A Comprehensive Assessment Protocol for Swallowing (CAPS): Paving the Way towards Computer-Aided Dysphagia Screening
Abstract
1. Introduction
1.1. Background of Dysphagia
1.2. Traditional Swallowing Assessments for Dysphagia
1.3. Emerging Computer-Aided Screening Technologies for Dysphagia
1.4. Scope and Objectives
2. Methods
2.1. Protocol Overview
2.2. Pre-Test Phase to Determine the Size of Bolus Intake for Assessment
2.3. Assessment Phase
2.4. Evaluation/Assessment Methods
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Malagelada, J.-R.; Bazzoli, F.; Boeckxstaens, G.; De Looze, D.; Fried, M.; Kahrilas, P.; Lindberg, G.; Malfertheiner, P.; Salis, G.; Sharma, P. World gastroenterology organisation global guidelines: Dysphagia—Global guidelines and cascades update September 2014. J. Clin. Gastroenterol. 2015, 49, 370–378. [Google Scholar] [CrossRef]
- Hammond, C.A.S.; Goldstein, L.B. Cough and aspiration of food and liquids due to oral-pharyngeal dysphagia: ACCP evidence-based clinical practice guidelines. Chest 2006, 129, 154S–168S. [Google Scholar] [CrossRef]
- Crary, M.A.; Carnaby, G.D.; Sia, I.; Khanna, A.; Waters, M.F. Spontaneous swallowing frequency has potential to identify dysphagia in acute stroke. Stroke 2013, 44, 3452–3457. [Google Scholar] [CrossRef] [PubMed]
- Auyeung, M.; Tsoi, T.; Mok, V.; Cheung, C.; Lee, C.; Li, R.; Yeung, E. Ten year survival and outcomes in a prospective cohort of new onset Chinese Parkinson’s disease patients. J. Neurol. Neurosurg. Psychiatry 2012, 83, 607–611. [Google Scholar] [CrossRef]
- Rogus-Pulia, N.; Malandraki, G.A.; Johnson, S.; Robbins, J. Understanding dysphagia in dementia: The present and the future. Curr. Phys. Med. Rehabil. Rep. 2015, 3, 86–97. [Google Scholar] [CrossRef]
- Bhattacharyya, N. The prevalence of dysphagia among adults in the United States. Otolaryngol.—Head Neck Surg. 2014, 151, 765–769. [Google Scholar] [CrossRef]
- Takizawa, C.; Gemmell, E.; Kenworthy, J.; Speyer, R. A systematic review of the prevalence of oropharyngeal dysphagia in stroke, Parkinson’s disease, Alzheimer’s disease, head injury, and pneumonia. Dysphagia 2016, 31, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.; Krishnaswami, S.; Steger, E.; Conover, E.; Vaezi, M.; Ciucci, M.; Francis, D. Economic and survival burden of dysphagia among inpatients in the United States. Dis. Esophagus 2018, 31, 131. [Google Scholar] [CrossRef]
- Baijens, L.W.; Clavé, P.; Cras, P.; Ekberg, O.; Forster, A.; Kolb, G.F.; Leners, J.-C.; Masiero, S.; Mateos-Nozal, J.; Ortega, O. European Society for Swallowing Disorders–European Union Geriatric Medicine Society white paper: Oropharyngeal dysphagia as a geriatric syndrome. Clin. Interv. Aging 2016, 11, 1403. [Google Scholar] [CrossRef] [PubMed]
