Abstract
Rates of non-alcoholic fatty liver disease (NAFLD) vary dramatically among Hispanic subpopulations, with Mexican-origin (MO) Hispanics experiencing a disproportionate burden. This study examined dietary fatty acid (FA) intake among overweight and obese MO Hispanic adults in the United States (US) and evaluated its association with liver steatosis and fibrosis. Participants (N = 285, MO Hispanic adults) completed 24-h dietary recalls to assess dietary FA exposure. Liver steatosis and fibrosis were estimated using transient elastography (FibroScan®). Multiple regression analysis tested relationships between FA intakes and liver steatosis or fibrosis, adjusting for age, sex, body mass index (BMI) and total energy. A total of 51% (n = 145) of participants were suspected to have NAFLD and 20% self-reported a type 2 diabetes diagnosis. No significant association was observed between Linoleic Acid and α-Linolenic Acid (LA:ALA) ratio, or omega-6 to omega-3 (n-6:n-3) ratio and liver steatosis. However, a one-point increase in the LA:ALA ratio resulted in a 1.01% increase in the liver fibrosis scores (95% CI: [1.00, 1.03]; p = 0.03), and a one-point increase in the n-6:n-3 ratio resulted in a 1.02% increase in liver fibrosis score (95% CI: [1.01, 1.03]; p = 0.01). Further research is needed to determine if modulation of FA intake could reduce NAFLD risk in this high-risk population.
1. Introduction
Hispanics of Mexican origin (MO) are the largest subpopulation in the United States (U.S.) and account for nearly two-thirds of the Hispanic population [1]. MO Hispanics have among the highest rates of metabolic conditions that are strongly associated with non-alcoholic fatty liver disease (NAFLD) including obesity and type 2 diabetes [2]. In fact, rates of NAFLD vary dramatically among Hispanic subpopulations, with MO Hispanics experiencing a disproportionate (33%) burden compared with Hispanics of Dominican (16%) or Puerto Rican origin (18%) [3]. Nearly 30% of individuals with NAFLD are at increased risk of developing cirrhosis, portal hypertension and hepatocellular carcinoma (HCC). HCC is projected to become the leading cause of liver-related morbidity and mortality in the U.S. [4]. Lifestyle changes, including improvements in diet quality and increased physical activity, are prevention and treatment strategies recommended for NAFLD [5].
Nutritional components hypothesized to impact NAFLD development include an imbalance in the consumption of omega-6 (n-6) relative to omega-3 (n-3) polyunsaturated fatty acids (PUFAs) that resulted from a marked increase in n-6 PUFA containing cooking oils and other processed foods in the past century [6,7,8]. Ratios of dietary n-6 to n-3 have been reported to be ~10:1 in most western populations; this is significantly higher than the 1:1 to 4:1 ratios reported to have health benefits [9,10]. This dietary shift has been observed in MO Hispanics, largely as a result of immigration and increasing acculturation to westernized diets of the U.S. [11,12]. Specifically, moving away from a traditional Mexican dietary pattern, shown to improve metabolic health [13,14,15,16], to a western dietary pattern associated with increased NAFLD risk and other obesity-related diseases, has been proposed to have devastating health effects and contribute to health disparities observed among MO Hispanics [17,18].
The vast majority of ingested PUFAs are n-6 and n-3 18-carbon (18C-) PUFAs such as linoleic acid (LA; 18:2, n-6) and α-linolenic acid (ALA, 18:3, n-3). These in turn compete in a biosynthetic pathway during conversion to biologically active highly unsaturated fatty acids (HUFAs) such as arachidonic acid (ARA; 20:4, n-6), eicosapentaenoic acid (EPA; 20:5, n-3) and docosahexaenoic acid (DHA; 22:6, n-3). ARA and its oxylipin metabolites including eicosanoids such as prostaglandins, thromboxanes, and leukotrienes, typically have pro-inflammatory and pro-coagulant properties, while n-3 HUFAs (EPA and DHA) and their metabolites have anti-inflammatory and pro-resolving activities [19,20]. Consequently, the levels and ratios of dietary 18C-PUFAs that enter this pathway dictate the balance of n-6 to n-3 HUFAs that are synthesized, and the dramatic shifts in 18C-PUFA ratios discussed above have great capacity to alter the balance of n-6 and n-3 HUFAs and their metabolites. This imbalance may be further exacerbated by the fact that MO Hispanics have high frequencies of ancestral fatty acid desaturase (FADS) variants that potentially limit their capacity to produce HUFAs and particularly n-3 HUFAs [21]. This gene by diet interaction may be linked to an observed depletion in n-3 HUFA levels observed among patients with NAFLD [22,23]. Given these genetic and dietary factors, the MO population may benefit from dietary interventions that focus on n-3 HUFA consumption or supplementation to reduce the imbalance of n-6 and n-3 HUFAs and their metabolites and thus prevent NAFLD progression.
To date, little information is known about the current intake of fatty acids (FAs) among MO Hispanics, despite the elevated risks for NAFLD and adverse HCC outcomes. The objective of the present study was to describe the intake of dietary fat, saturated FAs, monounsaturated FAs and n-6 and n-3 PUFAs among MO Hispanics, and to evaluate potential associations of these fats with liver steatosis and fibrosis in this population. This information addresses a vital gap in in the literature that will serve to inform the design and development of future dietary interventions to address the NAFLD burden in MO Hispanics.
