± 1.1 | 19.6 ± 4.1 |
| VT (L) * | 2.0 ± 0.4 | 1.7 ± 0.2 | 1.9 ± 0.5 | 2.7 ± 0.3 | 2.2 ± 0.5 | 2.3 ± 0.5 | 2.0 ± 0.3 | 1.7 ± 0.2 | 2.1 ± 0.5 |
| VE (L/min) | 37.9 ± 5.8 | 41.1 ± 4.8 | 39.3 ± 6.9 | 37.3 ± 3.5 | 40.2 ± 6.9 | 38.0 ± 8.6 | 37.8 ± 8.0 | 38.0 ± 3.6 | 39.6 ± 7.5 |
| VO2/kg (mL/min kg) | 24.2 ± 3.1 | 23.1 ± 4.1 | 23.5 ± 2.5 | 23.4 ± 2.9 | 24.1 ± 3.1 | 23.5 ± 2.7 | 21.8 ± 3.6 | 24.4 ± 1.3 | 23.2 ± 2.4 |
| VCO2/kg (mL/min kg) | 21.2 ± 2.9 | 21.8 ± 3.9 | 22.3 ± 2.9 | 21.5 ± 3.4 | 22.2 ± 3.0 | 21.2 ± 2.4 | 19.7 ± 3.0 | 22.4 ± 1.6 | 22.4 ± 2.9 |
| RQ | 0.88 ± 0.04 | 0.93 ± 0.06 | 0.94 ± 0.08 | 0.92 ± 0.06 | 0.93 ± 0.03 | 0.95 ± 0.07 | 0.89 ± 0.06 | 0.92 ± 0.03 | 0.93 ± 0.08 |
| eqO2 * | 21.3 ± 1.1 | 25.9 ± 2.3 | 25.8 ± 4.6 | 21.9 ± 1.0 | 24.8 ± 3.2 | 24.3 ± 3.3 | 22.6 ± 2.4 | 24.9 ± 4.9 | 24.4 ± 3.0 |
| eqCO2 * | 23.7 ± 0.9 | 27.9 ± 1.5 | 27.5 ± 3.2 | 23.3 ± 1.0 | 26.7 ± 1.7 | 26.1 ± 3.9 | 25.0 ± 1.6 | 27.4 ± 3.5 | 26.2 ± 4.2 |
| EPOC (mL) | 764 ± 132 | 779 ± 70 | 718 ± 66 | 695 ± 126 | 724 ± 147 | 663 ± 154 | 793 ± 190 | 795 ± 207 | 720 ± 153 |
| HRR30 (bpm) | 25.1 ± 7.9 | 25.4 ± 6.7 | 23.6 ± 9.2 | 23.3 ± 9.3 | 27.1 ± 8.2 | 24.8 ± 8.4 | 32.4 ± 8.2 | 24.1 ± 8.7 | 23.8 ± 8.4 |
| HRR60 (bpm) | 37.3 ± 8.0 | 34.4 ± 11.0 | 38.7 ± 13.8 | 41.4 ± 11.2 | 41.0 ± 7.3 | 37.2 ± 9.5 | 44.8 ± 10.6 | 39.4 ± 5.5 | 45.4 ± 11.3 |
| MAP (mmHg) | 125 ± 28 | 128 ± 25 | 124 ± 18 | 112 ± 29 | 123 ± 11 | 128 ± 22 | 120 ± 23 | 133 ± 15 | 124 ± 30 |
| O2pulse | 12.0 ± 2.8 | 11.8 ± 3.9 | 10.2 ± 2.6 | 12.1 ± 1.6 | 12.3 ± 3.2 | 11.3 ± 3.5 | 12.3 ± 2.8 | 10.2 ± 3.3 | 12.4 ± 2.6 |
| RPE * | 10.13 ± 1.36 | 11.20 ± 0.45 | 9.88 ± 2.48 | 13.50 ± 1.51 | 10.83 ± 0.41 | 12.75 ± 2.25 | 11.63 ± 1.18 | 12.17 ± 1.33 | 12.13 ± 3.04 |
4. Discussion
4.1. PEF and Phonation
4.2. RR and Entrainment during Exercise at Different BrPs
4.3. Other Respiratory and Metabolic Variables
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dallam, G.; Kies, B. The Effect of Nasal Breathing Versus Oral and Oronasal Breathing During Exercise: A Review. J. Sports Res. 2020, 7, 1–10. [Google Scholar] [CrossRef]
- Harbour, E.; Stöggl, T.; Schwameder, H.; Finkenzeller, T. Breath Tools: A Synthesis of Evidence-Based Breathing Strategies to Enhance Human Running. Front. Physiol. 2022, 13, 233. [Google Scholar] [CrossRef]
- Giuliodori, M.J.; Lujan, H.L.; Briggs, W.S.; DiCarlo, S.E. A Model of Locomotor-Respiratory Coupling in Quadrupeds. Adv. Physiol. Educ. 2009, 33, 315–318. [Google Scholar] [CrossRef] [PubMed]
- Plowman, S.A.; Smith, D.L. Exercise Physiology for Health, Fitness, and Performance; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2013; p. 771. [Google Scholar]
- Hoffmann, C.P.; Torregrosa, G.; Bardy, B.G. Sound Stabilizes Locomotor-Respiratory Coupling and Reduces Energy Cost. PLoS ONE 2012, 7, e45206. [Google Scholar] [CrossRef]
