Facile Phase Control and Photocatalytic Performance of BiVO4 Crystals for Methylene Blue Degradation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Preparation Methods
2.3. Characterizations
2.4. Photocatalytic Activity Tests
3. Results and Discussion
3.1. X-ray Diffraction (XRD)
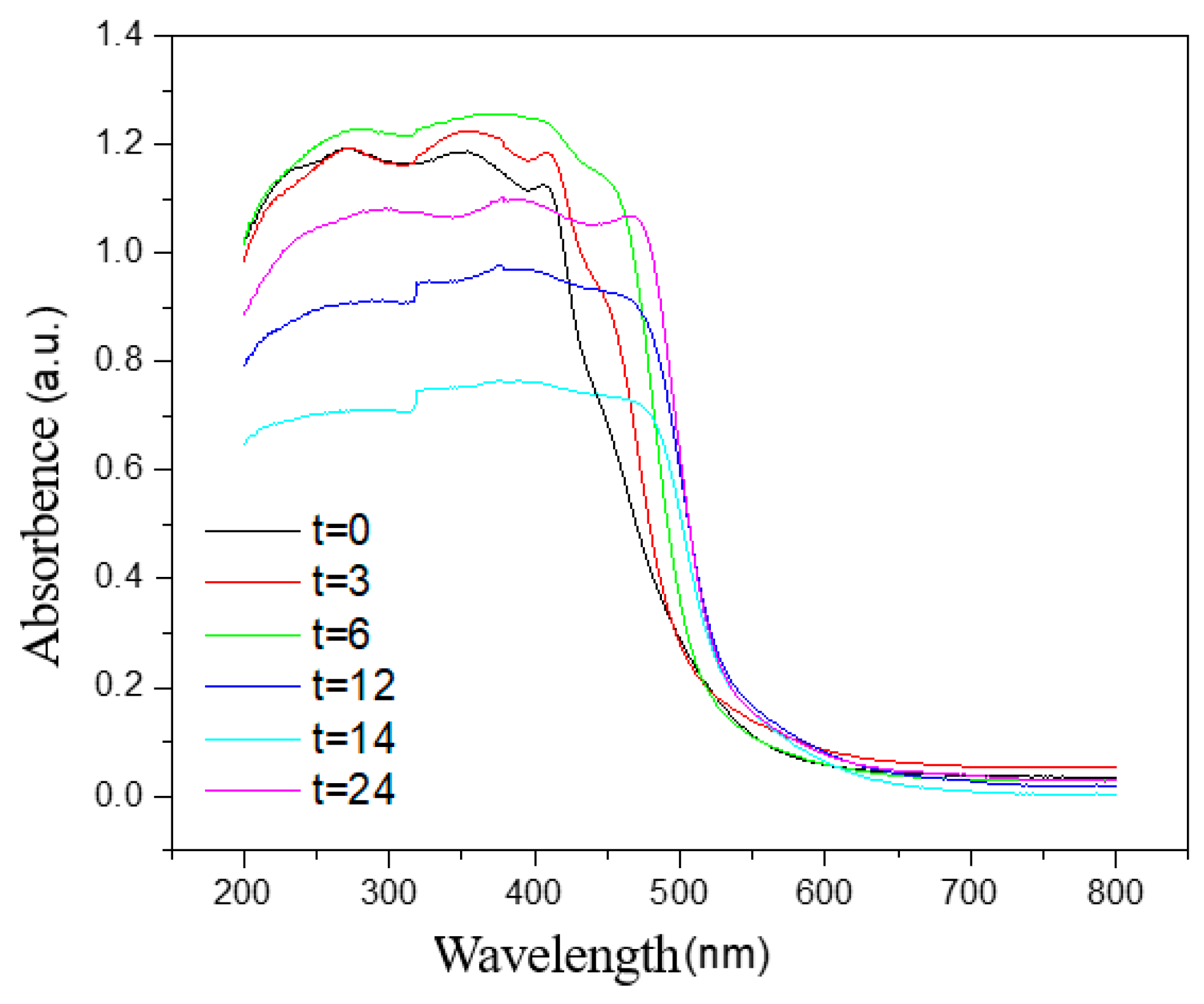
3.2. UV-Vis Test Analysis
3.3. SEM Test Analysis
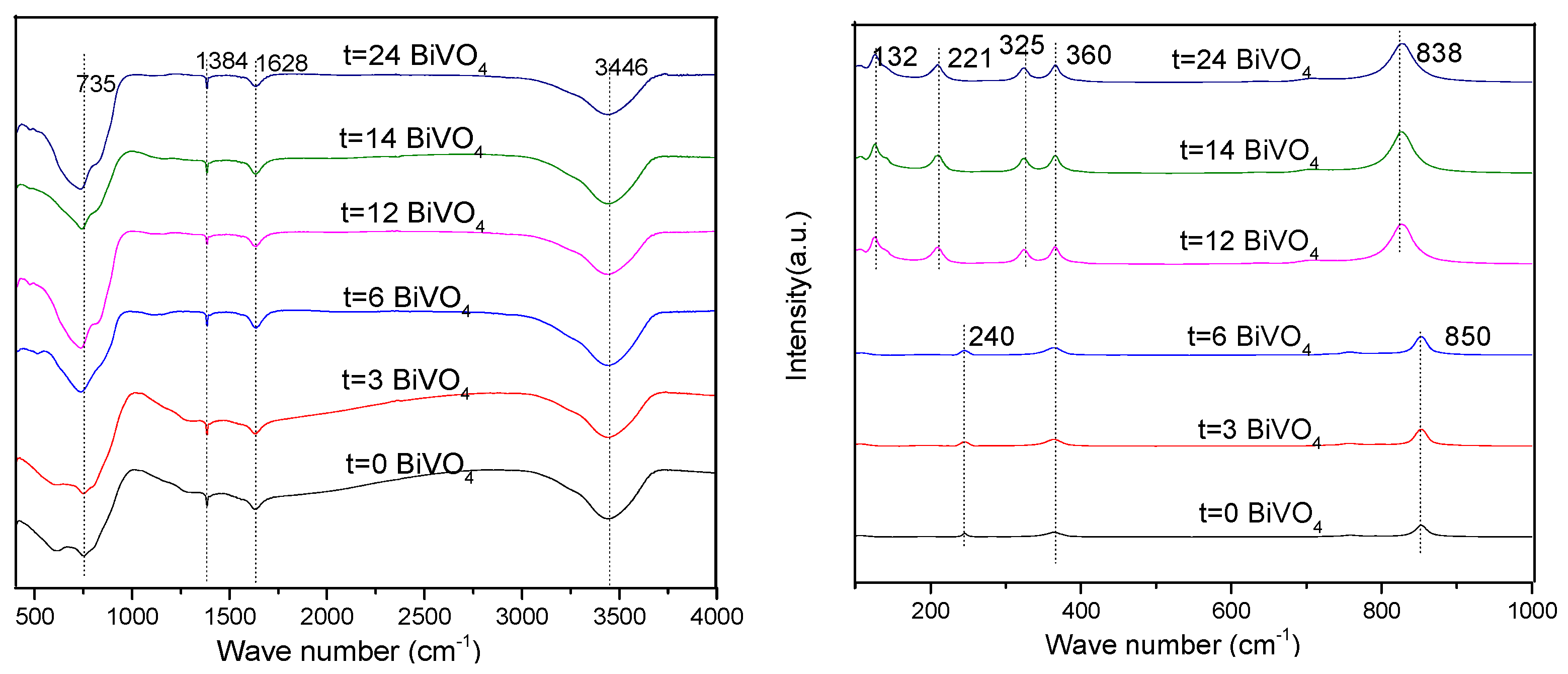
3.4. FT-IR and Raman Analyses
3.5. BiVO4 Generation Mechanism
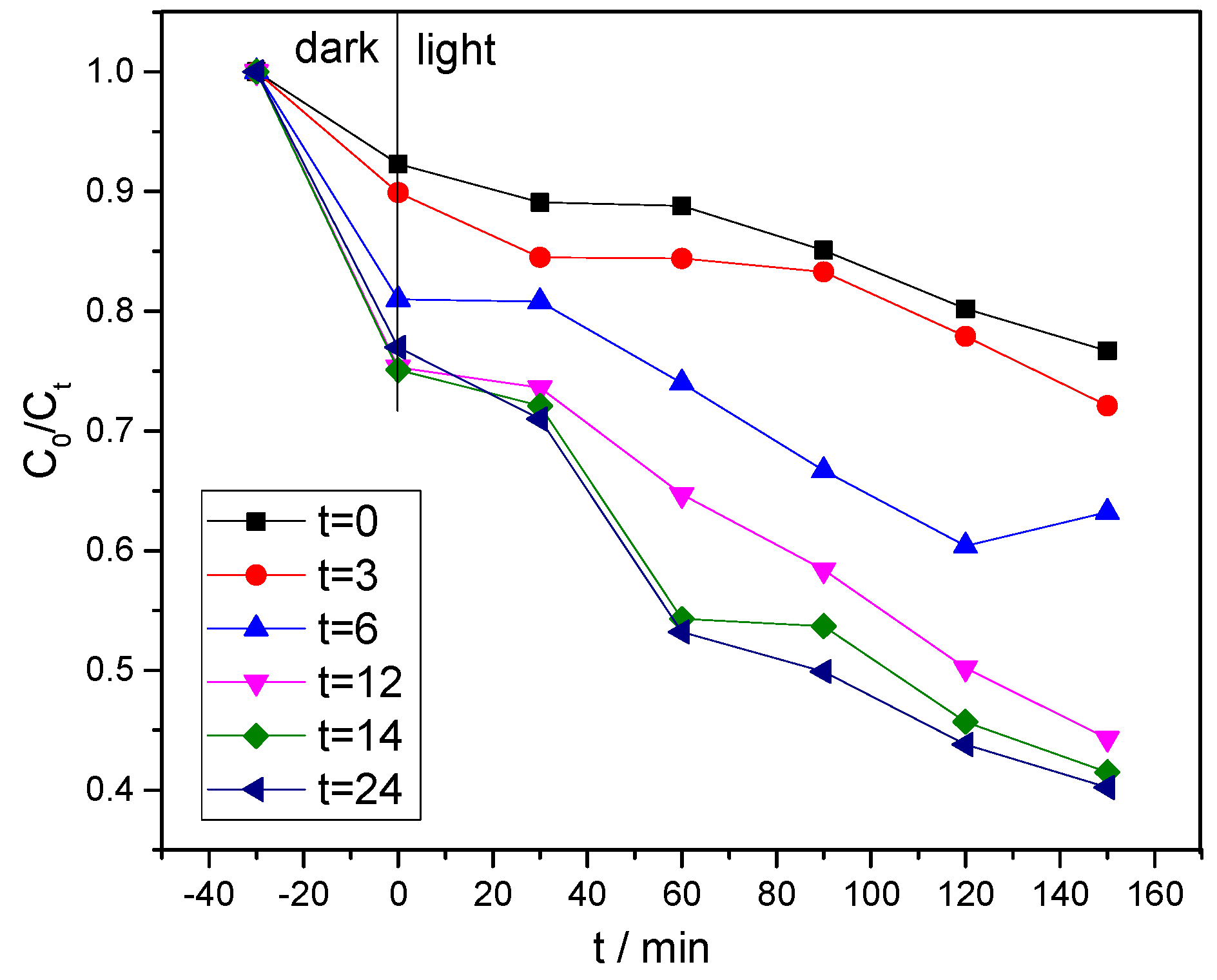
3.6. Photocatalytic Performance Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yurong, G.; Fang, Z.; Zwieten, L.V.; Bolan, N.; Dong, D.; Quin, B.F.; Meng, J.; Li, F.; Wu, F.; Wang, H.; et al. A critical review of biochar-based nitrogen fertilizers and their effects on crop production and the environment. Biochar 2022, 4, 36. [Google Scholar]
- Deng, F.; Shi, H.; Guo, Y.; Luo, X.; Zhou, J. Engineering paths of sustainable and green photocatalytic degradation technology for pharmaceuticals and organic contaminants of emerging concern. Curr. Opin. Green Sustain. Chem. 2021, 29, 100465. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, B.; Chen, H.; Yuan, R. Heterogeneous photocatalytic oxidation for the removal of organophosphorus pollutants from aqueous solutions: A review. Sci. Total Environ. 2023, 856, 159048. [Google Scholar] [CrossRef] [PubMed]
- Weng, B.; Lu, K.Q.; Tang, Z.; Chen, H.M.; Xu, Y.J. Stabilizing ultrasmall Au clusters for enhanced photoredox catalysis. Nat. Commun. 2018, 9, 1543. [Google Scholar] [CrossRef] [PubMed]