2. Methods
2.1. Participants and Procedures
The study sample for this analysis was derived from a previous observational cross-sectional study of MO men and women in Tucson, AZ [24]. Participants were eligible to participate in this study if they self-identified as being of Mexican origin and were between the ages of 18 and 64. Additionally, given excess body weight (BMI) is a risk factor for NAFLD and the primary study targeted high-risk individuals only, eligibility criteria were limited to those with BMI ≥ 25 kg/m2. Participants were asked to self-report any comorbid conditions, including type 2 diabetes, that were required to be under control at the time of the study. Participants with excessive alcohol intake, as defined as more than 21 standard drinks per week for men and 14 drinks per week for women, were not eligible to participate in the study [5]. A total of 285 (n = 103 men, 182 women) participants were successfully recruited and completed the study. Participants attended a single in-person visit to a specialized liver clinic where data were collected using standardized protocols. Participants were given a variety of study questionnaires to complete in English or Spanish based on their language of preference. The study protocol was approved by the Institutional Review Board (IRB) (IRB #1902380787) and all participants provided written informed consent prior to enrolling in the study.
2.2. Liver Assessment and NAFLD
Liver steatosis and fibrosis were measured by a trained physician or technician using transient elastography (TE) (FibroScan®). TE measures liver fat infiltration (steatosis) as controlled attenuation parameter (CAP) scores in units of dB/m and liver stiffness (a validated proxy for fibrosis [25,26]) expressed as kilopascals (kPa). A CAP score cutoff of 288 dB/m, equivalent to 5% hepatic steatosis, was used to determine the presence of suspected NAFLD based on the recommendation from Caussy et al., given its relevancy to our study population [25]. Liver fibrosis measurements can range from 1.5 kPa to 75 kPa, with higher values indicating more severe fibrosis and values of 7.9 kPa or greater indicating significant fibrosis [26]. Cut-off fibrosis severity values were selected based on previous literature as follows; <7.9 kPa (F0-F1), 7.9 to <8.8 kPa (F2), 8.8 to <11.7 kPa (F3), and ≥11.7 kPa (F4) [26]. Participants were asked to fast for at least three hours before their liver ultrasound. Measurements were obtained at a single time point during the participant’s clinical study and a minimum of 10 measurements were taken from each participant using an established CAP interquartile range of <30 dB/m for quality assurance across measurements [25,27].
2.3. Dietary Intake Assessment
Dietary intake was assessed through 24-h dietary recalls using the United States Department of Agriculture multiple-pass method [28]. For each participant, three recalls were conducted over a two-week period taking place on three non-sequential days including two weekdays and one weekend day. Initiation of the dietary recalls was within a week following the participant’s study visit. All 285 participants completed all three separate dietary recalls on different days of the week to account for variability in dietary habits. Participants were provided a food amounts booklet to facilitate reporting of portions and amounts. All 24-h recalls were administered by trained, bilingual and bicultural personnel familiar with the diets of MO Hispanics in the region, via telephone using validated protocols. Dietary data were processed using the Nutrition Data System for Research (NDSR-2019). Primary outcome variables for this analysis included total intake of dietary fat, saturated fat, MUFA, PUFA, and n-6 to n-3 PUFA ratios.
3. Statistical Analysis
