Relationships between Physical Activity Frequency and Self-Perceived Health, Self-Reported Depression, and Depressive Symptoms in Spanish Older Adults with Diabetes: A Cross-Sectional Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Design and Ethical Concerns
2.2. Participants
2.3. Procedures
2.4. Statistical Analysis
3. Results
4. Discussion
4.1. Association between Sex and Body Mass Index (Overweight and Obesity)
4.2. Physical Activity Frequency
4.3. Self-Reported Depression Prevalence
4.4. Self-Perceived Health
4.5. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zimmet, P.; Alberti, K.G.M.M.; Shaw, J. Global and Societal Implications of the Diabetes Epidemic. Nature 2001, 414, 782–787. [Google Scholar] [CrossRef]
- Cho, N.H.; Shaw, J.E.; Karuranga, S.; Huang, Y.; da Rocha Fernandes, J.D.; Ohlrogge, A.W.; Malanda, B. IDF Diabetes Atlas: Global Estimates of Diabetes Prevalence for 2017 and Projections for 2045. Diabetes Res.Clin. Pract. 2018, 138, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Arnold, S.V.; Khunti, K.; Tang, F.; Chen, H.; Cid-Ruzafa, J.; Cooper, A.; Fenici, P.; Gomes, M.B.; Hammar, N.; Ji, L.; et al. Incidence Rates and Predictors of Microvascular and Macrovascular Complications in Patients with Type 2 Diabetes: Results from the Longitudinal Global Discover Study. Am. Heart J. 2022, 243, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Polonsky, W.H.; Fisher, L.; Earles, J.; Dudl, R.J.; Lees, J.; Mullan, J.; Jackson, R.A. Assessing Psychosocial Distress in Diabetes. Diabetes Care 2005, 28, 626–631. [Google Scholar] [CrossRef]
- Lloyd, C.E. Diabetes and Mental Health; the Problem of Co-Morbidity. Diabet. Med. 2010, 27, 853–854. [Google Scholar] [CrossRef] [PubMed]
- Robinson, D.J.; Coons, M.; Haensel, H.; Vallis, M.; Yale, J.-F. Diabetes and Mental Health. Can. J. Diabetes 2018, 42, S130–S141. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Stone, M.A.; Peters, J.L.; Davies, M.J.; Khunti, K. The Prevalence of Co-Morbid Depression in Adults with Type 2 Diabetes: A Systematic Review and Meta-Analysis. Diabet. Med. 2006, 23, 1165–1173. [Google Scholar] [CrossRef]
- Asociación Americana de Psiquiatría. Manual Diagnóstico y Estadístico de Los Trastornos Mentales: DSM-5, 5th ed.; Editorial Médica Panamericana; Asociación Americana de Psiquiatría: Arlington, VA, USA, 2016. [Google Scholar]
- Barnard, K.D.; Skinner, T.C.; Peveler, R. The Prevalence of Co-Morbid Depression in Adults with Type 1 Diabetes: Systematic Literature Review. Diabet. Med. 2006, 23, 445–448. [Google Scholar] [CrossRef]
- Habib, S.; Sangaraju, S.L.; Yepez, D.; Grandes, X.A.; Talanki Manjunatha, R. The Nexus Between Diabetes and Depression: A Narrative Review. Cureus 2022, 14, e25611. [Google Scholar] [CrossRef]
- Hermanns, N.; Kulzer, B.; Krichbaum, M.; Kubiak, T.; Haak, T. Affective and Anxiety Disorders in a German Sample of Diabetic Patients: Prevalence, Comorbidity and Risk Factors. Diabet. Med. 2005, 22, 293–300. [Google Scholar] [CrossRef]
- Pibernik-Okanovic, M.; Peros, K.; Szabo, S.; Begic, D.; Metelko, Z. Depression in Croatian Type 2 Diabetic Patients: Prevalence and Risk Factors. A Croatian Survey from the European Depression in Diabetes (EDID) Research Consortium. Diabet. Med. 2005, 22, 942–945. [Google Scholar] [CrossRef] [PubMed]
- Pouwer, F.; Geelhoed-Duijvestijn, P.H.L.M.; Tack, C.J.; Bazelmans, E.; Beekman, A.-J.; Heine, R.J.; Snoek, F.J. Prevalence of Comorbid Depression Is High in Out-Patients with Type 1 or Type 2 Diabetes Mellitus. Results from Three out-Patient Clinics in the Netherlands. Diabet. Med. 2010, 27, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Sotiropoulos, A.; Papazafiropoulou, A.; Apostolou, O.; Kokolaki, A.; Gikas, A.; Pappas, S. Prevalence of Depressive Symptoms among Non Insulin Treated Greek Type 2 Diabetic Subjects. BMC Res. Notes 2008, 1, 101. [Google Scholar] [CrossRef]
- Egede, L.E.; Nietert, P.J.; Zheng, D. Depression and All-Cause and Coronary Heart Disease Mortality among Adults With and Without Diabetes. Diabetes Care 2005, 28, 1339–1345. [Google Scholar] [CrossRef] [PubMed]