- Vezos, N.; Gourgoulis, V.; Aggeloussis, N.; Kasimatis, P.; Christoforidis, C.; Mavromatis, G. Underwater Stroke Kinematics During Breathing and Breath-Holding Front Crawl Swimming. J. Sports Sci. Med. 2007, 6, 58–62. [Google Scholar] [PubMed]
- Daley, M.A.; Bramble, D.M.; Carrier, D.R. Impact Loading and Locomotor-Respiratory Coordination Significantly Influence Breathing Dynamics in Running Humans. PLoS ONE 2013, 8, e70752. [Google Scholar] [CrossRef] [PubMed]
- Garlando, F.; Kohl, J.; Koller, E.A.; Pietsch, P. Effect of Coupling the Breathing- and Cycling Rhythms on Oxygen Uptake during Bicycle Ergometry. Eur. J. Appl. Physiol. Occup. Physiol. 1985, 54, 497–501. [Google Scholar] [CrossRef]
- Villard, S.; Casties, J.F.; Mottet, D. Dynamic Stability of Locomotor Respiratory Coupling during Cycling in Humans. Neurosci. Lett. 2005, 383, 333–338. [Google Scholar] [CrossRef]
- Bonsignore, M.R.; Morici, G.; Abate, P.; Romano, S.; Bonsignore, G. Ventilation and Entrainment of Breathing during Cycling and Running in Triathletes. Med. Sci. Sports Exerc. 1998, 30, 239–245. [Google Scholar] [CrossRef]
- Takano, N.; Deguchi, H. Sensation of Breathlessness and Respiratory Oxygen Cost during Cycle Exercise with and without Conscious Entrainment of the Breathing Rhythm. Eur. J. Appl. Physiol. Occup. Physiol. 1997, 76, 209–213. [Google Scholar] [CrossRef]
- Maclennan, S.E.; Silvestri, G.A.; Ward, J.; Mahler, D.A. Does Entrained Breathing Improve the Economy of Rowing? Med. Sci. Sport. Exerc. 1994, 26, 610–614. [Google Scholar] [CrossRef]
- Francis, K.T.; Brasher, J. Physiological Effects of Wearing Mouthguards. Br. J. Sports Med. 1991, 25, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Olsén, M.F.; Lannefors, L.; Westerdahl, E. Positive Expiratory Pressure—Common Clinical Applications and Physiological Effects. Respir. Med. 2015, 109, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Tiep, B.L.; Burns, M.; Kao, D.; Madison, R.; Herrera, J. Pursed Lips Breathing Training Using Ear Oximetry. Chest 1986, 90, 218–221. [Google Scholar] [CrossRef]
- Lässing, J.; Falz, R.; Schulze, A.; Pökel, C.; Vondran, M.; Schröter, T.; Borger, M.A.; Busse, M. Decreased Exercise Capacity in Young Athletes Using Self-Adapted Mouthguards. Eur. J. Appl. Physiol. 2021, 121, 1881–1888. [Google Scholar] [CrossRef]
- Cardoso, F.; Monteiro, A.S.; Vilas-Boas, J.P.; Pinho, J.C.; Pyne, D.B.; Fernandes, R.J. Effects of Wearing a 50% Lower Jaw Advancement Splint on Biophysical and Perceptual Responses at Low to Severe Running Intensities. Life 2022, 12, 253. [Google Scholar] [CrossRef]
- Traser, L.; Knab, J.; Echternach, M.; Fuhrer, H.; Richter, B.; Buerkle, H.; Schumann, S. Regional Ventilation during Phonation in Professional Male and Female Singers. Respir. Physiol. Neurobiol. 2017, 239, 26–33. [Google Scholar] [CrossRef]