For each participant, intake values for each nutrient were computed as the mean of the three individual dietary recalls. Histograms of intakes were investigated and, in some cases, data were log-transformed due to skewedness. Additional values of interest, for example, the LA to ALA ratio and the n-6 to n-3 ratio, were computed from the individual level nutrient intake data. The (possibly transformed) intake values were compared between suspected NAFLD cases and controls using t-tests. Multiple regression was used to examine relationships between nutrient intakes (as % of total) or ratios and liver steatosis or fibrosis scores. Three models were conducted; crude unadjusted (model 1), adjusted for age, sex, BMI, and total energy intake (kcals) as covariates (model 2), and covariates shown to have a significant interaction in a stepwise regression analysis, which included sex and BMI only (model 3). p-values were not adjusted. All statistical computations were performed with R 4.0.
4. Results
4.1. Sample Characteristics
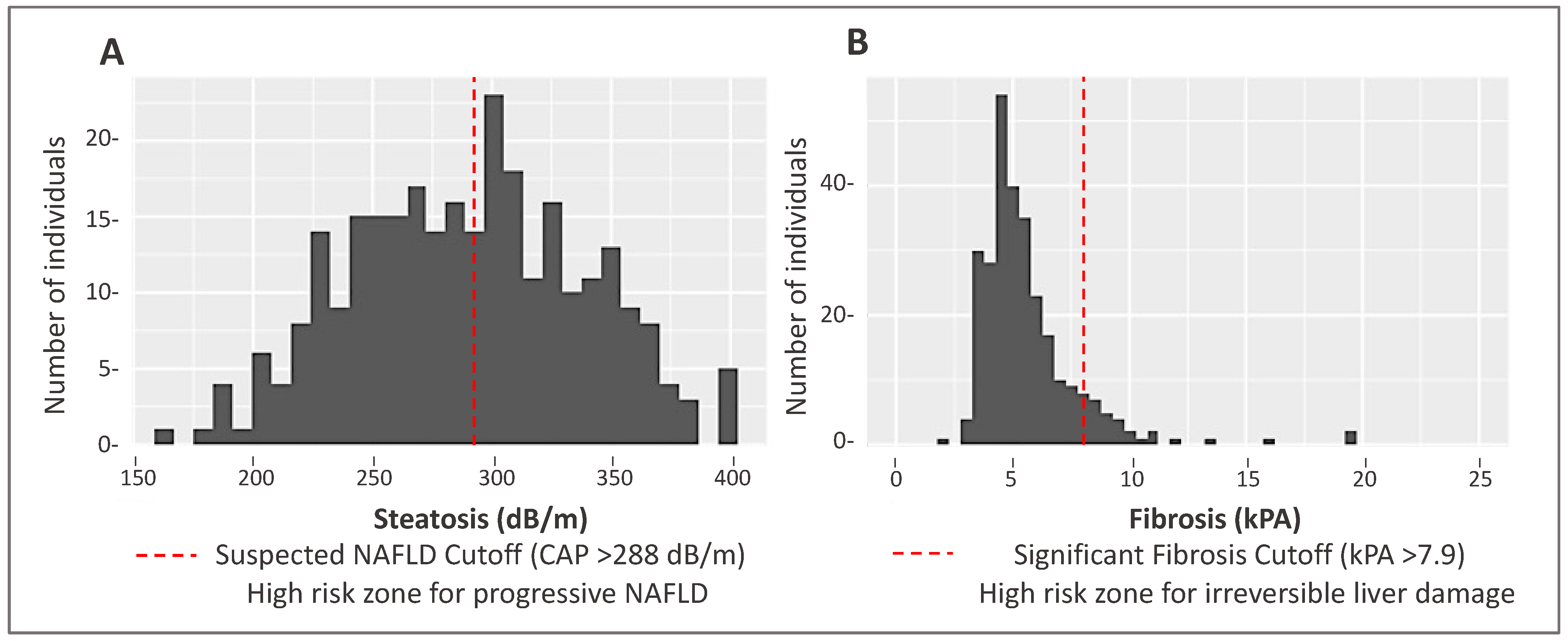
Demographic and clinical data for MO Hispanic participants (n = 285) are presented in Table 1. Participants were on average 45 years of age and 64% were women. In total, 145 participants (51%) had a CAP score ≥ 288 dB/m indicating the suspected presence of NAFLD. Significant differences were observed for several characteristics between non-NAFLD and suspected NAFLD cases. Specifically, suspected NAFLD participants had a higher proportion of individuals with obesity and type 2 diabetes. The distribution of liver steatosis and fibrosis scores are shown in Figure 1.

Table 1.
Demographic and clinical characteristics of a Mexican-origin Hispanic sample from Southern Arizona by suspected NAFLD status (N = 285).
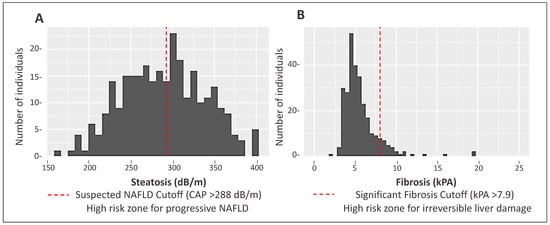
Figure 1.
Distribution of steatosis and fibrosis scores as measured by transient elastography (FibroScan®) in a Mexican-origin Hispanic sample from Southern Arizona (N = 285). (A) Distribution of steatosis CAP scores (Mean: 289.5 dB/m, Standard Deviation [SD]: 48.9 dB/m). (B) Distribution of liver fibrosis values (Mean: 55.67 kPa, SD: 2.83 kPa).
4.2. Dietary Intake
Dietary intake values by suspected NAFLD status are presented in Table 2. Overall mean caloric intake in our study sample was 1492 ± 539 kcal/day. After adjustment for multiple testing, there were no statistically significant differences between groups for total caloric intake (95% CI: −13.8, 237; p = 0.08), and percent calories from carbohydrates (95% CI −0.18, 3.77; p = 0.16). However, statistically significant differences were observed between the groups for total carbohydrate and sugar intake with the suspected NAFLD group consuming 19.2 g (95% CI: [3.01, 35.3]; p = 0.02) and 7.4 g ([−0.89, 15.8]; p = 0.02) lower, respectively, than the non-NAFLD group. Given the alcohol exclusion criteria for the original study, alcohol consumption was overall low with a mean consumption of 1.65 ± 4.9 g, among the suspected NAFLD group consuming approximately half the consumption compared to non-NAFLD participants. Intakes of other nutrients did not appear to vary by NAFLD status.

Table 2.
Dietary intake values by suspected NAFLD Status in a Mexican-origin Hispanic sample from Southern Arizona (N = 285).
4.3. Fat Intake
Overall, approximately 35% of calories came from fat and total fat intake averaged 59.6 g/day; 54.1 g/day for women and 69.4 g/day for men. Approximately 10–15% of daily calories came from saturated fatty acid (SFA), another 10–15% from monounsaturated fatty acid (MUFA), and an additional 5–10% of energy from PUFAs (Table 2). The primary SFA were palmitic acid (C16:0), stearic acid (C18:0), and myristic acid (C14:0). Oleic acid (C18:1, n-9) was the primary MUFA (90% of the total) and the fatty acid with the highest intake overall (~20 g/day).