- Katon, W.; Lin, E.H.B.; Kroenke, K. The Association of Depression and Anxiety with Medical Symptom Burden in Patients with Chronic Medical Illness. Gen. Hosp. Psychiatry 2007, 29, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Oyedeji, A.D.; Ullah, I.; Weich, S.; Bentall, R.; Booth, A. Effectiveness of Non-Specialist Delivered Psychological Interventions on Glycemic Control and Mental Health Problems in Individuals with Type 2 Diabetes: A Systematic Review and Meta-Analysis. Int. J. Ment. Health Syst. 2022, 16, 9. [Google Scholar] [CrossRef]
- Sullivan, M. The New Subjective Medicine: Taking the Patient’s Point of View on Health Care and Health. Soc. Sci. Med. 2003, 56, 1595–1604. [Google Scholar] [CrossRef]
- Jankowska, A.; Golicki, D. Self-Reported Diabetes and Quality of Life: Findings from a General Population Survey with the Short Form-12 (SF-12) Health Survey. Arch. Med. Sci. 2021, 18, 1157–1168. [Google Scholar] [CrossRef]
- Machón, M.; Vergara, I.; Dorronsoro, M.; Vrotsou, K.; Larrañaga, I. Self-Perceived Health in Functionally Independent Older People: Associated Factors. BMC Geriatr. 2016, 16, 66. [Google Scholar] [CrossRef]
- Bonner, W.I.A.; Weiler, R.; Orisatoki, R.; Lu, X.; Andkhoie, M.; Ramsay, D.; Yaghoubi, M.; Steeves, M.; Szafron, M.; Farag, M. Determinants of Self-Perceived Health for Canadians Aged 40 and Older and Policy Implications. Int. J. Equity Health 2017, 16, 94. [Google Scholar] [CrossRef]
- Pereira-de-Sousa, A.M.; López-Rodríguez, J.A. Salud Autopercibida En Ancianos Jóvenes Españoles y Portugueses Tras La Recesión Según La Encuesta Europea de Salud: Un Estudio Transversal. Aten. Primaria 2021, 53, 102064. [Google Scholar] [CrossRef] [PubMed]
- Caspersen, C.J.; Powell, K.E.; Christenson, G.M. Physical Activity, Exercise, and Physical Fitness: Definitions and Distinctions for Health-Related Research. Public Health Rep. 1985, 100, 126–131. [Google Scholar] [PubMed]
- Colberg, S.R.; Sigal, R.J.; Yardley, J.E.; Riddell, M.C.; Dunstan, D.W.; Dempsey, P.C.; Horton, E.S.; Castorino, K.; Tate, D.F. Physical Activity/Exercise and Diabetes: A Position Statement of the American Diabetes Association. Diabetes Care 2016, 39, 2065–2079. [Google Scholar] [CrossRef] [PubMed]
- Pfeifer, L.O.; de Nardi, A.T.; da Silva, L.X.N.; Botton, C.E.; do Nascimento, D.M.; Teodoro, J.L.; Schaan, B.D.; Umpierre, D. Association Between Physical Exercise Interventions Participation and Functional Capacity in Individuals with Type 2 Diabetes: A Systematic Review and Meta-Analysis of Controlled Trials. Sport. Med. Open 2022, 8, 34. [Google Scholar] [CrossRef]
- Wake, A.D. Protective Effects of Physical Activity against Health Risks Associated with Type 1 Diabetes: “Health Benefits Outweigh the Risks”. World J. Diabetes 2022, 13, 161–184. [Google Scholar] [CrossRef]
- Zhao, X.; He, Q.; Zeng, Y.; Cheng, L. Effectiveness of Combined Exercise in People with Type 2 Diabetes and Concurrent Overweight/Obesity: A Systematic Review and Meta-Analysis. BMJ Open 2021, 11, e046252. [Google Scholar] [CrossRef]
- Mousavi Gilani, S.R.; Khazaei Feizabad, A. The Effects of Aerobic Exercise Training on Mental Health and Self-Esteem of Type 2 Diabetes Mellitus Patients. Health Psychol. Res. 2019, 7, 6576. [Google Scholar] [CrossRef]
- Shamus, E.; Cohen, G. Depressed, Low Self-Esteem: What Can Exercise Do For You? Internet J. Allied Health Sci. Pract. 2009, 7, 2. [Google Scholar] [CrossRef]
- Chimen, M.; Kennedy, A.; Nirantharakumar, K.; Pang, T.T.; Andrews, R.; Narendran, P. What Are the Health Benefits of Physical Activity in Type 1 Diabetes Mellitus? A Literature Review. Diabetologia 2012, 55, 542–551. [Google Scholar] [CrossRef]
- Zoppini, G.; Carlini, M.; Muggeo, M. Self-Reported Exercise and Quality of Life in Young Type 1 Diabetic Subjects. Diabetes Nutr. Metab. 2003, 16, 77–80. [Google Scholar]
- Instituto Nacional de Estadística. Encuesta Europea de Salud En España 2014 EESE-2014; Instituto Nacional de Estadística: Madrid, Spain, 2014. [Google Scholar]
- Instituto Nacional de Estadística. Encuesta Europea de Salud En España 2020 EESE-2020; Instituto Nacional de Estadística: Madrid, Spain, 2020. [Google Scholar]