- Pouw, W.; Fuchs, S. Origins of Vocal-Entangled Gesture. Neurosci. Biobehav. Rev. 2022, 141, 104836. [Google Scholar] [CrossRef]
- Danieli, A.; Lusa, L.; Potočnik, N.; Meglič, B.; Grad, A.; Bajrović, F.F. Resting Heart Rate Variability and Heart Rate Recovery after Submaximal Exercise. Clin. Auton. Res. 2014, 24, 53–61. [Google Scholar] [CrossRef]
- Robergs, R.A.; Landwehr, R. Commentary The Surprising History of the “HRmax=220-Age ” Equation. J. Exerc. Physiol. 2002, 5, 1–10. [Google Scholar]
- Hughson, R.L.; Morrissey, M.A. Delayed Kinetics of VO2 in the Transition from Prior Exercise. Evidence for O2 Transport Limitation of VO2 Kinetics: A Review. Int. J. Sports Med. 1983, 4, 31–39. [Google Scholar] [CrossRef]
- Hopkins, W.G.; Marshall, S.W.; Batterham, A.M.; Hanin, J. Progressive Statistics for Studies in Sports Medicine and Exercise Science. Med. Sci. Sports Exerc. 2009, 41, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. Statistical Power for the Behaviour Sciences; Routledge: New York, NY, USA, 1977; ISBN 9780121790608. [Google Scholar]
- Mortola, J.P. How to Breathe? Respiratory Mechanics and Breathing Pattern. Respir. Physiol. Neurobiol. 2019, 261, 48–54. [Google Scholar] [CrossRef]
- Schmidt, J.; Martin, A.; Wenzel, C.; Weber, J.; Wirth, S.; Schumann, S. Control of the Expiratory Flow in a Lung Model and in Healthy Volunteers with an Adjustable Flow Regulator: A Combined Bench and Randomized Crossover Study. Respir. Res. 2021, 22, 292. [Google Scholar] [CrossRef] [PubMed]
- Nicolò, A.; Massaroni, C.; Passfield, L. Respiratory Frequency during Exercise: The Neglected Physiological Measure. Front. Physiol. 2017, 8, 922. [Google Scholar] [CrossRef] [PubMed]
- Schulze, A.; Kwast, S.; Busse, M. Influence of Mouthguards on Physiological Responses in Rugby. Sport. Med. Int. Open 2019, 3, E25–E31. [Google Scholar] [CrossRef]
- Garner, D.P.; Lamira, J. Respiratory Outcomes with the Use of a Lower Custom Fit Genioglossal-Effecting Oral Appliance. Clin. Exp. Dent. Res. 2020, 6, 100–106. [Google Scholar] [CrossRef]
- Matsumoto, T.; Masuda, T.; Hotta, K.; Shimizu, R.; Ishii, A.; Kutsuna, T.; Yamamoto, K.; Hara, M.; Takahira, N.; Matsunaga, A. Effects of Prolonged Expiration Breathing on Cardiopulmonary Responses during Incremental Exercise. Respir. Physiol. Neurobiol. 2011, 178, 275–282. [Google Scholar] [CrossRef]
- Bernasconi, P.; Kohl, J. Analysis of Co-Ordination between Breathing and Exercise Rhythms in Man. J. Physiol. 1993, 471, 693–706. [Google Scholar] [CrossRef]
- McDermott, W.J.; Van Emmerik, R.E.A.; Hamill, J. Running Training and Adaptive Strategies of Locomotor-Respiratory Coordination. Eur. J. Appl. Physiol. 2003, 89, 435–444. [Google Scholar] [CrossRef]
