Causal Model Building in the Context of Cardiac Rehabilitation: A Systematic Review
Abstract
:1. Introduction
2. Methods
2.1. Sources of Information and Search Strategy
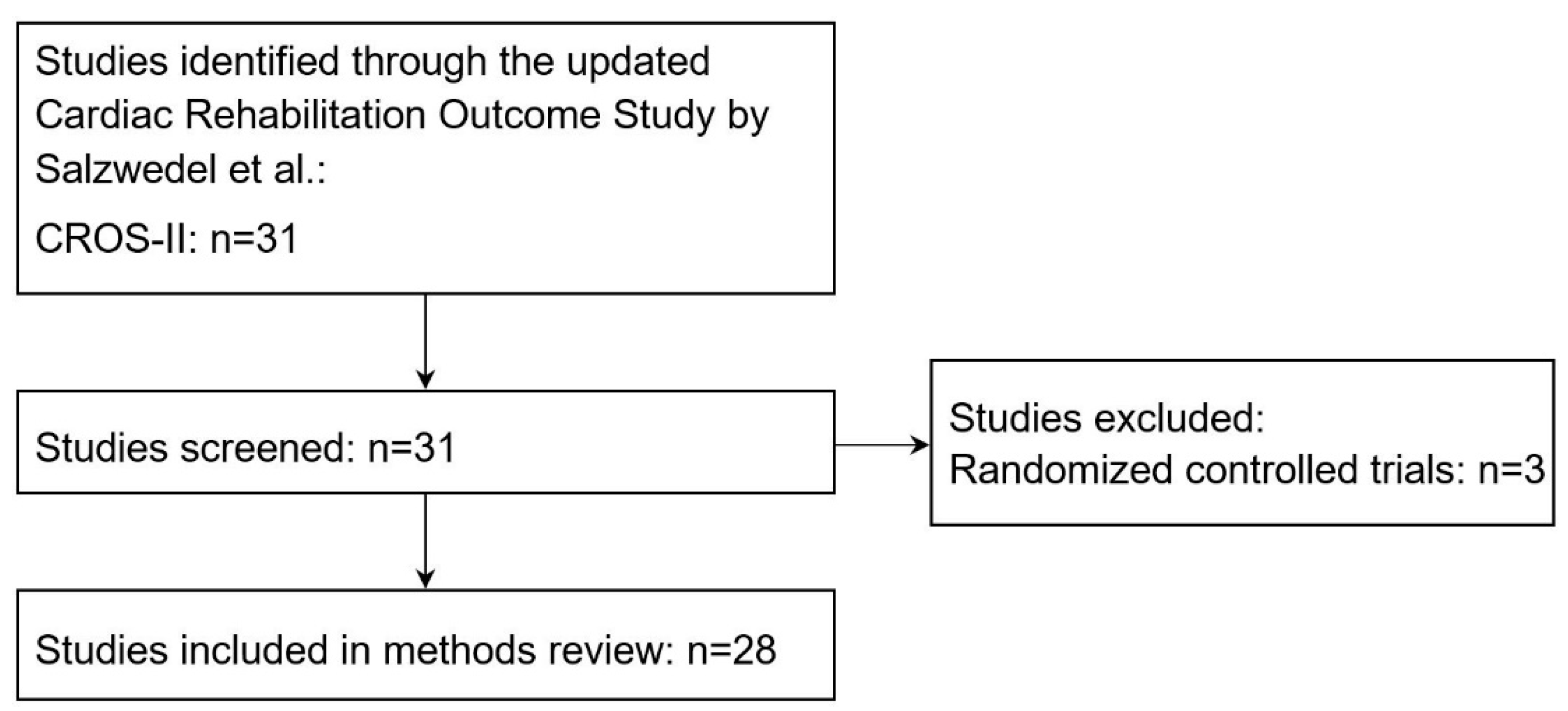
2.2. Selection of Studies for the Systematic Methods Review
2.3. Data Management, Collection Process and Data Items
2.4. Summary Measures and Risk of Bias Assessment
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dalal, H.M.; Doherty, P.; Taylor, R.S. Cardiac rehabilitation. BMJ 2015, 351, h5000. [Google Scholar] [CrossRef]
- Rauch, B.; Davos, C.H.; Doherty, P.; Saure, D.; Metzendorf, M.-I.; Salzwedel, A.; Voeller, H.; Jensen, K.; Schmid, J.-P. The prognostic effect of cardiac rehabilitation in the era of acute revascularisation and statin therapy: A systematic review and meta-analysis of randomized and non-randomized studies–The Cardiac Rehabilitation Outcome Study (CROS). Eur. J. Prev. Cardiol. 2016, 23, 1914–1939. [Google Scholar] [CrossRef]
- Rauch, B.; Riemer, T.; Schwaab, B.; Schneider, S.; Diller, F.; Gohlke, H.; Schiele, R.; Katus, H.; Gitt, A.; Senges, J.; et al. Short-term comprehensive cardiac rehabilitation after AMI is associated with reduced 1-year mortality: Results from the OMEGA study. Eur. J. Prev. Cardiol. 2014, 21, 1060–1069. [Google Scholar] [CrossRef]
- Shmueli, G. To Explain or to Predict? Stat. Sci. 2011, 25, 289–310. [Google Scholar]
- Greenland, S.; Pearl, J.; Robins, J.M. Causal diagrams for epidemiologic research. Epidemiology 1999, 10, 37–48. [Google Scholar] [CrossRef]
- Luijken, K.; Groenwold, R.H.; van Smeden, M.; Strohmaier, S.; Heinze, G. A comparison of full model specification and backward elimination of potential confounders when estimating marginal and conditional causal effects on binary outcomes from observational data. Biom. J. 2022. [Google Scholar] [CrossRef]
- Witte, J.; Didelez, V. Covariate selection strategies for causal inference: Classification and comparison. Biom. J. 2019, 61, 1270–1289. [Google Scholar] [CrossRef]
- Heinze, G.; Wallisch, C.; Dunkler, D. Variable selection—A review and recommendations for the practicing statistician. Biom. J. 2018, 60, 431–449. [Google Scholar] [CrossRef]
- Sauerbrei, W.; Perperoglou, A.; Schmid, M.; Abrahamowicz, M.; Becher, H.; Binder, H.; Dunkler, D.; Harrell, F.; Royston, P.; Heinze, G. State of the art in selection of variables and functional forms in multivariable analysis—Outstanding issues. Diagn. Progn. Res. 2020, 4, 3. [Google Scholar] [CrossRef]
- Lee, K.J.; Tilling, K.M.; Cornish, R.P.; Little, R.J.; Bell, M.L.; Goetghebeur, E.; Hogan, J.W.; Carpenter, J.R. Framework for the treatment and reporting of missing data in observational studies: The Treatment And Reporting of Missing data in Observational Studies framework. J. Clin. Epidemiol. 2021, 134, 79–88. [Google Scholar] [CrossRef]
- Box, G.E. Robustness in the strategy of scientific model building. In Robustness in Statistics; Academic Press: Cambridge, MA, USA, 1979; pp. 201–236. [Google Scholar]