4.4. High n-6 to n-3 Ratio
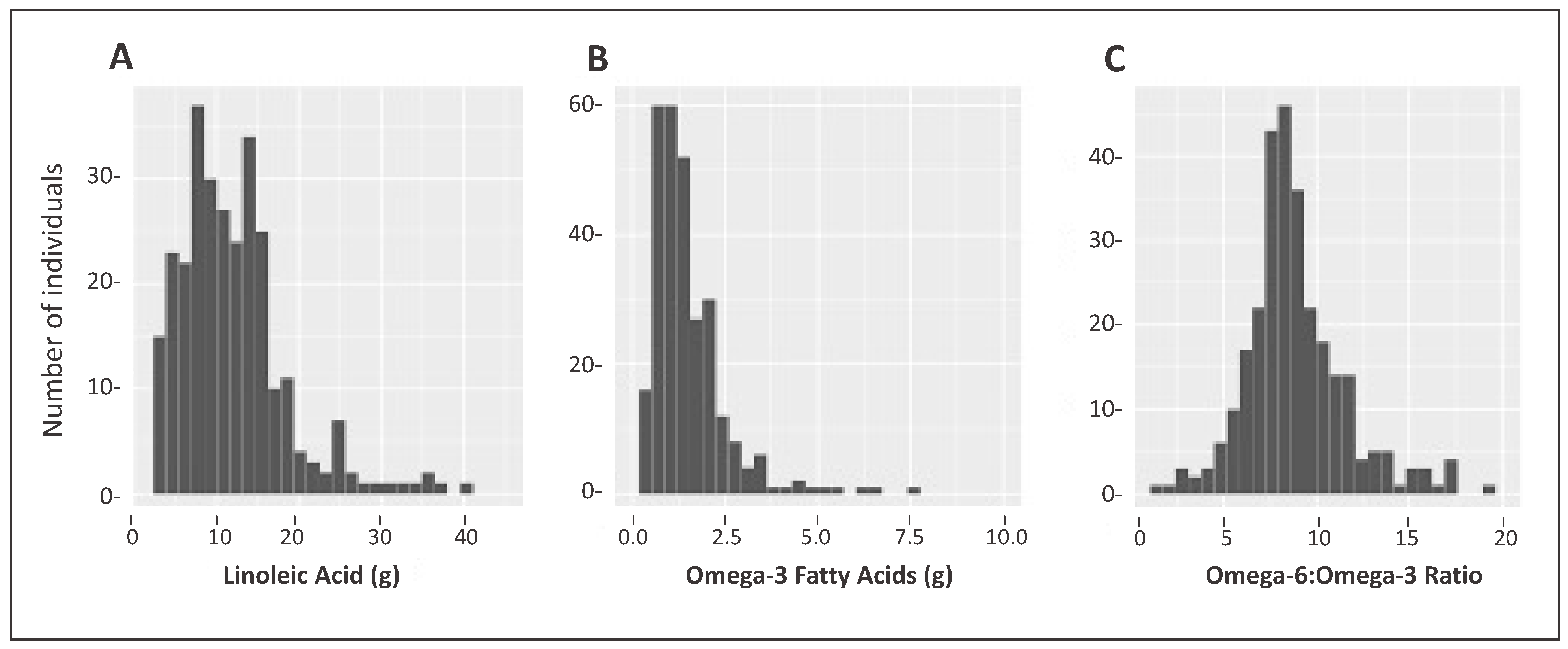
The majority of PUFA intake was n-6 fatty acid LA; C18:2, with a mean intake of 12 ± 6.5 g/day, accounting for 6.9 ± 2.2% of total energy (Table 2). Figure 2A shows the distribution of LA intake. In contrast, relative to n-6, n-3 fatty acid intake was overall very low, with the majority consisting of ALA 1.50 g/day ± 1.01 accounting for 0.82 ± 0.37% of total energy, with low intakes of EPA and DHA (both less than 0.1 g/day on average). The mean n-6 to n-3 PUFA ratio for the non-NAFLD group was 8.95 ± 2.65 compared to 8.71 ± 2.70 (p = 0.44) for suspected-NAFLD cases. The distribution of all n-3s combined is plotted in Figure 2B. These intakes resulted in an average n-6 to n-3 ratio of 8.9 (range: 2.46–19.8), with only nine subjects having ratios less than 5 (Figure 2C).
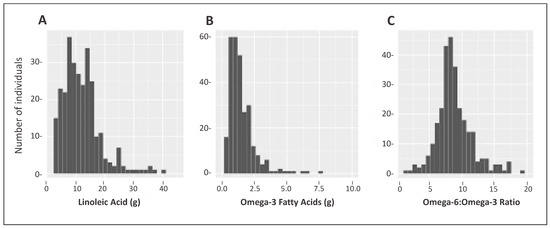
Figure 2.
Intake distribution of linoleic acid (g), omega-3 fatty acids, (g) and Omega-6 to Omega-3 ratio in a Mexican-origin Hispanic sample from Southern Arizona (N = 285). (A) Distribution of Omega-6 (predominantly Linoleic Acid); (B) Distribution of Omega-3 (α-Linolenic Acid, Eicosapentaenoic Acid, Docosapentaenoic Acid, and Docosahexaenoic Acid); (C) Distribution of Omega-6 to Omega-3 ratio. Note the difference in the scales for both axes.
4.5. Fatty Acid Intake and Liver Steatosis and Fibrosis
Interaction analysis showed BMI was strongly associated with both steatosis and fibrosis, explaining 17.6% (p < 0.001) and 11.7% (p < 0.001) of their variation, respectively. For this reason, regression models 2 and 3 included BMI as a confounder. Overall, none of the nutrient intakes (as % of total) explained more than 2% of the total variation (R2) in steatosis or fibrosis scores and the regression F-tests were not significant (i.e., all p > 0.05).
Our results show the LA:ALA ratio was not statistically significantly associated with liver steatosis in Model 1-crude (p = 0.87) nor Model 2 (p = 0.83). Similarly, the n-6:n-3 ratios were not statistically associated with liver steatosis in either models (Model 1: Estimate = −0.51, p = 0.64; Model 2: Estimate = −0.11, p = 0.91). Stepwise regression for steatosis yielded no significant covariates (as in the crude model); therefore, we do not report results for Model 3. Our results also indicate that both the LA:ALA ratio and the n-6:n-3 ratio were positively associated with liver fibrosis in both Model 2, which adjusts for literature-derived covariates known to influence NAFLD risk, and Model 3, which includes covariates with a significant interaction in a stepwise regression analysis (Table 3). In particular, a one-point increase in the LA:ALA ratio resulted in a 1.01% increase in liver fibrosis scores (95% CI: [1.00, 1.03]; p = 0.03), and a one-point increase in the n-6:n-3 resulted in a 1.02% increase in liver fibrosis score (95% CI: [1.01, 1.03]; p = 0.01).