- Zahmatkesh, S.; Bokhari, A.; Karimian, M.; Zahra, M.M.A.; Sillanpaa, M.; Panchal, H.; Alrubaie, A.J.; Rezakhani, Y. A comprehensive review of various approaches for treatment of tertiary wastewater with emerging contaminants: What do we know? Environ. Monit. Assess. 2022, 194, 884. [Google Scholar] [CrossRef]
- Tian, C.; Lv, J.; Zhang, W.; Wang, H.; Chao, J.; Chai, L.; Lin, Z. Accelerated Degradation of Microplastics at the Liquid Interface of Ice Crystals in Frozen Aqueous Solutions. Angew. Chem. Int. Ed. 2022, 61, e202206947. [Google Scholar] [CrossRef]
- Fang, Z.; Gao, Y.; Bolan, N.; Shaheen, S.M.; Xu, S.; Wu, X.; Xu, X.; Hu, H.; Lin, J.; Zhang, F.; et al. Conversion of biological solid waste to graphene-containing biochar for water remediation: A critical review. Chem. Eng. J. 2020, 390, 124611–124626. [Google Scholar] [CrossRef]
- Long, Z.; Song, H.; Zhang, G.; Gao, J.; Zhu, J. Fabrication of Bi-Bi3O4Cl plasmon photocatalysts for removal of aqueous emerging contaminants under visible light. J. Environ. Sci. 2022, 118, 87–100. [Google Scholar] [CrossRef]
- Fang, Z.; Gao, Y.; Zhang, F.; Zhu, K.; Shen, Z.; Liang, H.; Xie, Y.; Yu, C.; Bao, Y.; Feng, B.; et al. The adsorption mechanisms of oriental plane tree biochar toward bisphenol S: A combined thermodynamic evidence, spectroscopic analysis and theoretical calculations. Environ. Pollut. 2022, 310, 119819. [Google Scholar] [CrossRef]