- Nuttall, F.Q. Body Mass Index. Nutr. Today 2015, 50, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Kroenke, K.; Strine, T.W.; Spitzer, R.L.; Williams, J.B.W.; Berry, J.T.; Mokdad, A.H. The PHQ-8 as a Measure of Current Depression in the General Population. J. Affect. Disord. 2009, 114, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Diez-Quevedo, C.; Rangil, T.; Sanchez-Planell, L.; Kroenke, K.; Spitzer, R.L. Validation and Utility of the Patient Health Questionnaire in Diagnosing Mental Disorders in 1003 General Hospital Spanish Inpatients. Psychosom. Med. 2001, 63, 679–686. [Google Scholar] [CrossRef]
- Schubert, P.; Leimstoll, U. Importance and Use of Information Technology in Small and Medium-Sized Companies. Electron. Mark. 2007, 17, 38–55. [Google Scholar] [CrossRef]
- International Diabetes Federation. IDF Diabetes Atlas; IDF: Brussels, Belgium, 2017. [Google Scholar]
- Haugaard, S.B.; Mu, H.; Vaag, A.; Madsbad, S. Intramyocellular Triglyceride Content in Man, Influence of Sex, Obesity and Glycaemic Control. Eur. J. Endocrinol. 2009, 161, 57–64. [Google Scholar] [CrossRef]
- Jackson, A.; Stanforth, P.; Gagnon, J.; Rankinen, T.; Leon, A.; Rao, D.; Skinner, J.; Bouchard, C.; Wilmore, J. The Effect of Sex, Age and Race on Estimating Percentage Body Fat from Body Mass Index: The Heritage Family Study. Int. J. Obes. 2002, 26, 789–796. [Google Scholar] [CrossRef]
- Zillikens, M.C.; Yazdanpanah, M.; Pardo, L.M.; Rivadeneira, F.; Aulchenko, Y.S.; Oostra, B.A.; Uitterlinden, A.G.; Pols, H.A.P.; van Duijn, C.M. Sex-Specific Genetic Effects Influence Variation in Body Composition. Diabetologia 2008, 51, 2233–2241. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, A.; Peeters, A.; de Courten, M.; Stoelwinder, J. The Magnitude of Association between Overweight and Obesity and the Risk of Diabetes: A Meta-Analysis of Prospective Cohort Studies. Diabetes Res. Clin. Pract. 2010, 89, 309–319. [Google Scholar] [CrossRef]
- Vazquez, G.; Duval, S.; Jacobs, D.R.; Silventoinen, K. Comparison of Body Mass Index, Waist Circumference, and Waist/Hip Ratio in Predicting Incident Diabetes: A Meta-Analysis. Epidemiol. Rev. 2007, 29, 115–128. [Google Scholar] [CrossRef]
- Clayton, J.A.; Tannenbaum, C. Reporting Sex, Gender, or Both in Clinical Research? JAMA 2016, 316, 1863. [Google Scholar] [CrossRef]
- Miller, L.R.; Marks, C.; Becker, J.B.; Hurn, P.D.; Chen, W.; Woodruff, T.; McCarthy, M.M.; Sohrabji, F.; Schiebinger, L.; Wetherington, C.L.; et al. Considering Sex as a Biological Variable in Preclinical Research. FASEB J. 2017, 31, 29–34. [Google Scholar] [CrossRef]
- Bassuk, S.S.; Manson, J.E. Epidemiological Evidence for the Role of Physical Activity in Reducing Risk of Type 2 Diabetes and Cardiovascular Disease. J. Appl. Physiol. 2005, 99, 1193–1204. [Google Scholar] [CrossRef] [PubMed]
- Daniele, T.M.d.C.; de Bruin, V.M.S.; de Oliveira, D.S.N.; Pompeu, C.M.R.; Forti, A.C.E. Associations among Physical Activity, Comorbidities, Depressive Symptoms and Health-Related Quality of Life in Type 2 Diabetes. Arq. Bras. Endocrinol. Metabol. 2013, 57, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Chen, Y.; Krewski, D. Gender-Related Differences in the Association between Socioeconomic Status and Self-Reported Diabetes. Int. J. Epidemiol. 2003, 32, 381–385. [Google Scholar] [CrossRef] [PubMed]
- Thiel, D.M.; al Sayah, F.; Vallance, J.K.; Johnson, S.T.; Johnson, J.A. Association between Physical Activity and Health-Related Quality of Life in Adults with Type 2 Diabetes. Can. J. Diabetes 2017, 41, 58–63. [Google Scholar] [CrossRef]
- Minister of Industry National Population Health Survey Overview 1996/97; Government of Canada Publications: Ottawa, ON, Canada, 1998.
- Cvecka, J.; Tirpakova, V.; Sedliak, M.; Kern, H.; Mayr, W.; Hamar, D. Physical Activity in Elderly. Eur. J. Transl. Myol. 2015, 25, 249. [Google Scholar] [CrossRef]
- Ivy, J.L. Role of Exercise Training in the Prevention and Treatment of Insulin Resistance and Non-Insulin-Dependent Diabetes Mellitus. Sport. Med. 1997, 24, 321–336. [Google Scholar] [CrossRef]
- Koeneman, M.A.; Verheijden, M.W.; Chinapaw, M.J.M.; Hopman-Rock, M. Determinants of Physical Activity and Exercise in Healthy Older Adults: A Systematic Review. Int. J. Behav. Nutr. Phys. Act. 2011, 8, 142. [Google Scholar] [CrossRef]