- Wallisch, C.; Bach, P.; Hafermann, L.; Klein, N.; Sauerbrei, W.; Steyerberg, E.W.; Heinze, G.; Rauch, G.; topic group 2 of the STRATOS initiative. Review of guidance papers on regression modeling in statistical series of medical journals. PLoS ONE 2022, 17, e0262918. [Google Scholar] [CrossRef]
- Harrell, F.E. Regression Modeling Strategies: With Applications to Linear Models, Logistic Regression, and Survival Analysis; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Salzwedel, A.; Jensen, K.; Rauch, B.; Doherty, P.; Metzendorf, M.-I.; Hackbusch, M.; Völler, H.; Schmid, J.-P.; Davos, C.H. Effectiveness of comprehensive cardiac rehabilitation in coronary artery disease patients treated according to contemporary evidence based medicine: Update of the Cardiac Rehabilitation Outcome Study (CROS-II). Eur. J. Prev. Cardiol. 2020, 27, 1756–1774. [Google Scholar] [CrossRef]
- West, R.R.; Jones, D.A.; Henderson, A.H. Rehabilitation after myocardial infarction trial (RAMIT): Multi-centre randomised controlled trial of comprehensive cardiac rehabilitation in patients following acute myocardial infarction. Heart 2012, 98, 637–644. [Google Scholar] [CrossRef]
- Aronov, D.M.; Bubnova, M.G.; Ioseliani, D.G.; Krasnitsky, V.B.; Shovkun, T.V.; Novikova, N.K.; Yarnykh, E.V. The complex program of rehabilitation of patients with ischemic heart disease after coronary artery bypass surgery in ambulatory cardiorehabilitational department: Clinical effects of third stage of rehabilitation. Kardiologiia 2017, 57, 10–19. [Google Scholar]
- Hautala, A.J.; Kiviniemi, A.M.; Mäkikallio, T.; Koistinen, P.; Ryynänen, O.P.; Martikainen, J.A.; Seppänen, T.; Huikuri, H.V.; Tulppo, M.P. Economic evaluation of exercise-based cardiac rehabilitation in patients with a recent acute coronary syndrome. Scand. J. Med. Sci. Sport. 2017, 27, 1395–1403. [Google Scholar] [CrossRef]
- Higgins, J.P.; Ramsay, C.; Reeves, B.C.; Deeks, J.J.; Shea, B.; Valentine, J.C.; Tugwell, P.; Wells, G. Issues relating to study design and risk of bias when including non-randomized studies in systematic reviews on the effects of interventions. Res. Synth. Methods 2013, 4, 12–25. [Google Scholar] [CrossRef]
- Wells, G.A.; Shea, B.; Higgins, J.P.; Sterne, J.; Tugwell, P.; Reeves, B.C. Checklists of methodological issues for review authors to consider when including non-randomized studies in systematic reviews. Res. Synth. Methods 2013, 4, 63–77. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef] [PubMed]
- Hafermann, L.; Becher, H.; Herrmann, C.; Klein, N.; Heinze, G.; Rauch, G. Statistical model building: Background “knowledge” based on inappropriate preselection causes misspecification. BMC Med. Res. Methodol. 2021, 21, 196. [Google Scholar] [CrossRef] [PubMed]
- Hernán, M.A.; Robins, R.J. Causal Inference: What If; Chapman & Hall/CRC: Boca Raton, FL, USA, 2020. [Google Scholar]
- Petersen, M.L.; Porter, K.E.; Gruber, S.; Wang, Y.; Van Der Laan, M.J. Diagnosing and responding to violations in the positivity assumption. Stat. Methods Med. Res. 2012, 21, 31–54. [Google Scholar] [CrossRef]
- Sauerbrei, W.; Royston, P.; Binder, H. Selection of important variables and determination of functional form for continuous predictors in multivariable model building. Stat. Med. 2007, 26, 5512–5528. [Google Scholar] [CrossRef]