- Gao, Y.; Fang, Z.; Lin, W.; Chen, H.; Bhatnagar, A.; Li, J.; Xie, Y.; Bao, Y.; Chen, J.; Zhao, H.; et al. Large-flake graphene-modified biochar for the removal of bisphenol S from water: Rapid oxygen escape mechanism for synthesis and improved adsorption performance. Environ. Pollut. 2023, 317, 120847. [Google Scholar] [CrossRef]
- Qin, N.; Zhang, S.; He, J.; Long, F.; Wang, L. In situ synthesis of BiVO4/BiOBr microsphere heterojunction with enhanced photocatalytic performance. J. Alloys Compd. 2022, 927, 166661. [Google Scholar] [CrossRef]
- Malathi, A.; Madhavan, J.; Ashokkumar, M.; Arunachalam, P. A review on BiVO4 photocatalyst: Activity enhancement methods for solar photocatalytic applications. Appl. Catal. A Gen. 2018, 555, 47–74. [Google Scholar] [CrossRef]
- Biswas, M.R.U.D.; Oh, W.-C. Synthesis of BiVO4-GO-PVDF nanocomposite: An excellent, newly designed material for high photocatalytic activity towards organic dye degradation by tuning band gap energies. Solid State Sci. 2018, 80, 22–30. [Google Scholar] [CrossRef]
- Ullah, H.; Tahir, A.A.; Mallick, T.K. Structural and electronic properties of oxygen defective and Se-doped p-type BiVO4(001) thin film for the applications of photocatalysis. Appl. Catal. B Environ. 2018, 224, 895–903. [Google Scholar] [CrossRef]
- Kshetri, Y.K.; Chaudhary, B.; Kamiyama, T.; Kim, T.-H.; Rosei, F.; Lee, S.W. Determination of ferroelastic phase transition temperature in BiVO4 by Raman spectroscopy. Mater. Lett. 2021, 291, 129519. [Google Scholar] [CrossRef]
- Samran, B.; Lunput, S.; Tonnonchiang, S.; Chaiwichian, S. BiFeO3/BiVO4 nanocomposite photocatalysts with highly enhanced photocatalytic activity for rhodamine B degradation under visible light irradiation. Phys. B Condens. Matter 2019, 561, 23–28. [Google Scholar] [CrossRef]