- Milanovic, Z.; Jorgić, B.; Trajković, N.; Sporis, G.; Pantelić, S.; James, N. Age-Related Decrease in Physical Activity and Functional Fitness among Elderly Men and Women. Clin. Interv. Aging 2013, 8, 549. [Google Scholar] [CrossRef]
- Mulder, M. The Stability of Lifestyle Behaviour. Int. J. Epidemiol. 1998, 27, 199–207. [Google Scholar] [CrossRef]
- Soriguer, F.; Goday, A.; Bosch-Comas, A.; Bordiú, E.; Calle-Pascual, A.; Carmena, R.; Casamitjana, R.; Castaño, L.; Castell, C.; Catalá, M.; et al. Prevalence of Diabetes Mellitus and Impaired Glucose Regulation in Spain: The Di@bet.Es Study. Diabetologia 2012, 55, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Brugnara, L.; Murillo, S.; Novials, A.; Rojo-Martínez, G.; Soriguer, F.; Goday, A.; Calle-Pascual, A.; Castaño, L.; Gaztambide, S.; Valdés, S.; et al. Low Physical Activity and Its Association with Diabetes and Other Cardiovascular Risk Factors: A Nationwide, Population-Based Study. PLoS ONE 2016, 11, e0160959. [Google Scholar] [CrossRef] [PubMed]
- Aekplakorn, W.; Chariyalertsak, S.; Kessomboon, P.; Assanangkornchai, S.; Taneepanichskul, S.; Putwatana, P. Prevalence of Diabetes and Relationship with Socioeconomic Status in the Thai Population: National Health Examination Survey, 2004–2014. J. Diabetes Res. 2018, 2018, 1654530. [Google Scholar] [CrossRef] [PubMed]
- Shang, X.; Li, J.; Tao, Q.; Li, J.; Li, X.; Zhang, L.; Liu, X.; Wang, Q.; Shi, X.; Zhao, Y.; et al. Educational Level, Obesity and Incidence of Diabetes among Chinese Adult Men and Women Aged 18–59 Years Old: An 11-Year Follow-Up Study. PLoS ONE 2013, 8, e66479. [Google Scholar] [CrossRef]
- Sone, H.; Tanaka, S.; Iimuro, S.; Tanaka, S.; Oida, K.; Yamasaki, Y.; Oikawa, S.; Ishibashi, S.; Katayama, S.; Yamashita, H.; et al. Long-Term Lifestyle Intervention Lowers the Incidence of Stroke in Japanese Patients with Type 2 Diabetes: A Nationwide Multicentre Randomised Controlled Trial (the Japan Diabetes Complications Study). Diabetologia 2010, 53, 419–428. [Google Scholar] [CrossRef]
- The Look AHEAD Research Group Cardiovascular Effects of Intensive Lifestyle Intervention in Type 2 Diabetes. N. Engl. J. Med. 2013, 369, 145–154. [CrossRef]
- Scholes, S.; Bann, D. Education-Related Disparities in Reported Physical Activity during Leisure-Time, Active Transportation, and Work among US Adults: Repeated Cross-Sectional Analysis from the National Health and Nutrition Examination Surveys, 2007 to 2016. BMC Public Health 2018, 18, 926. [Google Scholar] [CrossRef]
- Icks, A.; Kruse, J.; Dragano, N.; Broecker-Preuss, M.; Slomiany, U.; Mann, K.; Jöckel, K.; Erbel, R.; Giani, G.; Moebus, S. Are Symptoms of Depression Commoner in Diabetes? Results from the Heinz Nixdorf Recall Study. Diabet. Med. 2008, 25, 1330–1336. [Google Scholar] [CrossRef]
- Anderson, R.J.; Freedland, K.E.; Clouse, R.E.; Lustman, P.J. The Prevalence of Comorbid Depression in Adults With Diabetes. Diabetes Care 2001, 24, 1069–1078. [Google Scholar] [CrossRef]
- Lin, E.H.B.; Heckbert, S.R.; Rutter, C.M.; Katon, W.J.; Ciechanowski, P.; Ludman, E.J.; Oliver, M.; Young, B.A.; McCulloch, D.K.; von Korff, M. Depression and Increased Mortality in Diabetes: Unexpected Causes of Death. Ann. Fam. Med. 2009, 7, 414–421. [Google Scholar] [CrossRef]
- Roy, T.; Lloyd, C.E. Epidemiology of Depression and Diabetes: A Systematic Review. J. Affect. Disord. 2012, 142, S8–S21. [Google Scholar] [CrossRef] [PubMed]
- Eren, İ.; Erdi, Ö.; Şahin, M. The Effect of Depression on Quality of Life of Patients with Type II Diabetes Mellitus. Depress Anxiety 2008, 25, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Green, A.J.; Fox, K.M.; Grandy, S. Self-Reported Hypoglycemia and Impact on Quality of Life and Depression among Adults with Type 2 Diabetes Mellitus. Diabetes Res. Clin. Pract. 2012, 96, 313–318. [Google Scholar] [CrossRef]
- Gregg, E.W.; Beckles, G.L.; Williamson, D.F.; Leveille, S.G.; Langlois, J.A.; Engelgau, M.M.; Narayan, K.M. Diabetes and Physical Disability among Older U.S. Adults. Diabetes Care 2000, 23, 1272–1277. [Google Scholar] [CrossRef]