Table 3.
Multiple regression results for liver steatosis and fibrosis in a Mexican-origin Hispanic sample from Southern, Arizona (N = 285).
5. Discussion
The present study sought to describe dietary fatty acid intake among MO Hispanics in Southern Arizona and to determine whether intakes were associated with suspected NAFLD status, particularly liver steatosis and fibrosis. Findings from this work show there was no significant association between fatty acid intake and liver steatosis. However, ratios of LA:ALA and n-6:n-3 were both found to be significantly associated with liver fibrosis. To our knowledge, this is the first study to investigate these associations in a sample of exclusively MO Hispanic participants.
This study was a secondary analysis of an observational study in MO Hispanics who were overweight or obese. Over half of participants (51%) were suspected of having NAFLD based on transient elastography and 20% self-reported a type 2 diabetes diagnosis. Research has shown that these obesity-related metabolic diseases are highly interconnected and engage in a positive feedback loop where NAFLD increases risk of type 2 diabetes and diabetes exacerbates severity of NAFLD that can lead to more serious conditions such as cirrhosis and HCC [29]. Interestingly, we found that a higher proportion of suspected NAFLD cases were women (66%) compared to men (34%). This observation could potentially be explained by the fact that a higher proportion of women had obesity (69% vs. 58%) and were carriers of at least one PNPLA3 risk allele (66% vs. 34%). Previous studies indicate that carriers of the risk allele show > 2-fold higher levels of hepatic fat than non-carriers [30]. Therefore, this finding highlights the intricate relationship of various factors that play a role in the development and severity of NAFLD that should be taken into consideration in future efforts seeking to address alarming NAFLD rates.
Similar to previous findings with PUFAs [31], LA intake accounted for the majority of n-6 PUFA consumption and ALA was the most consumed n-3 PUFA. Intake of n-3 HUFAs, both EPA and DHA, were very low in this population. The mean n-6:n-3 FA ratio of 8.9 (range: 2.46–19.8) reported in the current study is substantially lower than the ratio of 16.0 found by Ramirez-Silva in another Mexican sample [31]. The ratios n-6:n-3 PUFAs in the current study were similar to the compositions and ratios observed throughout the U.S. and to a western diet pattern [9,10,32].
Findings in this study differ from those in the literature. It is important to highlight that previous studies have mainly focused on n-3 intake and supplementation. Additionally, such studies have included populations other than MO Hispanics and different outcome measures of liver disease; therefore, direct comparison across study findings are limited. In our study, no associations were observed between dietary intake of n-3, or the ratio of n-6:n-3 and liver steatosis. In contrast, numerous clinical trials have shown association between n-3 HUFAs and improvement of liver steatosis and/or other markers of NAFLD [33,34,35]. Congruent results were observed in one study investigating fatty acid intake ratios and disease severity in NAFLD patients where no associations were observed [36] as well as in a controlled study testing high fat diets differing in n-6:n-3 ratios in a Maurine model [37]. While findings for FA intake ratios and steatosis were null in our study, significant associations were found between FA intake ratio and liver fibrosis, which are congruent with others in the literature. Specifically, in the current analysis, significant association between n-6:n-3 PUFA ratios and liver fibrosis scores was observed, such that a greater n-6:n-3 ratio corresponded to a significantly higher percentage of fibrosis scores. Such results are in line with several in the literature where supplementation of n-3 intake, and subsequent reduction in n-6:n-3 ratio, was associated with improvement in hepatic fibrosis [38]. On the other hand, recent studies have shown no association between n-3 intake and any histological feature of NASH [39] highlighting the need for more robust evaluation of this association. Although it was not feasible to confirm NAFLD diagnosis in the current study, results show similar associations to studies where NAFLD cases were confirmed. Araya et al., 2004, tested the hypothesis that depletion of hepatic HUFAs is a major mechanism contributing to the pathogenesis of fatty liver in NAFLD patients and showed that higher n-6:n-3 ratios within the liver of NAFLD patients caused a derangement in the capacity of the liver to regulate lipid metabolism [23]. Han et al., 2014, reported that low levels of dietary n-3 PUFAs were associated with elevated NAFLD in Korean men (OR: 2.16; p-trend = 0.030) [32]. Collectively, these findings point out the need for further studies to evaluate the effects of n-6 and n-3 intakes on liver steatosis and fibrosis. In particular, these studies emphasize a critical need to investigate the effects of n-6 and n-3 PUFA in diverse populations such as MO Hispanics where metabolic responses to dietary PUFAs may vary.
Differences in other non-lipid dietary factors, such as carbohydrate and sugar intake, were observed in suspected NAFLD cases versus non-NAFLD participants. Carbohydrate intake, particularly, sugar in the form fructose, has been reported to stimulate the development of NAFLD [40]. However, our study results show that participants in the suspected NAFLD group had a statistically significant lower intake of total carbohydrate and total sugars compared to participants in the non-NAFLD group. While robust efforts were undertaken by the research team to address commonly known issues with dietary reporting, it is suspected that this played a role in our current findings. Participants in the suspected NAFLD group were overall more obese and more likely to have diabetes compared to the non-NAFLD group. In addition, the suspected NAFLD group had a larger proportion of women. Previous studies have reported that such sociodemographic characteristics are associated with dietary underreporting [41,42]. Specifically, underreporting of carbohydrate intake is particularly relevant among Spanish-speaking Hispanic women [43]. Underreporting sugar could also be related to the large number of individuals with diabetes among this group, many of whom know sugar consumption is recommended to be limited for the management of this disease.