- Lv, C.; Chen, G.; Sun, J.; Zhou, Y.; Fan, S.; Zhang, C. Realizing nanosized interfacial contact via constructing BiVO4/Bi4V2O11 element-copied heterojunction nanofibres for superior photocatalytic properties. Appl. Catal. B Environ. 2015, 179, 54–60. [Google Scholar] [CrossRef]
- Samsudin, M.F.R.; Bashiri, R.; Mohamed, N.M.; Ng, Y.H.; Sufian, S. Tailoring the morphological structure of BiVO4 photocatalyst for enhanced photoelectrochemical solar hydrogen production from natural lake water. Appl. Surf. Sci. 2020, 504, 144417. [Google Scholar] [CrossRef]
- Guo, Z.; Wei, J.; Zhang, B.; Ruan, M.; Liu, Z. Construction and photoelectrocatalytic performance of TiO2/BiVO4 heterojunction modified with cobalt phosphate. J. Alloys Compd. 2020, 821, 153225. [Google Scholar] [CrossRef]
- Regmi, C.; Kshetri, Y.K.; Ray, S.K.; Pandey, R.P.; Lee, S.W. Utilization of visible to NIR light energy by Yb+3, Er+3 and Tm+3 doped BiVO4 for the photocatalytic degradation of methylene blue. Appl. Surf. Sci. 2017, 392, 61–70. [Google Scholar] [CrossRef]
- Tailor, N.K.; Abdi-Jalebi, M.; Gupta, V.; Hu, H.; Dar, M.I.; Li, G.; Satapathi, S. Recent progress in morphology optimization in perovskite solar cell. J. Mater. Chem. A 2020, 8, 21356–21386. [Google Scholar] [CrossRef]
- Wang, M.; Lu, W.; Chen, D.; Liu, J.; Hu, B.; Jin, L.; Lin, Y.; Yue, D.; Huang, J.; Wang, Z. Synthesis of dendritic-like BiVO4:Ag heterostructure for enhanced and fast photocatalytic degradation of RhB solution. Mater. Res. Bull. 2016, 84, 414–421. [Google Scholar] [CrossRef]