- Mayfield, J.A.; Deb, P.; Whitecotton, L. Work Disability and Diabetes. Diabetes Care 1999, 22, 1105–1109. [Google Scholar] [CrossRef] [PubMed]
- Miettola, J.; Niskanen, L.K.; Viinamäki, H.; Kumpusalo, E. Metabolic Syndrome Is Associated with Self-Perceived Depression. Scand. J. Prim. Health Care 2008, 26, 203–210. [Google Scholar] [CrossRef]
- Pan, A.; Lucas, M.; Sun, Q.; van Dam, R.M.; Franco, O.H.; Willett, W.C.; Manson, J.E.; Rexrode, K.M.; Ascherio, A.; Hu, F.B. Increased Mortality Risk in Women With Depression and Diabetes Mellitus. Arch. Gen. Psychiatry 2011, 68, 42. [Google Scholar] [CrossRef] [PubMed]
- Paschalides, C.; Wearden, A.J.; Dunkerley, R.; Bundy, C.; Davies, R.; Dickens, C.M. The Associations of Anxiety, Depression and Personal Illness Representations with Glycaemic Control and Health-Related Quality of Life in Patients with Type 2 Diabetes Mellitus. J. Psychosom. Res. 2004, 57, 557–564. [Google Scholar] [CrossRef]
- Pawaskar, M.D.; Anderson, R.T.; Balkrishnan, R. Self-Reported Predictors of Depressive Symptomatology in an Elderly Population with Type 2 Diabetes Mellitus: A Prospective Cohort Study. Health Qual. Life Outcomes 2007, 5, 50. [Google Scholar] [CrossRef]
- Xuan, J.; Kirchdoerfer, L.J.; Boyer, J.G.; Norwood, G.J. Effects of Comorbidity on Health-Related Quality-of-Life Scores: An Analysis of Clinical Trial Data. Clin. Ther. 1999, 21, 383–403. [Google Scholar] [CrossRef]
- van der Feltz-Cornelis, C.M.; Nuyen, J.; Stoop, C.; Chan, J.; Jacobson, A.M.; Katon, W.; Snoek, F.; Sartorius, N. Effect of Interventions for Major Depressive Disorder and Significant Depressive Symptoms in Patients with Diabetes Mellitus: A Systematic Review and Meta-Analysis. Gen. Hosp. Psychiatry 2010, 32, 380–395. [Google Scholar] [CrossRef] [PubMed]
- Engum, A. The Role of Depression and Anxiety in Onset of Diabetes in a Large Population-Based Study. J. Psychosom. Res. 2007, 62, 31–38. [Google Scholar] [CrossRef]
- Golden, S.H. Examining a Bidirectional Association Between Depressive Symptoms and Diabetes. JAMA 2008, 299, 2751. [Google Scholar] [CrossRef] [PubMed]
- Holt, R.I.G.; Phillips, D.I.W.; Jameson, K.A.; Cooper, C.; Dennison, E.M.; Peveler, R.C. The Relationship between Depression and Diabetes Mellitus: Findings from the Hertfordshire Cohort Study. Diabet. Med. 2009, 26, 641–648. [Google Scholar] [CrossRef]
- Brown, L.C. Type 2 Diabetes Does Not Increase Risk of Depression. Can. Med. Assoc. J. 2006, 175, 42–46. [Google Scholar] [CrossRef]
- Paddison, C.A.M.; Eborall, H.C.; French, D.P.; Kinmonth, A.L.; Prevost, A.T.; Griffin, S.J.; Sutton, S. Predictors of Anxiety and Depression among People Attending Diabetes Screening: A Prospective Cohort Study Embedded in the ADDITION (Cambridge) Randomized Control Trial. Br. J. Health Psychol. 2011, 16, 213–226. [Google Scholar] [CrossRef]
- Brasil, C.H.G.; Maia, L.C.; Caldeira, A.P.; Brito, M.F.S.F.; Pinho, L. de Autopercepção Positiva de Saúde Entre Idosos Não Longevos e Longevos e Fatores Associados. Cien. Saude Colet. 2021, 26, 5157–5170. [Google Scholar] [CrossRef]
- Pagotto, V.; Bachion, M.M.; Silveira, E.A. da Autoavaliação Da Saúde Por Idosos Brasileiros: Revisão Sistemática Da Literatura. Rev. Panam. De Salud Pública 2013, 33, 302–310. [Google Scholar] [CrossRef]
- Ferraro, K.F.; Farmer, M.M. Utility of Health Data from Social Surveys: Is There a Gold Standard for Measuring Morbidity? Am. Sociol. Rev. 1999, 64, 303. [Google Scholar] [CrossRef]
- Kaleta, D.; Makowiec-Dąbrowska, T.; Dziankowska-Zaborszczyk, E.; Jegier, A. Physical Activity and Self-Perceived Health Status. Int. J. Occup. Med. Env. Health 2006, 19, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Miilunpalo, S.; Vuori, I.; Oja, P.; Pasanen, M.; Urponen, H. Self-Rated Health Status as a Health Measure: The Predictive Value of Self-Reported Health Status on the Use of Physician Services and on Mortality in the Working-Age Population. J. Clin. Epidemiol. 1997, 50, 517–528. [Google Scholar] [CrossRef]
- Cheval, B.; Sivaramakrishnan, H.; Maltagliati, S.; Fessler, L.; Forestier, C.; Sarrazin, P.; Orsholits, D.; Chalabaev, A.; Sander, D.; Ntoumanis, N.; et al. Relationships between Changes in Self-Reported Physical Activity, Sedentary Behaviour and Health during the Coronavirus (COVID-19) Pandemic in France and Switzerland. J. Sport. Sci. 2021, 39, 699–704. [Google Scholar] [CrossRef] [PubMed]