While there are strengths in our study including the study sample of specifically MO U.S. Hispanic adults, measured liver steatosis and fibrosis estimates, representation across sexes, and data collection (including repeat 24-h recalls) in the language of preference, several limitations also exist. First, our study sample was composed of only individuals with overweight or obesity (BMI ≥ 25 kg/m2) and therefore, the generalizability of our findings should not be extended to those who do not meet this clinical criterion. It is also well recognized that increased plasma fatty acid levels are elevated in obesity due to numerous factors [44]. Such high levels of endogenous circulating fatty acids may limit the impact of dietary fatty acids on total exposure. This work should expand to normal BMI individuals to determine if dietary exposure and elevated circulating FAs drive an at-risk phenotype of normal BMI individuals. Second, while the 24-h recalls were administered by trained, bilingual and bicultural personnel familiar with the diets of MO Hispanics in the region using validated protocols, there is a need to continue to improve on dietary assessment measures for diverse populations such as MO Hispanics in order to overcome existing dietary misreporting. Importantly, future studies focused on understanding the association of dietary fatty acid exposure and the levels of circulating fatty acids in MO Hispanics are important next steps in understanding the overall impact of circulating levels of fatty acids. Lastly, measurements of liver steatosis and fibrosis were obtained using transient elastography (FibroScan®), which, while robust, is not the gold standard and therefore MRI confirmation of disease severity is warranted.
6. Conclusions
Our findings indicate that the dietary n-6:n-3 PUFA ratio is associated with liver fibrosis in a sample of MO Hispanic adults with overweight or obesity. Dietary n-6 and n-3 PUFA intakes were comparable to what is observed in general U.S. populations and were not associated with liver steatosis. Additional studies are warranted to include large samples, normal weight individuals and even more robust and serial measures of liver pathology to determine whether modulation of fatty acid intake is an appropriate dietary strategy to reduce NAFLD risk in this high-risk population.
Author Contributions
The authors confirm contribution to the paper as follows: study conception and design: M.L.-P., B.H., F.C. and D.O.G.; data collection: M.L.-P. and D.O.G.; analysis and interpretation of results: M.L.-P., B.H., C.A.T., F.C. and D.O.G.; draft manuscript preparation: M.L.-P. and B.H. All authors have read and agreed to the published version of the manuscript.
Funding
Research reported in this publication was supported by the National Cancer Institute under award number T32CA251064, the National Institute on Minority Health and Health Disparities (Award Numbers F31MD016283 and 1 K01 MD014761-01), the American Cancer Society (Institutional Cancer Research grant number IRG-16-124-37), the National Center for Complementary and Integrative Health (NCCIH) (R01 grant number AT008621), the University of Arizona Cancer Center’s Behavioral Measurements and Intervention Shared Resources (P30 CA023074), the University of Arizona Health Sciences, the Center for Health Disparities, and the University of Arizona Graduate Professional Student Council. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Institutional Review Board Statement
This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving research study participants were approved by the University of Arizona Institutional Review Board (IRB #1902380787).
Informed Consent Statement
Written informed consent was obtained from all subjects/participants.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
The authors thank the participants who took part in this study, the Arizona Liver Health partners for their collaboration in the original study, and all study staff and students who participated in the development, implementation, and data collection for the study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- United States Census Bureau. Profile America Facts for Features: Hispanic Heritage Month 2015; U.S. Department of Commerce, Economics and Statistics Administration: Washington, DC, USA, 2015; pp. 1–6.
- Lazo, M.; Bilal, U.; Perez-Escamilla, R. Epidemiology of NAFLD and Type 2 Diabetes: Health Disparities among Persons of Hispanic Origin. Curr. Diab. Rep. 2015, 15, 116. [Google Scholar] [CrossRef] [PubMed]
- Fleischman, M.W.; Budoff, M.; Zeb, I.; Li, D.; Foster, T. NAFLD prevalence differs among hispanic subgroups: The Multi-Ethnic Study of Atherosclerosis. World J. Gastroenterol. 2014, 20, 4987–4993. [Google Scholar] [CrossRef] [PubMed]
- Riazi, K.; Raman, M.; Taylor, L.; Swain, M.G.; Shaheen, A.A. Dietary Patterns and Components in Nonalcoholic Fatty Liver Disease (NAFLD): What Key Messages Can Health Care Providers Offer? Nutrients 2019, 11, 2878. [Google Scholar] [CrossRef]