- Reli, M.; Troppová, I.; Šihor, M.; Pavlovský, J.; Praus, P.; Kočí, K. Photocatalytic decomposition of N2O over g-C3N4/BiVO4 composite. Appl. Surf. Sci. 2019, 469, 181–191. [Google Scholar] [CrossRef]
- Yu, X.; Wang, Z.; Li, E.; Li, X.; Cui, M.; Guo, C. Preparation of Different BiVO4 Catalysts and Their Photocatalytic Performance in the Coupling Reaction Between Alcohols and Amines. Catal. Lett. 2022, 152, 1244–1255. [Google Scholar] [CrossRef]
- Kshetri, Y.K.; Regmi, C.; Chaudhary, B.; Kim, H.-S.; Kim, T.-H.; Rosei, F.; Lee, S.W. BiVO4 ceramics for high-sensitivity and high-temperature optical thermometry. J. Lumin. 2021, 230, 117739. [Google Scholar] [CrossRef]
- Houas, A.; Lachheb, H.; Ksibi, M.; Elaloui, E.; Guillard, C.; Herrmann, J.-M. Photocatalytic degradation pathway of methylene blue in water. Appl. Catal. B Environ. 2001, 31, 145–157. [Google Scholar] [CrossRef]
- Yan, M.; Yan, Y.; Wu, Y.; Shi, W.; Hua, Y. Microwave-assisted synthesis of monoclinic–tetragonal BiVO4 heterojunctions with enhanced visible-light-driven photocatalytic degradation of tetracycline. RSC Adv. 2015, 5, 90255–90264. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, Y.; Ma, F.; Zhang, Z.; Wei, X. Influences of vacancies on the structural, electronic and optical properties of monoclinic BiVO4. J. Phys. Chem. Solids 2018, 121, 85–92. [Google Scholar] [CrossRef]
- Teimori, H.; Balouchi, H.; Moradi, A.; Soltani, E. Quantifying seed germination response of deteriorated Trigonella foenum-graecum L. seed to temperatures and water potentials: Thermal time, hydrotime and hydrothermal time models. J. Appl. Res. Med. Aromat. Plants 2021, 20, 100276. [Google Scholar] [CrossRef]