- Buecker, S.; Simacek, T.; Ingwersen, B.; Terwiel, S.; Simonsmeier, B.A. Physical Activity and Subjective Well-Being in Healthy Individuals: A Meta-Analytic Review. Health Psychol. Rev. 2021, 15, 574–592. [Google Scholar] [CrossRef] [PubMed]
- Stover, J.C.; Skelly, A.H.; Holditch-Davis, D.; Dunn, P.F. Perceptions of Health and Their Relationship to Symptoms in African American Women with Type 2 Diabetes. Appl. Nurs. Res. 2001, 14, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Kautzky-Willer, A.; Harreiter, J.; Pacini, G. Sex and Gender Differences in Risk, Pathophysiology and Complications of Type 2 Diabetes Mellitus. Endocr. Rev. 2016, 37, 278–316. [Google Scholar] [CrossRef]
- Sattar, N. Gender Aspects in Type 2 Diabetes Mellitus and Cardiometabolic Risk. Best Pract. Res. Clin. Endocrinol. Metab. 2013, 27, 501–507. [Google Scholar] [CrossRef]
- Seghieri, G.; Policardo, L.; Anichini, R.; Franconi, F.; Campesi, I.; Cherchi, S.; Tonolo, G. The Effect of Sex and Gender on Diabetic Complications. Curr. Diabetes Rev. 2017, 13, 148–160. [Google Scholar] [CrossRef]
- Espelt, A.; Goday, A.; Franch, J.; Borrell, C. Validity of Self-Reported Diabetes in Health Interview Surveys for Measuring Social Inequalities in the Prevalence of Diabetes: Table 1. J. Epidemiol. Community Health 2012, 66, e15. [Google Scholar] [CrossRef] [PubMed]
- Schneider, A.L.C.; Pankow, J.S.; Heiss, G.; Selvin, E. Validity and Reliability of Self-Reported Diabetes in the Atherosclerosis Risk in Communities Study. Am. J. Epidemiol. 2012, 176, 738–743. [Google Scholar] [CrossRef]
- Yuan, X.; Liu, T.; Wu, L.; Zou, Z.-Y.; Li, C. Validity of Self-Reported Diabetes among Middle-Aged and Older Chinese Adults: The China Health and Retirement Longitudinal Study. BMJ Open 2015, 5, e006633. [Google Scholar] [CrossRef]
- Chen, Y.; Rennie, D.; Lockinger, L.; Dosman, J. Association between Obesity and High Blood Pressure: Reporting Bias Related to Gender and Age. Int. J. Obes. 1998, 22, 771–777. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.H.; Macfarlane, D.J.; Lam, T.H.H.; Stewart, S.M. Validity of the International Physical Activity Questionnaire Short Form (IPAQ-SF): A Systematic Review. Int. J. Behav. Nutr. Phys. Act. 2011, 8, 115. [Google Scholar] [CrossRef] [PubMed]
- Bakker, E.A.; Hartman, Y.A.W.; Hopman, M.T.E.; Hopkins, N.D.; Graves, L.E.F.; Dunstan, D.W.; Healy, G.N.; Eijsvogels, T.M.H.; Thijssen, D.H.J. Validity and Reliability of Subjective Methods to Assess Sedentary Behaviour in Adults: A Systematic Review and Meta-Analysis. Int. J. Behav. Nutr. Phys. Act. 2020, 17, 75. [Google Scholar] [CrossRef] [PubMed]
- Félix-Redondo, F.J.; Grau, M.; Baena-Díez, J.M.; Dégano, I.R.; de León, A.C.; Guembe, M.J.; Alzamora, M.T.; Vega-Alonso, T.; Robles, N.R.; Ortiz, H.; et al. Prevalence of Obesity and Associated Cardiovascular Risk: The DARIOS Study. BMC Public Health 2013, 13, 542. [Google Scholar] [CrossRef]
- Strath, S.J.; Kaminsky, L.A.; Ainsworth, B.E.; Ekelund, U.; Freedson, P.S.; Gary, R.A.; Richardson, C.R.; Smith, D.T.; Swartz, A.M. Guide to the Assessment of Physical Activity: Clinical and Research Applications. Circulation 2013, 128, 2259–2279. [Google Scholar] [CrossRef] [PubMed]




| Overall (n = 1319) | Men (n = 687) | Women (n = 632) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variables | Mdn | IQR | Mdn | IQR | Mdn | IQR | x2 | df | p | CC |
| Age | 67 | 12 | 66 | 12 | 69 | 12 | n.a. | n.a. | <0.001 | n.a. |
| BMI | 28.4 | 6.4 | 28 | 5.6 | 29 | 7.4 | n.a. | n.a. | 0.003 | n.a. |
| BMI Group | n | % | n | % | n | % | p * | |||
| Underweight | 6 | 0.5% | 2 | 0.3% | 4 | 0.7% | 21.8 | 3 | <0.001 | 0.132 |
| Normal | 240 | 19.4% | 125 | 18.7% | 115 | 20.2% | ||||
| Overweight | 534 | 43.1% | 327 | 48.8% | 207 | 36.3% * | ||||
| Obesity | 460 | 37.1% | 216 | 32.2% | 244 | 42.8% * | ||||
| Physical Activity Frequency | n | % | n | % | n | % | p * | |||
| Inactive | 621 | 47.1% | 269 | 39.2% | 352 | 55.7% * | 42.2 | 3 | <0.001 | 0.176 |