- Chalasani, N.; Younossi, Z.; Lavine, J.E.; Charlton, M.; Cusi, K.; Rinella, M.; Harrison, S.A.; Brunt, E.M.; Sanyal, A.J. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018, 67, 328–357. [Google Scholar] [CrossRef] [PubMed]
- Jandacek, R.J. Linoleic Acid: A Nutritional Quandary. Healthcare 2017, 5, 25. [Google Scholar] [CrossRef]
- Khadge, S.; Sharp, J.G.; Thiele, G.M.; McGuire, T.R.; Klassen, L.W.; Duryee, M.J.; Britton, H.C.; Dafferner, A.J.; Beck, J.; Black, P.N.; et al. Dietary omega-3 and omega-6 polyunsaturated fatty acids modulate hepatic pathology. J. Nutr. Biochem. 2018, 52, 92–102. [Google Scholar] [CrossRef]
- Tobin, D.; Brevik-Andersen, M.; Qin, Y.; Innes, J.K.; Calder, P.C. Evaluation of a High Concentrate Omega-3 for Correcting the Omega-3 Fatty Acid Nutritional Deficiency in Non-Alcoholic Fatty Liver Disease (CONDIN). Nutrients 2018, 10, 1126. [Google Scholar] [CrossRef]
- Simopoulos, A.P. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed. Pharmacother. 2002, 56, 365–379. [Google Scholar] [CrossRef]
- Saini, R.K.; Keum, Y.S. Omega-3 and omega-6 polyunsaturated fatty acids: Dietary sources, metabolism, and significance—A review. Life Sci. 2018, 203, 255–267. [Google Scholar] [CrossRef]
- Perez-Escamilla, R.; Putnik, P. The role of acculturation in nutrition, lifestyle, and incidence of type 2 diabetes among Latinos. J. Nutr. 2007, 137, 860–870. [Google Scholar] [CrossRef]
- Perez-Escamilla, R. Dietary quality among Latinos: Is acculturation making us sick? J. Am. Diet. Assoc. 2009, 109, 988–991. [Google Scholar] [CrossRef] [PubMed]
- Santiago-Torres, M.; Tinker, L.F.; Allison, M.A.; Breymeyer, K.L.; Garcia, L.; Kroenke, C.H.; Lampe, J.W.; Shikany, J.M.; Van Horn, L.; Neuhouser, M.L. Development and Use of a Traditional Mexican Diet Score in Relation to Systemic Inflammation and Insulin Resistance among Women of Mexican Descent. J. Nutr. 2015, 145, 2732–2740. [Google Scholar] [CrossRef]
- Santiago-Torres, M.; Kratz, M.; Lampe, J.W.; Tapsoba Jde, D.; Breymeyer, K.L.; Levy, L.; Villasenor, A.; Wang, C.Y.; Song, X.; Neuhouser, M.L. Metabolic responses to a traditional Mexican diet compared with a commonly consumed US diet in women of Mexican descent: A randomized crossover feeding trial. Am. J. Clin. Nutr. 2016, 103, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Valerino-Perea, S.; Lara-Castor, L.; Armstrong, M.E.G.; Papadaki, A. Definition of the Traditional Mexican Diet and Its Role in Health: A Systematic Review. Nutrients 2019, 11, 2803. [Google Scholar] [CrossRef] [PubMed]
- Valerino-Perea, S.; Armstrong, M.E.G.; Papadaki, A. Adherence to a traditional Mexican diet and non-communicable disease-related outcomes: Secondary data analysis of the cross-sectional Mexican National Health and Nutrition Survey. Br. J. Nutr. 2022, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Mirmiran, P.; Amirhamidi, Z.; Ejtahed, H.S.; Bahadoran, Z.; Azizi, F. Relationship between Diet and Non-alcoholic Fatty Liver Disease: A Review Article. Iran. J. Public Health 2017, 46, 1007–1017. [Google Scholar]
- Drake, I.; Sonestedt, E.; Ericson, U.; Wallstrom, P.; Orho-Melander, M. A Western dietary pattern is prospectively associated with cardio-metabolic traits and incidence of the metabolic syndrome. Br. J. Nutr. 2018, 119, 1168–1176. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N. Pro-resolving lipid mediators are leads for resolution physiology. Nature 2014, 510, 92–101. [Google Scholar] [CrossRef]
- Serhan, C.N.; Chiang, N.; Van Dyke, T.E. Resolving inflammation: Dual anti-inflammatory and pro-resolution lipid mediators. Nat. Rev. Immunol. 2008, 8, 349–361. [Google Scholar] [CrossRef]
- Blomquist, S.; Coletta, D.; Mandarino, L.J.; Hallmark, B.; Yang, C.; Rich, S.; Manichaikul, A.W.; Chilton, F. Fatty Acid Desaturase Gene-Induced Omega-3 Deficiency in Amerindian-Ancestry Hispanic Populations. FASEB J. 2020, 34, 1-1. [Google Scholar] [CrossRef]
- Elizondo, A.; Araya, J.; Rodrigo, R.; Poniachik, J.; Csendes, A.; Maluenda, F.; Diaz, J.C.; Signorini, C.; Sgherri, C.; Comporti, M.; et al. Polyunsaturated fatty acid pattern in liver and erythrocyte phospholipids from obese patients. Obesity 2007, 15, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Araya, J.; Rodrigo, R.; Videla, L.A.; Thielemann, L.; Orellana, M.; Pettinelli, P.; Poniachik, J. Increase in long-chain polyunsaturated fatty acid n-6/n-3 ratio in relation to hepatic steatosis in patients with non-alcoholic fatty liver disease. Clin. Sci. 2004, 106, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Garcia, D.O.; Morrill, K.E.; Lopez-Pentecost, M.; Villavicencio, E.A.; Vogel, R.M.; Bell, M.L.; Klimentidis, Y.C.; Marrero, D.G.; Thomson, C.A. Nonalcoholic Fatty Liver Disease and Associated Risk Factors in a Community-Based Sample of Mexican-Origin Adults. Hepatol. Commun. 2022, 6, 1322–1335. [Google Scholar] [CrossRef] [PubMed]