- Warnecke, T.; Dziewas, R.; Langmore, S.; Warnecke, T.; Dziewas, R.; Langmore, S. Neurogenic Dysphagia; FEES and Other Instrumental Methods for Swallowing Evaluation; Karger Publishers: Basel, Switzerland, 2021; pp. 55–107. [Google Scholar]
- Maccarini, A.R.; Filippini, A.; Padovani, D.; Limarzi, M.; Loffredo, M.; Casolino, D. Clinical non-instrumental evaluation of dysphagia. Acta Otolaryngol. Ital. 2007, 27, 299–305. [Google Scholar]
- DePippo, K.L.; Holas, M.A.; Reding, M.J. Validation of the 3-oz water swallow test for aspiration following stroke. Arch. Neurol. 1992, 49, 1259–1261. [Google Scholar] [CrossRef]
- Dudik, J.M.; Coyle, J.L.; Sejdić, E. Dysphagia screening: Contributions of cervical auscultation signals and modern signal-processing techniques. IEEE Trans. Hum.—Mach. Syst. 2015, 45, 465–477. [Google Scholar] [CrossRef] [PubMed]
- O’Horo, J.C.; Rogus-Pulia, N.; Garcia-Arguello, L.; Robbins, J.; Safdar, N. Bedside diagnosis of dysphagia: A systematic review. J. Hosp. Med. 2015, 10, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Lagarde, M.L.; Kamalski, D.M.; Van Den Engel-Hoek, L. The reliability and validity of cervical auscultation in the diagnosis of dysphagia: A systematic review. Clin. Rehabil. 2016, 30, 199–207. [Google Scholar] [CrossRef]
- Santos, B.P.; Andrade, M.J.C.; Silva, R.O.; da Menezes, E.C. Dysphagia in the elderly in long-stay institutions-a systematic literature review. Rev. CEFAC 2018, 20, 123–130. [Google Scholar] [CrossRef]
- Namasivayam-MacDonald, A.M.; Alomari, N.; Attner, L.; Benjamin, R.D.; Chill, A.; Doka, S.; Guastella, R.; Marchese, J.; Oppedisano, S.; Ressa, K. A retrospective analysis of swallowing function and physiology in patients living with dementia. Dysphagia 2022, 37, 900–908. [Google Scholar] [CrossRef]
- Okuni, I.; Ebihara, S. Are Oropharyngeal Dysphagia Screening Tests Effective in Preventing Pneumonia? J. Clin. Med. 2022, 11, 370. [Google Scholar] [CrossRef]
- So, B.P.-H.; Chan, T.T.-C.; Liu, L.; Yip, C.C.-K.; Lim, H.-J.; Lam, W.-K.; Wong, D.W.-C.; Cheung, D.S.-K.; Cheung, J.C.-W. Swallow Detection with Acoustics and Accelerometric-Based Wearable Technology: A Scoping Review. Int. J. Environ. Res. Public Health 2023, 20, 170. [Google Scholar] [CrossRef]
- The International Dysphagia Diet Standardisation Initiative 2019. The IDDSI Framework. Available online: https://iddsi.org/framework/ (accessed on 2 January 2023).