- Sun, Z.; Yu, Z.; Liu, Y.; Shi, C.; Zhu, M.; Wang, A. Construction of 2D/2D BiVO4/g-C3N4 nanosheet heterostructures with improved photocatalytic activity. J. Colloid Interface Sci. 2019, 533, 251–258. [Google Scholar] [CrossRef]
- Suwanchawalit, C.; Buddee, S.; Wongnawa, S. Triton X-100 induced cuboid-like BiVO4 microsphere with high photocatalytic performance. J. Environ. Sci. 2017, 55, 257–265. [Google Scholar] [CrossRef]
- Kadam, A.N.; Babu, B.; Lee, S.-W.; Kim, J.; Yoo, K. Morphological guided sphere to dendrite BiVO4 for highly efficient organic pollutant removal and photoelectrochemical performance under solar light. Chemosphere 2022, 305, 135461. [Google Scholar] [CrossRef]
- Obregón, S.; Caballero, A.; Colón, G. Hydrothermal synthesis of BiVO4: Structural and morphological influence on the photocatalytic activity. Appl. Catal. B-Environ. 2012, 117–118, 59–66. [Google Scholar] [CrossRef]
- Ying, Y.; Tao, F.; Hong, T.; Wang, L. Controlled fabrication of bismuth vanadium oxide hierarchical microtubes with enhanced visible light photocatalytic activity. Mater. Sci. Semicond. Process. 2015, 32, 82–89. [Google Scholar] [CrossRef]
- Lu, Y.; Luo, Y.-S.; Xiao, H.-M.; Fu, S.-Y. Novel core–shell structured BiVO4 hollow spheres with an ultra-high surface area as visible-light-driven catalyst. CrystEngComm 2014, 16, 6059–6065. [Google Scholar] [CrossRef]

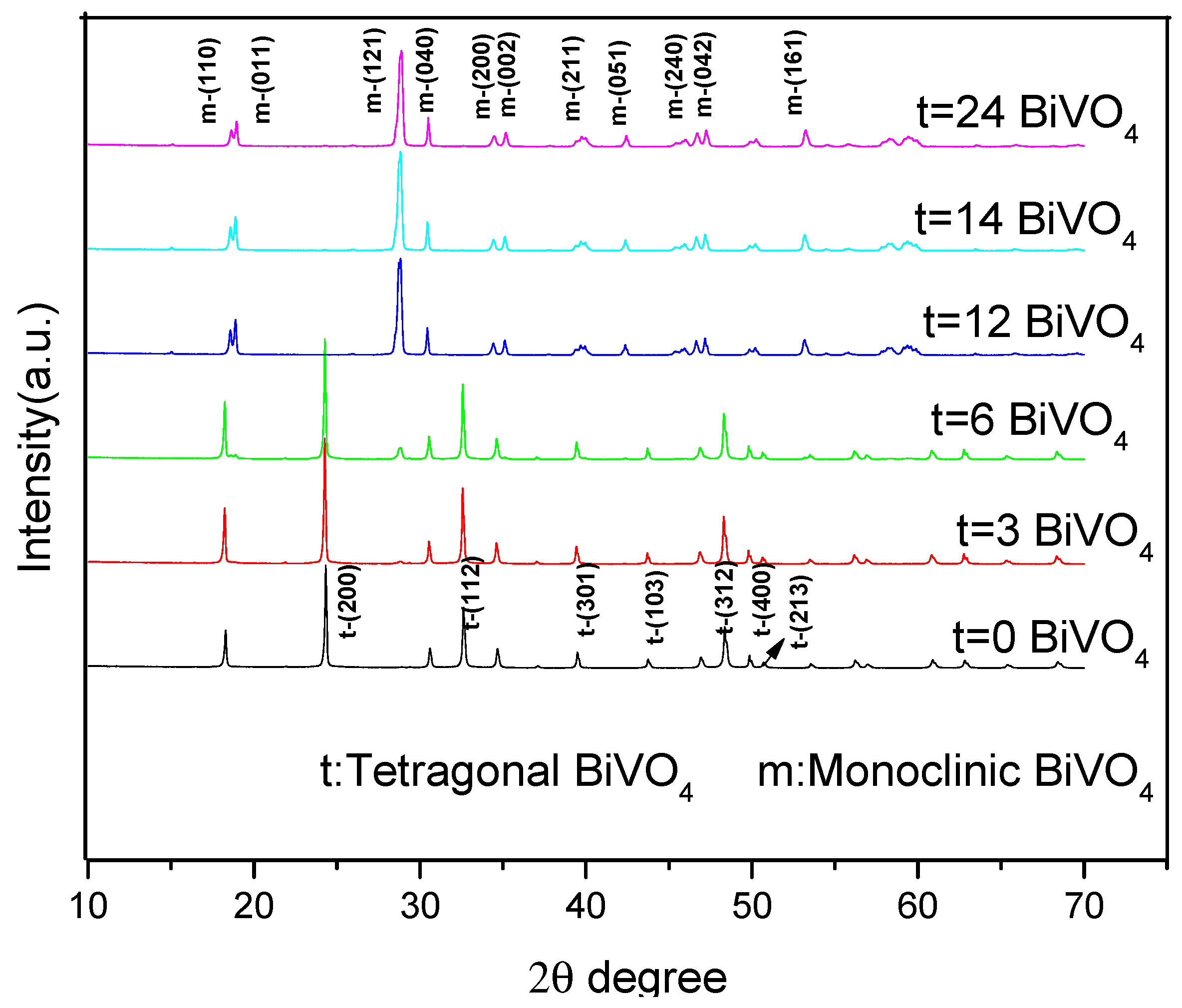