| Occasional | 601 | 45.6% | 349 | 50.8% | 252 | 39.9% * | ||||
| Active | 46 | 3.5% | 33 | 4.8% | 13 | 2.1% * | ||||
| Very active | 50 | 3.8% | 35 | 5.1% | 15 | 2.4% * | ||||
| Depression | n | % | n | % | n | % | p * | |||
| Yes | 291 | 22.1% | 94 | 13.7% | 197 | 31.2% * | 58.8 | 1 | <0.001 | 0.207 |
| No | 1027 | 77.9% | 593 | 86.3% | 434 | 68.8% * | ||||
| Depressive symptoms | n | % | n | % | n | % | p * | |||
| None | 915 | 69.9% | 551 | 80.7% | 364 | 58.1% * | 80.5 | 2 | <0.001 | 0.241 |
| Minor | 306 | 23.4% | 108 | 15.8% | 198 | 31.6% * | ||||
| Severe | 88 | 6.7% | 24 | 3.5% | 64 | 10.2% * | ||||
| Self-Perceived Health | n | % | n | % | n | % | p * | |||
| Positive | 465 | 35.3% | 288 | 41.9% | 177 | 28% * | 36.1 | 2 | <0.001 | 0.163 |
| Fair | 539 | 40.9% | 272 | 39.6% | 267 | 42.2% | ||||
| Negative | 315 | 23.9% | 127 | 18.5% | 188 | 29.7% * | ||||
| Overall (n = 1480) | Men (n = 799) | Women (n = 681) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variables | Mdn | IQR | Mdn | IQR | Mdn | IQR | x2 | df | p | CC |
| Age | 68 | 12 | 68 | 12 | 69 | 12 | n.a. | n.a. | 0.003 | n.a |
| BMI | 27.9 | 6.0 | 27.7 | 5.4 | 28.1 | 6.8 | n.a. | n.a. | 0.060 | n.a. |
| BMI Group | n | % | n | % | n | % | x2 | df | p * | CC |
| Underweight | 3 | 0.2% | 0 | 0.0% | 3 | 0.5% | 13.0 | 3 | 0.005 | 0.096 |
| Normal | 321 | 22.9% | 176 | 22.7% | 145 | 23% | ||||
| Overweight | 622 | 44.3% | 369 | 47.7% | 253 | 40.2% * | ||||
| Obesity | 458 | 32.6% | 229 | 29.6% | 229 | 36.3% * | ||||
| Physical Activity Frequency | n | % | n | % | n | % | x2 | df | p * | CC |
| Inactive | 637 | 43% | 310 | 38.8% | 327 | 48% * | 14.4 | 3 | 0.002 | 0.096 |
| Occasional | 657 | 44.4% | 384 | 48.1% | 273 | 40.1% * | ||||
| Active | 78 | 5.3% | 40 | 5% | 38 | 5.6% | ||||
| Very active | 108 | 7.3% | 65 | 8.1% | 43 | 6.3% | ||||
| Depression | n | % | n | % | n | % | x2 | df | p * | CC |
| Yes | 261 | 17.7% | 89 | 11.2% | 172 | 25.3% * | 50.7 | 1 | <0.001 | 0.182 |
| No | 1216 | 82.3% | 709 + 229 | 88.8% | 507 | 74.7% * | ||||
| Depressive symptoms | n | % | n | % | n | % | x2 | df | p * | CC |
| None | 1137 | 77.4% | 659 | 83.1% | 478 | 70.7% * | 34.3 | 2 | <0.001 | 0.151 |
| Minor | 280 | 19.1% | 118 | 14.9% | 162 | 24% * | ||||
| Severe | 52 | 3.5% | 16 | 2% | 36 | 5.3% * | ||||
| Self-Perceived Health | n | % | n | % | n | % | x2 | df | p * | CC |
| Positive | 656 | 44.3% | 406 | 50.8% | 250 | 21.7% * | 30.1 | 2 | <0.001 | 0.141 |
| Fair | 550 | 47.2% | 267 | 33.4% | 283 | 41.6% | ||||
| Negative | 274 | 18.5% | 126 | 15.8% | 148 | 29.7% * | ||||
| EHIS2014 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Never | Occasionally | Several Times/Month | Several Times/Week | |||||||||
| Depression | n | (%) | n | (%) | n | (%) | n | (%) | x2 | df | p | CC |
| Yes | 172 | (27.7%) | 110 | (18.3%) | 5 | (10.9%) | 4 | (8%) | 25.6 | 3 | <0.001 | 0.138 |
| No | 448 | (72.3%) | 491 | (81.7%) | 41 | (89.1%) | 46 | (92%) | ||||
| Depressive symptoms | n | (%) | n | (%) | n | (%) | n | (%) | x2 | df | p | CC |
| None | 348 | (56.8%) | 481 | (80.3%) | 38 | (82.6%) | 47 | (94%) | 104.6 | 6 | <0.001 | 0.272 |
| Minor | 196 | (32%) | 99 | (16.5%) | 8 | (17.4%) | 3 | (6%) | ||||
| Severe | 69 | (11.3%) | 19 | (3.2%) | 0 | (0%) | 0 | (0%) | ||||
| Self-Perceived Health | n | (%) | n | (%) | n | (%) | n | (%) | x2 | df | p | CC |
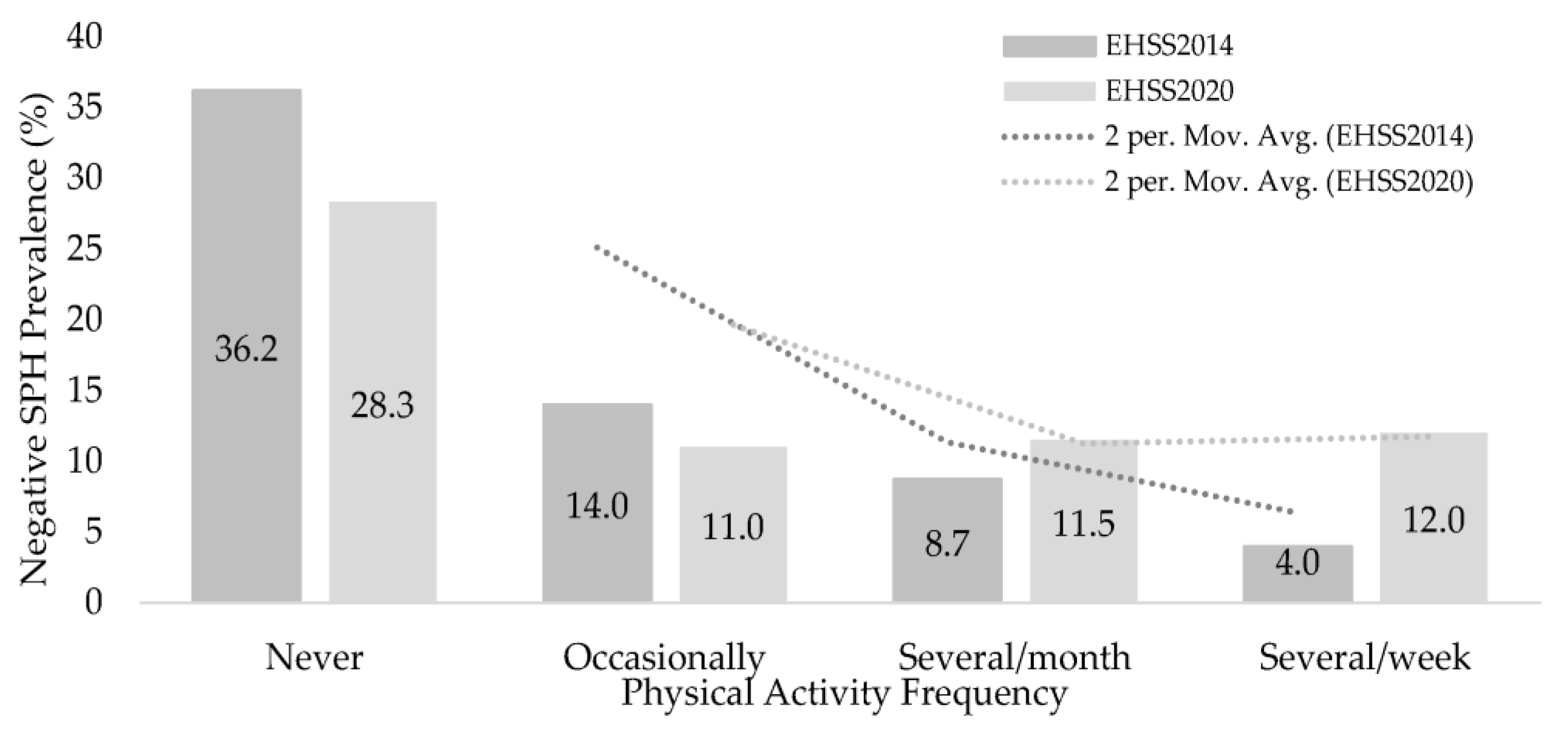
| Negative | 225 | (36.2%) | 84 | (14.0%) | 4 | (8.7%) | 2 | (4%) | 143.4 | 6 | <0.001 | 0.313 |
| Fair | 258 | (41.5%) | 248 | (41.3%) | 31 | (39.1%) | 35 | (28%) | ||||
| Positive | 138 | (22.2%) | 269 | (44.8%) | 24 | (52.2%) | 34 | (68%) | ||||
| EHISS2020 | ||||||||||||
| Never | Occasionally | Several times/month | Several times/week | |||||||||
| Depression | n | (%) | n | (%) | n | (%) | n | (%) | x2 | df | p | CC |
| Yes | 152 | (23.9%) | 83 | (12.7%) | 10 | (12.8%) | 16 | (15%) | 30.1 | 3 | <0.001 | 0.141 |
| No | 484 | (76.1%) | 573 | (87.3%) | 68 | (87.2%) | 91 | (85%) | ||||
| Depressive symptoms | n | (%) | n | (%) | n | (%) | n | (%) | x2 | df | p | CC |
| None | 428 | (67.9%) | 550 | (84.2%) | 65 | (83.3%) | 94 | (87%) | 60.9 | 6 | <0.001 | 0.200 |