- Bennett, D.A. How can I deal with missing data in my study? Aust. N. Z. J. Public Health 2001, 25, 464–469. [Google Scholar] [CrossRef]
- Cousineau, D.; Chartier, S. Outliers detection and treatment: A review. Int. J. Psychol. Res. 2010, 3, 58–67. [Google Scholar] [CrossRef]
- Heinze, G.; Dunkler, D. Five myths about variable selection. Transpl. Int. 2017, 30, 6–10. [Google Scholar] [CrossRef]
- Van Smeden, M.; de Groot, J.A.; Moons, K.G.; Collins, G.S.; Altman, D.G.; Eijkemans, M.J.; Reitsma, J.B. No rationale for 1 variable per 10 events criterion for binary logistic regression analysis. BMC Med. Res. Methodol. 2016, 16, 163. [Google Scholar] [CrossRef]
- Royston, P.; Altman, D.G.; Sauerbrei, W. Dichotomizing continuous predictors in multiple regression: A bad idea. Stat. Med. 2006, 25, 127–141. [Google Scholar] [CrossRef]
- Boulay, P.; Prud’homme, D. Health-care consumption and recurrent myocardial infarction after 1 year of conventional treatment versus short-and long-term cardiac rehabilitation. Prev. Med. 2004, 38, 586–593. [Google Scholar] [CrossRef]
- Espinosa Caliani, S.; Bravo Navas, J.C.; Collantes Rivera, R.; González Jiménez, B.; Martínez Lao, M.; de Teresa Galván, E.; Gómez-Doblas, J.J. Rehabilitación cardiaca postinfarto de miocardio en enfermos de bajo riesgo. Resultados de un programa de coordinación entre cardiología y atención primaria. Rev. Española De Cardiol. 2004, 57, 53–59. [Google Scholar] [CrossRef]
- Kim, C.; Kim, D.Y.; Moon, C.J. Prognostic influences of cardiac rehabilitation in Korean acute myocardial infarction patients. Ann. Rehabil. Med. 2011, 35, 375–380. [Google Scholar] [CrossRef]
- Lee, H.Y.; Kim, J.H.; Kim, B.O.; Byun, Y.-S.; Cho, S.; Goh, C.W.; Ahn, H.; Rhee, K.J.; Kim, C. Regular exercise training reduces coronary restenosis after percutaneous coronary intervention in patients with acute myocardial infarction. Int. J. Cardiol. 2013, 167, 2617–2622. [Google Scholar] [CrossRef]
- Norris, C.M.; Jensen, L.A.; Galbraith, P.D.; Graham, M.M.; Daub, W.D.; Knudtson, M.L.; Ghali, W.A. Referral rate and outcomes of cardiac rehabilitation after cardiac catheterization in a large Canadian city. J. Cardiopulm. Rehabil. Prev. 2004, 24, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Kutner, N.G.; Zhang, R.; Huang, Y.; Herzog, C.A. Cardiac rehabilitation and survival of dialysis patients after coronary bypass. J. Am. Soc. Nephrol. 2006, 17, 1175–1180. [Google Scholar] [CrossRef] [Green Version]
- Milani, R.V.; Lavie, C.J. Impact of cardiac rehabilitation on depression and its associated mortality. Am. J. Med. 2007, 120, 799–806. [Google Scholar] [CrossRef] [PubMed]
- Hansen, D.; Dendale, P.; Leenders, M.; Berger, J.; Raskin, A.; Vaes, J.; Meeusen, R. Reduction of cardiovascular event rate: Different effects of cardiac rehabilitation in CABG and PCI patients. Acta Cardiol. 2009, 64, 639–644. [Google Scholar] [CrossRef] [PubMed]
- Suaya, J.A.; Stason, W.B.; Ades, P.A.; Normand, S.-L.T.; Shepard, D.S. Cardiac rehabilitation and survival in older coronary patients. J. Am. Coll. Cardiol. 2009, 54, 25–33. [Google Scholar] [CrossRef]