- O’Halloran, J.; Hamill, J.; McDermott, W.J.; Remelius, J.G.; Van Emmerik, R.E.A.; Ward, S.A. Locomotor-Respiratory Coupling Patterns and Oxygen Consumption during Walking above and below Preferred Stride Frequency. Eur. J. Appl. Physiol. 2012, 112, 929–940. [Google Scholar] [CrossRef] [PubMed]
- Tabary, A.; Rassler, B. Increased Breathing Resistance Compromises the Time Course of Rhythmical Forearm Movements-a Pilot Study. J. Transl. Intern. Med. 2015, 3, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Breslin, E.H. The Pattern of Respiratory Muscle Recruitment during Pursed-Lip Breathing. Chest 1992, 101, 75–78. [Google Scholar] [CrossRef] [PubMed]
- Dechman, G.; Wilson, C.R. Evidence Underlying Breathing Retraining in People with Stable Chronic Obstructive Pulmonary Disease. Phys. Ther. 2004, 84, 1189–1197. [Google Scholar] [CrossRef] [PubMed]
- Faager, G.; Stähle, A.; Larsen, F.F. Influence of Spontaneous Pursed Lips Breathing on Walking Endurance and Oxygen Saturation in Patients with Moderate to Severe Chronic Obstructive Pulmonary Disease. Clin. Rehabil. 2008, 22, 675–683. [Google Scholar] [CrossRef]
- Sakhaei, S.; Sadagheyani, H.E.; Zinalpoor, S.; Markani, A.K.; Motaarefi, H. The Impact of Pursed-Lips Breathing Maneuver on Cardiac, Respiratory, and Oxygenation Parameters in COPD Patients. Open Access Maced. J. Med. Sci. 2018, 6, 1851–1856. [Google Scholar] [CrossRef]
- De Pereira Araujo, C.L.; Karloh, M.; Dos Reis, C.M.; Palú, M.; Mayer, A.F. Pursed-Lips Breathing Reduces Dynamic Hyperinflation Induced by Activities of Daily Living Test in Patients with Chronic Obstructive Pulmonary Disease: A Randomized Crossover Study. J. Rehabil. Med. 2015, 47, 957–962. [Google Scholar] [CrossRef]
- Schultz Martins, R.; Girouard, P.; Elliott, E.; Mekary, S. Physiological Responses of a Jaw-Repositioning Custom-Made Mouthguard on Airway and Their Effects on Athletic Performance. J. Strength Cond. Res. 2020, 34, 422–429. [Google Scholar] [CrossRef]
- Stucky, F.; Aliverti, A.; Kayser, B.; Uva, B. Priming the Cardiodynamic Phase of Pulmonary Oxygen Uptake through Voluntary Modulations of the Respiratory Pump at the Onset of Exercise. Exp. Physiol. 2021, 106, 555–566. [Google Scholar] [CrossRef]
- Salomoni, S.; van Den Hoorn, W.; Hodges, P. Breathing and Singing: Objective Characterization of Breathing Patterns in Classical Singers. PLoS ONE 2016, 11, e0155084. [Google Scholar] [CrossRef]
- Aliverti, A. The Respiratory Muscles during Exercise. Breathe 2016, 12, 165–168. [Google Scholar] [CrossRef] [PubMed]
- Bramble, D.M.; Carrier, D.R. Running and Breathing in Mammals. Science 1983, 219, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Carrier, D.R.; Deban, S.M.; Fischbein, T. Locomotor Function of Forelimb Protractor and Retractor Muscles of Dogs: Evidence of Strut-like Behavior at the Shoulder. J. Exp. Biol. 2008, 211, 150–162. [Google Scholar] [CrossRef]