| Minor | 164 | (26%) | 92 | (14.1%) | 12 | (15.4%) | 12 | (11.1%) | ||||
| Severe | 38 | (6.0%) | 11 | (1.7%) | 1 | (1.3%) | 2 | (1.9%) | ||||
| Self-Perceived Health | n | (%) | n | (%) | n | (%) | n (%) | x2 | df | p | CC | |
| Negative | 180 | (28.3%) | 72 | (11%) | 9 | (11.5%) | 13 | (12%) | 89.9 | 6 | <0.001 | 0.239 |
| Fair | 245 | (38.5%) | 239 | (36.4%) | 31 | (39.7%) | 35 | (32.4%) | ||||
| Positive | 212 | (33.3%) | 346 | (52.7%) | 38 | (48.7%) | 60 | (55.6%) | ||||
| EHIS 2014 | ||||||||
|---|---|---|---|---|---|---|---|---|
| β | S.E. | Wald | df. | Sig. | Exp(β) | 95% C.I. for EXP(β) | ||
| Lower | Upper | |||||||
| PAF (Never) | 9.901 | 3 | 0.019 | |||||
| Occasionally | −0.322 | 0.149 | 4.662 | 1 | 0.031 | 0.724 | 0.541 | 0.971 |
| Several/month | −0.796 | 0.494 | 2.594 | 1 | 0.107 | 0.451 | 0.171 | 1.189 |
| Several/week | −1.172 | 0.538 | 4.755 | 1 | 0.029 | 0.310 | 0.108 | 0.888 |
| Sex (Women) | 0.980 | 0.149 | 42.954 | 1 | 0.000 | 2.663 | 1.987 | 3.569 |
| Age | −0.008 | 0.009 | 0.733 | 1 | 0.392 | 0.992 | 0.975 | 1.010 |
| BMI | 0.022 | 0.014 | 2.352 | 1 | 0.125 | 1.022 | 0.994 | 1.051 |
| Constant | −1768.000 | 0.769 | 5.280 | 1 | 0.022 | 0.171 | ||
| EHIS 2020 | ||||||||
| β | S.E. | Wald | df. | Sig. | Exp(β) | 95% C.I. for EXP(β) | ||
| Lower | Upper | |||||||
| PAF (Never) | 18.481 | 3 | 0.000 | |||||
| Occasionally | −0.646 | 0.158 | 16.791 | 1 | 0.000 | 0.524 | 0.385 | 0.714 |
| Several/month | −0.696 | 0.359 | 3.762 | 1 | 0.052 | 0.498 | 0.247 | 1.007 |
| Several/week | −0.411 | 0.294 | 1.944 | 1 | 0.163 | 0.663 | 0.372 | 1.181 |
| Sex (Women) | 0.905 | 0.148 | 37.282 | 1 | 0.000 | 2.473 | 1.849 | 3.307 |
| Age | −0.014 | 0.009 | 2.152 | 1 | 0.142 | 0.987 | 0.969 | 1.005 |
| BMI | 0.023 | 0.014 | 2.603 | 1 | 0.107 | 1.023 | 0.995 | 1.053 |
| Constant | −1.465 | 0.788 | 3.458 | 1 | 0.063 | 0.231 | ||
| Hypothesis | Confirmation |
|---|---|
| Prevalence of overweight or obesity both from the EHIS2014 and EHIS2020 | confirmed |
| Relationship between sex and BMI groups both from the EHIS2014 and EHIS2020 | confirmed |
| Prevalence of inactive or occasional physical activity frequency both from the EHIS2014 and EHIS2020 | confirmed |
| Associations between sex and depression and depressive symptoms prevalence both from the EHIS2014 and EHIS2020 | confirmed |
| Associations between Self-Perceived Health and sex both from the EHIS2014 and EHIS2020 | confirmed |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Denche-Zamorano, A.; Perez-Gomez, J.; Barrios-Fernandez, S.; Oliveira, R.; Adsuar, J.C.; Brito, J.P. Relationships between Physical Activity Frequency and Self-Perceived Health, Self-Reported Depression, and Depressive Symptoms in Spanish Older Adults with Diabetes: A Cross-Sectional Study. Int. J. Environ. Res. Public Health 2023, 20, 2857. https://doi.org/10.3390/ijerph20042857
Denche-Zamorano A, Perez-Gomez J, Barrios-Fernandez S, Oliveira R, Adsuar JC, Brito JP. Relationships between Physical Activity Frequency and Self-Perceived Health, Self-Reported Depression, and Depressive Symptoms in Spanish Older Adults with Diabetes: A Cross-Sectional Study. International Journal of Environmental Research and Public Health. 2023; 20(4):2857. https://doi.org/10.3390/ijerph20042857
Chicago/Turabian StyleDenche-Zamorano, Angel, Jorge Perez-Gomez, Sabina Barrios-Fernandez, Rafael Oliveira, Jose C. Adsuar, and João Paulo Brito. 2023. "Relationships between Physical Activity Frequency and Self-Perceived Health, Self-Reported Depression, and Depressive Symptoms in Spanish Older Adults with Diabetes: A Cross-Sectional Study" International Journal of Environmental Research and Public Health 20, no. 4: 2857. https://doi.org/10.3390/ijerph20042857
APA StyleDenche-Zamorano, A., Perez-Gomez, J., Barrios-Fernandez, S., Oliveira, R., Adsuar, J. C., & Brito, J. P. (2023). Relationships between Physical Activity Frequency and Self-Perceived Health, Self-Reported Depression, and Depressive Symptoms in Spanish Older Adults with Diabetes: A Cross-Sectional Study. International Journal of Environmental Research and Public Health, 20(4), 2857. https://doi.org/10.3390/ijerph20042857