- Jünger, C.; Rauch, B.; Schneider, S.; Liebhart, N.; Rauch, G.; Senges, J.; Bestehorn, K. Effect of early short-term cardiac rehabilitation after acute ST-elevation and non-ST-elevation myocardial infarction on 1-year mortality. Curr. Med. Res. Opin. 2010, 26, 803–811. [Google Scholar] [CrossRef]
- Schwaab, B.; Waldmann, A.; Katalinic, A.; Sheikhzadeh, A.; Raspe, H. In-patient cardiac rehabilitation versus medical care–a prospective multicentre controlled 12 months follow-up in patients with coronary heart disease. Eur. J. Prev. Cardiol. 2011, 18, 581–586. [Google Scholar] [CrossRef]
- Martin, B.-J.; Hauer, T.; Arena, R.; Austford, L.D.; Galbraith, P.D.; Lewin, A.M.; Knudtson, M.L.; Ghali, W.A.; Stone, J.A.; Aggarwal, S.G. Cardiac rehabilitation attendance and outcomes in coronary artery disease patients. Circulation 2012, 126, 677–687. [Google Scholar] [CrossRef]
- Prince, D.Z.; Sobolev, M.; Gao, J.; Taub, C.C. Racial disparities in cardiac rehabilitation initiation and the effect on survival. PMR 2014, 6, 486–492. [Google Scholar] [CrossRef]
- Doimo, S.; Fabris, E.; Piepoli, M.; Barbati, G.; Antonini-Canterin, F.; Bernardi, G.; Maras, P.; Sinagra, G. Impact of ambulatory cardiac rehabilitation on cardiovascular outcomes: A long-term follow-up study. Eur. Heart J. 2019, 40, 678–685. [Google Scholar] [CrossRef]
- Sunamura, M.; Ter Hoeve, N.; van den Berg-Emons, R.J.G.; Boersma, E.; van Domburg, R.T.; Geleijnse, M.L. Cardiac rehabilitation in patients with acute coronary syndrome with primary percutaneous coronary intervention is associated with improved 10-year survival. Eur. Heart J. Qual. Care Clin. Outcomes 2018, 4, 168–172. [Google Scholar] [CrossRef]
- Goel, K.; Lennon, R.J.; Tilbury, R.T.; Squires, R.W.; Thomas, R.J. Impact of cardiac rehabilitation on mortality and cardiovascular events after percutaneous coronary intervention in the community. Circulation 2011, 123, 2344–2352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marzolini, S.; Leung, Y.W.; Alter, D.A.; Wu, G.; Grace, S.L. Outcomes associated with cardiac rehabilitation participation in patients with musculoskeletal comorbidities. Eur. J. Phys. Rehabil. Med. 2013, 49, 775–783. [Google Scholar]
- Schlitt, A.; Wischmann, P.; Wienke, A.; Hoepfner, F.; Noack, F.; Silber, R.-E.; Werdan, K. Rehabilitation in patients with coronary heart disease: Participation and its effect on prognosis. Dtsch. Ärzteblatt Int. 2015, 112, 527–534. [Google Scholar]
- Pack, Q.R.; Goel, K.; Lahr, B.D.; Greason, K.L.; Squires, R.W.; Lopez-Jimenez, F.; Zhang, Z.; Thomas, R.J. Participation in cardiac rehabilitation and survival after coronary artery bypass graft surgery: A community-based study. Circulation 2013, 128, 590–597. [Google Scholar] [CrossRef] [PubMed]
- Meurs, M.; Burger, H.; van Riezen, J.; Slaets, J.P.; Rosmalen, J.G.M.; van Melle, J.P.; Roest, A.M.; de Jonge, P. The association between cardiac rehabilitation and mortality risk for myocardial infarction patients with and without depressive symptoms. J. Affect. Disord. 2015, 188, 278–283. [Google Scholar] [CrossRef]