- Fontana, J.M.; Melo, P.L.; Sazonov, E.S. Swallowing detection by sonic and subsonic frequencies: A comparison. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Boston, MA, USA, 30 August–3 September 2011; pp. 6890–6893. [Google Scholar]
- Fukuike, C.; Kodama, N.; Manda, Y.; Hashimoto, Y.; Sugimoto, K.; Hirata, A.; Pan, Q.; Maeda, N.; Minagi, S. A novel automated detection system for swallowing sounds during eating and speech under everyday conditions. J. Oral Rehabil. 2015, 42, 340–347. [Google Scholar] [CrossRef]
- Garcia, J.M.; Chambers IV, E.; Yarrow, K. Thickened liquids for dysphagia management: A call to action in the development of educational and instructional strategies. J. Texture Stud. 2021, 52, 679–683. [Google Scholar] [CrossRef] [PubMed]
- Robbins, J.; Nicosia, M.; Hind, J.A.; Gill, G.D.; Blanco, R.; Logemann, J. Defining physical properties of fluids for dysphagia evaluation and treatment. Perspect. Swallowing Swallowing Disord. (Dysphagia) 2002, 11, 16–19. [Google Scholar] [CrossRef]
- Clavé, P.; De Kraa, M.; Arreola, V.; Girvent, M.; Farre, R.; Palomera, E.; Serra-Prat, M. The effect of bolus viscosity on swallowing function in neurogenic dysphagia. Aliment. Pharmacol. Ther. 2006, 24, 1385–1394. [Google Scholar] [CrossRef]
- Logemann, J.A. Swallowing physiology and pathophysiology. Otolaryngol. Clin. N. Am. 1988, 21, 613–623. [Google Scholar] [CrossRef]
- Logemann, J.A. Noninvasive approaches to deglutitive aspiration. Dysphagia 1993, 8, 331–333. [Google Scholar] [CrossRef]
- Clavé, P.; Rofes, L.; Carrión, S.; Ortega, O.; Cabré, M.; Serra-Prat, M.; Arreola, V. Pathophysiology, relevance and natural history of oropharyngeal dysphagia among older people. In Stepping Stones to Living Well with Dysphagia; Karger Publishers: Basel, Switzerland, 2012; Volume 72, pp. 57–66. [Google Scholar]
- Steele, C.M.; Huckabee, M.L. The influence of orolingual pressure on the timing of pharyngeal pressure events. Dysphagia 2007, 22, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Skowronski, M.D.; Crary, M.A.; Shrivastav, R. Acoustic discrimination of healthy swallows from upper airway movements. J. Acoust. Soc. Am. 2013, 134, EL127–EL132. [Google Scholar] [CrossRef]
- Roldan-Vasco, S.; Orozco-Duque, A.; Suarez-Escudero, J.C.; Orozco-Arroyave, J.R. Machine learning based analysis of speech dimensions in functional oropharyngeal dysphagia. Comput. Methods Programs Biomed. 2021, 208, 106248. [Google Scholar] [CrossRef]
- Chen, P.C.; Chuang, C.H.; Leong, C.P.; Guo, S.E.; Hsin, Y.J. Systematic review and meta-analysis of the diagnostic accuracy of the water swallow test for screening aspiration in stroke patients. J. Adv. Nurs. 2016, 72, 2575–2586. [Google Scholar] [CrossRef]