| Sample | Time | Crystal Structure | Diffraction Intensity/CPS | Relative Intensity Ratio | |||||
|---|---|---|---|---|---|---|---|---|---|
| m-(121) | m-(040) | t-(200) | t-(112) | (121)/011 | (040)/(011) | (040)/(121) | |||
| BiVO4 | 0 h | t + m | -- | 5909 | 29,744 | 17,675 | -- | 0.537 | -- |
| 3 h | t + m | -- | 6663 | 36,229 | 21,987 | -- | 0.410 | -- | |
| 6 h | t + m | 3610 | 6848 | 34,808 | 21,794 | 0.215 | 0.408 | 1.897 | |
| 12 h | m | 27,973 | 7297 | -- | -- | 3.758 | 0.942 | 0.261 | |
| 14 h | m | 28,627 | 8476 | -- | -- | 2.884 | 0.854 | 0.296 | |
| 24 h | m | 27,740 | 8516 | -- | -- | 3.754 | 1.152 | 0.307 | |
| Hydrothermal Time/h | Crystal Phase | Morphology | Specific Surface Areas m2/g | Reasons for Changes in Specific Surface Area |
|---|---|---|---|---|
| 0 | Tetragonal | Smooth spheroid | 2.5952 ± 0.1498 | Surface differentiation |
| 3 | Tetragonal | Relatively smooth spheroid | 2.6115 ± 0.1642 | |
| 6 | Tetragonal + minor monoclinic | Surface-differentiated polyhedron | 2.7727 ± 0.1581 | |
| 12 | Monoclinic | Clustered flower shape | 2.9271 ± 0.1085 | |
| 14 | Monoclinic | Clustered flower shape | 3.0971 ± 0.1525 | |
| 24 | Monoclinic | Polyhedron agglomeration | 2.0352 ± 0.1276 | Crystal agglomeration |
| Materials | Photocatalytic Parameters | Degradation Efficiency | Refs. |
|---|---|---|---|
| BiVO4 peanut, 1.0 mg/mL | MB, 10 mg/L, Xenon lamp with 35 W/m2 | 40% in 120 min | [33] |
| BiVO4 microtube, 1.0 mg/mL | MO, 20 mg/L, 250-W Xenon arc lamp | 95% in 180 min | [34] |
| BiVO4 spheres, 0.5 mg/mL | RhB, 10−5 mol/L, 500 W Xenon lamp | 27% in 150 min | [35] |