- Juvin, L.; Colnot, E.; Barrière, G.; Thoby-Brisson, M.; Morin, D. Neurogenic Mechanisms for Locomotor-Respiratory Coordination in Mammals. Front. Neuroanat. 2022, 16, 953746. [Google Scholar] [CrossRef] [PubMed]

| BrP | Entrainment | BrP * Entrainment | Gender | ||||
|---|---|---|---|---|---|---|---|
| p | η2 | p | η2 | p | η2 | ||
| HR | 0.701 | 0.960 | 0.626 | <0.001 | 0.391 | ||
| VT | 0.001 * | 0.175 | 0.023 # | 0.103 | 0.668 | <0.001 | 0.176 |
| BrP1/BrP2: D = 2.90 large | entMCUFR/entO: D = 0.66 moderate | ||||||
| BrP2/BrP3: D = 0.65 moderate | entMCUFR/NONentr: D = 0.46 small | ||||||
| VE | 0.837 | 0.558 | 0.817 | <0.001 | 0.378 | ||
| VO2/kg & | 0.974 | 0.428 | 0.800 | 0.883 | |||
| VCO2/kg | 0.782 | 0.292 | 0.922 | 0.502 | |||
| RQ | 0.611 | 0.154 | 0.669 | 0.0419 | |||
| eqO2 | 0.814 | 0.014 # | 0.116 | 0.820 | 0.231 | ||
| entMCUFR/entO: D = 1.17 large | |||||||
| entMCUFR/NONent: D = 1.00 large | |||||||
| eqCO2 | 0.558 | 0.006 # | 0.139 | 0.688 | 0.400 | ||
| entMCUFR/entO: D = 1.20 large | |||||||
| entMCUFR/NONent: D = 0.91 large | |||||||
| EPOC & | 0.372 | 0.627 | 0.754 | 0.798 | |||
| HRR30 | 0.662 | 0.454 | 0.648 | 0.918 | |||
| HRR60 | 0.555 | 0.493 | 0.672 | 0.111 | |||
| MAP | 0.587 | 0.694 | 0.966 | 0.432 | |||
| O2pulse | 0.955 | 0.386 | 0.510 | <0.001 | 0.758 | ||
| RPE | 0.011 * | 0.145 | 0.525 | 0.549 | 0.183 | ||
| BrP1/BrP2: D = 0.75 moderate | |||||||
| BrP1/BrP3: D = 0.91 large | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Potočnik, M.M.; Edwards, I.; Potočnik, N. Locomotor–Respiratory Entrainment upon Phonated Compared to Spontaneous Breathing during Submaximal Exercise. Int. J. Environ. Res. Public Health 2023, 20, 2838. https://doi.org/10.3390/ijerph20042838
Potočnik MM, Edwards I, Potočnik N. Locomotor–Respiratory Entrainment upon Phonated Compared to Spontaneous Breathing during Submaximal Exercise. International Journal of Environmental Research and Public Health. 2023; 20(4):2838. https://doi.org/10.3390/ijerph20042838
Chicago/Turabian StylePotočnik, Maja Marija, Ian Edwards, and Nejka Potočnik. 2023. "Locomotor–Respiratory Entrainment upon Phonated Compared to Spontaneous Breathing during Submaximal Exercise" International Journal of Environmental Research and Public Health 20, no. 4: 2838. https://doi.org/10.3390/ijerph20042838
APA StylePotočnik, M. M., Edwards, I., & Potočnik, N. (2023). Locomotor–Respiratory Entrainment upon Phonated Compared to Spontaneous Breathing during Submaximal Exercise. International Journal of Environmental Research and Public Health, 20(4), 2838. https://doi.org/10.3390/ijerph20042838