- Antonios, N.; Carnaby-Mann, G.; Crary, M.; Miller, L.; Hubbard, H.; Hood, K.; Sambandam, R.; Xavier, A.; Silliman, S. Analysis of a physician tool for evaluating dysphagia on an inpatient stroke unit: The modified Mann Assessment of Swallowing Ability. J. Stroke Cerebrovasc. Dis. 2010, 19, 49–57. [Google Scholar] [CrossRef]
- So, B.P.-H.; Lai, D.K.-H.; Cheung, D.S.-K.; Lam, W.-K.; Cheung, J.C.-W.; Wong, D.W.-C. Virtual Reality-Based Immersive Rehabilitation for Cognitive-and Behavioral-Impairment-Related Eating Disorders: A VREHAB Framework Scoping Review. Int. J. Environ. Res. Public Health 2022, 19, 5821. [Google Scholar] [CrossRef]
- Cheung, J.C.-W.; Tam, E.W.-C.; Mak, A.H.-Y.; Chan, T.T.-C.; Zheng, Y.-P. A night-time monitoring system (eNightLog) to prevent elderly wandering in hostels: A three-month field study. Int. J. Environ. Res. Public Health 2022, 19, 2103. [Google Scholar] [CrossRef]
- Cheung, J.C.-W.; So, B.P.-H.; Ho, K.H.M.; Wong, D.W.-C.; Lam, A.H.-F.; Cheung, D.S.K. Wrist accelerometry for monitoring dementia agitation behaviour in clinical settings: A scoping review. Front. Psychiatry 2022, 13, 913213. [Google Scholar] [CrossRef]
- Cheung, D.S.K.; Wang, S.S.; Li, Y.; Ho, K.H.M.; Kwok, R.K.H.; Mo, S.H.; Bressington, D. Sensory-based interventions for the immediate de-escalation of agitation in people with dementia: A systematic review. Aging Ment. Health 2022. [Google Scholar] [CrossRef]
- Ma, C.Z.-H.; Wong, D.W.-C.; Lam, W.K.; Wan, A.H.-P.; Lee, W.C.-C. Balance improvement effects of biofeedback systems with state-of-the-art wearable sensors: A systematic review. Sensors 2016, 16, 434. [Google Scholar] [CrossRef] [PubMed]
- Enshaeifar, S.; Barnaghi, P.; Skillman, S.; Markides, A.; Elsaleh, T.; Acton, S.T.; Nilforooshan, R.; Rostill, H. The internet of things for dementia care. IEEE Internet Comput. 2018, 22, 8–17. [Google Scholar] [CrossRef]
- Sheikhtaheri, A.; Sabermahani, F. Applications and Outcomes of Internet of Things for Patients with Alzheimer’s Disease/Dementia: A Scoping Review. BioMed Res. Int. 2022, 2022, 6274185. [Google Scholar] [CrossRef] [PubMed]
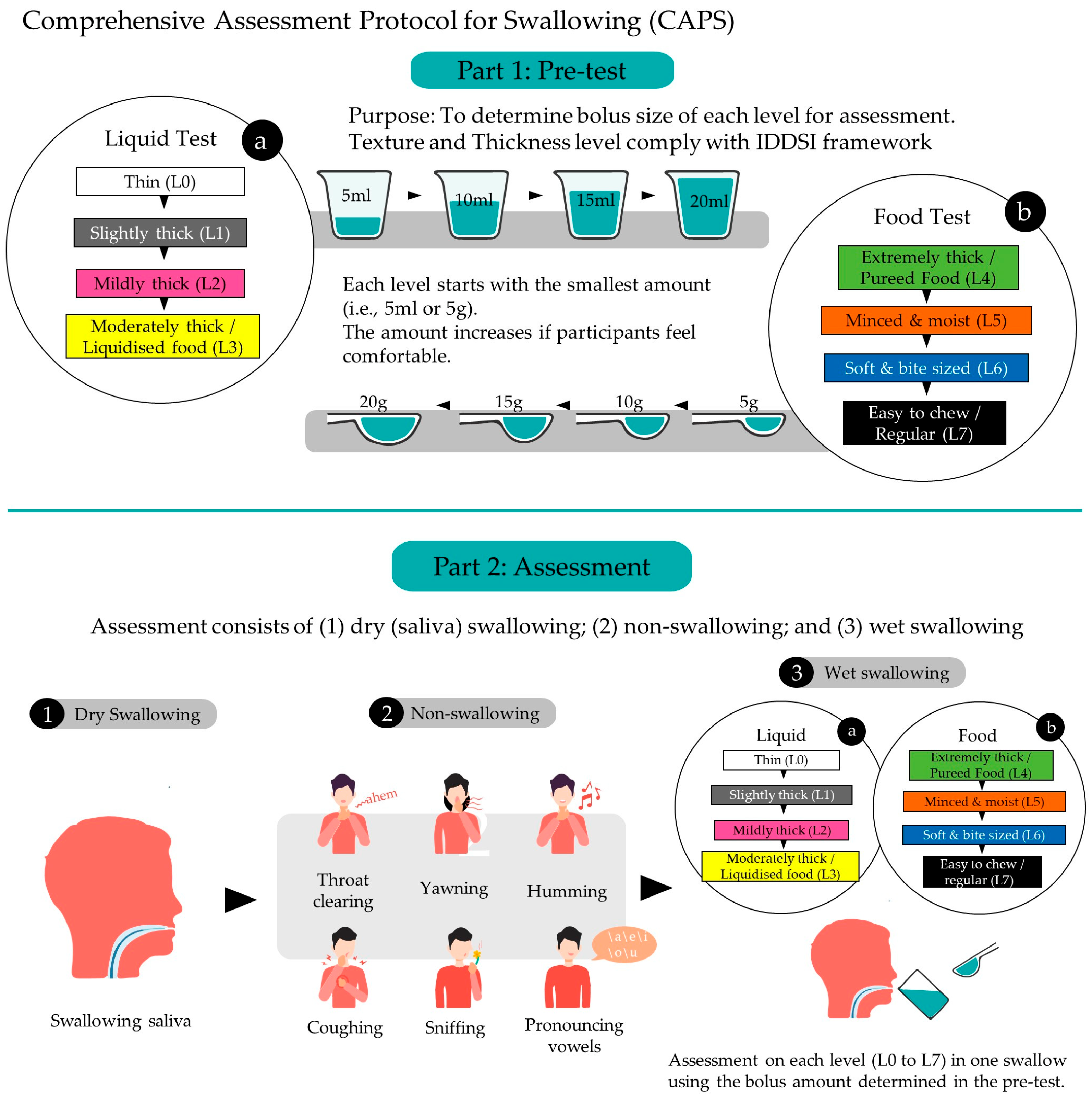

| Intake | IDDSI Level | Step | Food Texture and Drink Thickness | Examples |
|---|---|---|---|---|
| Liquids | Level 0 | 1 | Thin drink | Water, milk, tea |
| Level 1 | 2 | Slightly thick drink | Anti-regurgitation (AR) infant formulas | |
| Level 2 | 3 | Mildly thick drink | Milkshakes, thick shakes | |
| Level 3 | 4 | Moderately thick drink or liquidized food | Smooth yoghurt, fruit juice, liquidized poultry, fish, or vegetables | |