- Caussy, C.; Alquiraish, M.H.; Nguyen, P.; Hernandez, C.; Cepin, S.; Fortney, L.E.; Ajmera, V.; Bettencourt, R.; Collier, S.; Hooker, J.; et al. Optimal threshold of controlled attenuation parameter with MRI-PDFF as the gold standard for the detection of hepatic steatosis. Hepatology 2018, 67, 1348–1359. [Google Scholar] [CrossRef] [PubMed]
- Wong, V.W.; Vergniol, J.; Wong, G.L.; Foucher, J.; Chan, H.L.; Le Bail, B.; Choi, P.C.; Kowo, M.; Chan, A.W.; Merrouche, W.; et al. Diagnosis of fibrosis and cirrhosis using liver stiffness measurement in nonalcoholic fatty liver disease. Hepatology 2010, 51, 454–462. [Google Scholar] [CrossRef] [PubMed]
- Dang, H.W.; Kim, S.U.; Park, J.Y.; Ahn, S.H.; Han, K.H.; Chon, C.Y.; Park, Y.N.; Choi, E.H.; Kim, D.Y. How many valid measurements are necessary to assess liver fibrosis using FibroScan(R) in patients with chronic viral hepatitis? An analysis of subjects with at least 10 valid measurements. Yonsei Med. J. 2012, 53, 337–345. [Google Scholar] [CrossRef]
- Blanton, C.A.; Moshfegh, A.J.; Baer, D.J.; Kretsch, M.J. The USDA Automated Multiple-Pass Method accurately estimates group total energy and nutrient intake. J. Nutr. 2006, 136, 2594–2599. [Google Scholar] [CrossRef]
- Xia, M.F.; Bian, H.; Gao, X. NAFLD and Diabetes: Two Sides of the Same Coin? Rationale for Gene-Based Personalized NAFLD Treatment. Front. Pharmacol. 2019, 10, 877. [Google Scholar] [CrossRef]
- Romeo, S.; Kozlitina, J.; Xing, C.; Pertsemlidis, A.; Cox, D.; Pennacchio, L.A.; Boerwinkle, E.; Cohen, J.C.; Hobbs, H.H. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat. Genet. 2008, 40, 1461–1465. [Google Scholar] [CrossRef]
- Ramirez-Silva, I.; Villalpando, S.; Moreno-Saracho, J.E.; Bernal-Medina, D. Fatty acids intake in the Mexican population. Results of the National Nutrition Survey 2006. Nutr. Metab. 2011, 8, 33. [Google Scholar] [CrossRef]
- Han, J.M.; Jo, A.N.; Lee, S.M.; Bae, H.S.; Jun, D.W.; Cho, Y.K.; Suk, K.T.; Yoon, J.H.; Ahn, S.B.; Cho, Y.J.; et al. Associations between intakes of individual nutrients or whole food groups and non-alcoholic fatty liver disease among Korean adults. J. Gastroenterol. Hepatol. 2014, 29, 1265–1272. [Google Scholar] [CrossRef] [PubMed]
- de Castro, G.S.; Calder, P.C. Non-alcoholic fatty liver disease and its treatment with n-3 polyunsaturated fatty acids. Clin. Nutr. 2018, 37, 37–55. [Google Scholar] [CrossRef]
- Guo, X.F.; Yang, B.; Tang, J.; Li, D. Fatty acid and non-alcoholic fatty liver disease: Meta-analyses of case-control and randomized controlled trials. Clin. Nutr. 2018, 37, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Scorletti, E.; Byrne, C.D. Omega-3 fatty acids, hepatic lipid metabolism, and nonalcoholic fatty liver disease. Annu. Rev. Nutr. 2013, 33, 231–248. [Google Scholar] [CrossRef]
- Heinzer, K.; Lang, S.; Farowski, F.; Wisplinghoff, H.; Vehreschild, M.; Martin, A.; Nowag, A.; Kretzschmar, A.; Scholz, C.J.; Roderburg, C.; et al. Dietary omega-6/omega-3 ratio is not associated with gut microbiota composition and disease severity in patients with nonalcoholic fatty liver disease. Nutr. Res. 2022, 107, 12–25. [Google Scholar] [CrossRef] [PubMed]
- Enos, R.T.; Velazquez, K.T.; McClellan, J.L.; Cranford, T.L.; Walla, M.D.; Murphy, E.A. Lowering the dietary omega-6: Omega-3 does not hinder nonalcoholic fatty-liver disease development in a murine model. Nutr. Res. 2015, 35, 449–459. [Google Scholar] [CrossRef]
- Tanaka, N.; Sano, K.; Horiuchi, A.; Tanaka, E.; Kiyosawa, K.; Aoyama, T. Highly purified eicosapentaenoic acid treatment improves nonalcoholic steatohepatitis. J. Clin. Gastroenterol. 2008, 42, 413–418. [Google Scholar] [CrossRef]
- Sanyal, A.J.; Abdelmalek, M.F.; Suzuki, A.; Cummings, O.W.; Chojkier, M.; Group, E.-A.S. No significant effects of ethyl-eicosapentanoic acid on histologic features of nonalcoholic steatohepatitis in a phase 2 trial. Gastroenterology 2014, 147, 377–384.e1. [Google Scholar] [CrossRef]
- Basaranoglu, M.; Basaranoglu, G.; Bugianesi, E. Carbohydrate intake and nonalcoholic fatty liver disease: Fructose as a weapon of mass destruction. Hepatobiliary Surg. Nutr. 2015, 4, 109–116. [Google Scholar] [CrossRef]
- Bothwell, E.K.; Ayala, G.X.; Conway, T.L.; Rock, C.L.; Gallo, L.C.; Elder, J.P. Underreporting of food intake among Mexican/Mexican-American Women: Rates and correlates. J. Am. Diet. Assoc. 2009, 109, 624–632. [Google Scholar] [CrossRef] [PubMed]
- Macdiarmid, J.; Blundell, J. Assessing dietary intake: Who, what and why of under-reporting. Nutr. Res. Rev. 1998, 11, 231–253. [Google Scholar] [CrossRef] [PubMed]
- Banna, J.C.; Fialkowski, M.K.; Townsend, M.S. Misreporting of dietary intake affects estimated nutrient intakes in low-income Spanish-speaking women. J. Acad. Nutr. Diet. 2015, 115, 1124–1133. [Google Scholar] [CrossRef] [PubMed]
- Boden, G. Obesity and free fatty acids. Endocrinol. Metab. Clin. N. Am. 2008, 37, 635–646. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).