| BiVO4 biscuits, 0.5 mg/mL | RhB, 10−5 mol/L, 500 W Xenon lamp | 44% in 150 min | [35] |
| Tetragonal BiVO4, 1.0 mg/mL | MB, 20 mg/L, 550 W Xenon lamp | 20% in 150 min | This study |
| Monoclinic BiVO4, 1.0 mg/mL | MB, 20 mg/L, 550 W Xenon lamp | 45% in 150 min | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, H.; Cheng, L.; Chen, H.; Dou, R.; Chen, J.; Zhao, Y.; Li, F.; Fang, Z. Facile Phase Control and Photocatalytic Performance of BiVO4 Crystals for Methylene Blue Degradation. Int. J. Environ. Res. Public Health 2023, 20, 3093. https://doi.org/10.3390/ijerph20043093
Cai H, Cheng L, Chen H, Dou R, Chen J, Zhao Y, Li F, Fang Z. Facile Phase Control and Photocatalytic Performance of BiVO4 Crystals for Methylene Blue Degradation. International Journal of Environmental Research and Public Health. 2023; 20(4):3093. https://doi.org/10.3390/ijerph20043093
Chicago/Turabian StyleCai, Heshan, Linmei Cheng, Huacong Chen, Rongni Dou, Junfeng Chen, Yuxin Zhao, Fuhua Li, and Zheng Fang. 2023. "Facile Phase Control and Photocatalytic Performance of BiVO4 Crystals for Methylene Blue Degradation" International Journal of Environmental Research and Public Health 20, no. 4: 3093. https://doi.org/10.3390/ijerph20043093
APA StyleCai, H., Cheng, L., Chen, H., Dou, R., Chen, J., Zhao, Y., Li, F., & Fang, Z. (2023). Facile Phase Control and Photocatalytic Performance of BiVO4 Crystals for Methylene Blue Degradation. International Journal of Environmental Research and Public Health, 20(4), 3093. https://doi.org/10.3390/ijerph20043093