| Foods/Transitional Foods | Level 4 | 5 | Extremely thick drink or pureed food | Pureed mince and gravy, smooth mashed potatoes, pureed vegetables, thick cream |
| Level 5 | 6 | Minced and moist food | Mashed fruit or vegetables, scrambled egg, milk pudding | |
| Level 6 | 7 | Soft and bite-sized food | Steamed or boiled vegetables, cooked fish, tender meat less than 1.5 cm × 1.5 cm | |
| Level 7 | 8 | Easy to chew or regular food | Steamed or boiled vegetables, cooked fish, tender meat with no specific size requirements |
| Step | Maneuvers | Instructions |
|---|---|---|
| 1 | Throat clearing | Participants should make the sound “ahem” by inhaling slightly and then exhaling more forcibly. |
| 2 | Yawning | Participants should open their jaw widely, take in a deep breath, and then quickly exhale. |
| 3 | Sniffing | Participants should draw air into their nose in short breaths. |
| 4 | Coughing | Participants should take a deep breath, hold it for 2–3 s, and use their stomach muscles to forcefully expel the air. |
| 5 | Humming | Participants should hold their lips together and sing the alphabet song. |
| 6 | Pronouncing vowels | Pronouncing vowels: participants should slowly read out the vowels “\a\”, “\e\”, “\i\”, “\o\”, “\u\” and sustain for 3 s. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, H.-J.; Lai, D.K.-H.; So, B.P.-H.; Yip, C.C.-K.; Cheung, D.S.K.; Cheung, J.C.-W.; Wong, D.W.-C. A Comprehensive Assessment Protocol for Swallowing (CAPS): Paving the Way towards Computer-Aided Dysphagia Screening. Int. J. Environ. Res. Public Health 2023, 20, 2998. https://doi.org/10.3390/ijerph20042998
Lim H-J, Lai DK-H, So BP-H, Yip CC-K, Cheung DSK, Cheung JC-W, Wong DW-C. A Comprehensive Assessment Protocol for Swallowing (CAPS): Paving the Way towards Computer-Aided Dysphagia Screening. International Journal of Environmental Research and Public Health. 2023; 20(4):2998. https://doi.org/10.3390/ijerph20042998
Chicago/Turabian StyleLim, Hyo-Jung, Derek Ka-Hei Lai, Bryan Pak-Hei So, Calvin Chi-Kong Yip, Daphne Sze Ki Cheung, James Chung-Wai Cheung, and Duo Wai-Chi Wong. 2023. "A Comprehensive Assessment Protocol for Swallowing (CAPS): Paving the Way towards Computer-Aided Dysphagia Screening" International Journal of Environmental Research and Public Health 20, no. 4: 2998. https://doi.org/10.3390/ijerph20042998
APA StyleLim, H.-J., Lai, D. K.-H., So, B. P.-H., Yip, C. C.-K., Cheung, D. S. K., Cheung, J. C.-W., & Wong, D. W.-C. (2023). A Comprehensive Assessment Protocol for Swallowing (CAPS): Paving the Way towards Computer-Aided Dysphagia Screening. International Journal of Environmental Research and Public Health, 20(4), 2998. https://doi.org/10.3390/ijerph20042998