- Nielsen, K.M.; Faergeman, O.; Foldspang, A.; Larsen, M.L. Cardiac rehabilitation: Health characteristics and socio-economic status among those who do not attend. Eur. J. Public Health 2008, 18, 479–483. [Google Scholar] [CrossRef]
- Alter, D.A.; Oh, P.I.; Chong, A. Relationship between cardiac rehabilitation and survival after acute cardiac hospitalization within a universal health care system. Eur. J. Cardiovasc. Prev. Rehabil. 2009, 16, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Coll-Fernández, R.; Coll, R.; Pascual, T.; Muñoz-Torrero, J.F.S.; Sahuquillo, J.C.; Manzano, L.; Aguilar, E.; Alcala-Pedrajas, J.N.; Álvarez, L.R.; García-Díaz, A.M.A.; et al. Cardiac rehabilitation and outcome in stable outpatients with recent myocardial infarction. Arch. Phys. Med. Rehabil. 2014, 95, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-Y.; Ahn, J.-M.; Park, D.-W.; Kang, S.-J.; Kim, Y.-H.; Lee, S.-W.; Lee, C.W.; Park, S.-W.; Park, S.-J. Impact of exercise-based cardiac rehabilitation on long-term clinical outcomes in patients with left main coronary artery stenosis. Eur. J. Prev. Cardiol. 2016, 23, 1804–1813. [Google Scholar] [CrossRef]
- Beauchamp, A.; Worcester, M.; Ng, A.; Murphy, B.; Tatoulis, J.; Grigg, L.; Newman, R.; Goble, A. Attendance at cardiac rehabilitation is associated with lower all-cause mortality after 14 years of follow-up. Heart 2013, 99, 620–625. [Google Scholar] [CrossRef] [PubMed]
- Goel, K.; Pack, Q.R.; Lahr, B.; Greason, K.L.; Lopez-Jimenez, F.; Squires, R.W.; Zhang, Z.; Thomas, R.J. Cardiac rehabilitation is associated with reduced long-term mortality in patients undergoing combined heart valve and CABG surgery. Eur. J. Prev. Cardiol. 2015, 22, 159–168. [Google Scholar] [CrossRef]
- De Vries, H.; Kemps, H.M.C.; van Engen-Verheul, M.M.; Kraaijenhagen, R.A.; Peek, N. Cardiac rehabilitation and survival in a large representative community cohort of Dutch patients. Eur. Heart J. 2015, 36, 1519–1528. [Google Scholar] [CrossRef] [PubMed]
- Walter, S.; Tiemeier, H. Variable selection: Current practice in epidemiological studies. Eur. J. Epidemiol. 2009, 24, 733–736. [Google Scholar] [CrossRef] [Green Version]
- Mallett, S.; Royston, P.; Dutton, S.; Waters, R.; Altman, D. Reporting methods in studies developing prognostic models in cancer: A review. BMC Med. 2010, 8, 20. [Google Scholar] [CrossRef] [PubMed]
- Collins, G.S.; Mallett, S.; Omar, O.; Yu, L.M. Developing risk prediction models for type 2 diabetes: A systematic review of methodology and reporting. BMC Med. 2011, 9, 103. [Google Scholar] [CrossRef]
- Talbot, D.; Massamba, V. A descriptive review of variable selection methods in four epidemiologic journals: There is still room for improvement. Eur. J. Epidemiol. 2019, 34, 725–730. [Google Scholar] [CrossRef]
- Sun, G.-W.; Shook, T.L.; Kay, G.L. Inappropriate use of bivariable analysis to screen risk factors for use in multivariable analysis. J. Clin. Epidemiol. 1996, 49, 907–916. [Google Scholar] [CrossRef]
- Mantel, N. Why stepdown procedures in variable selection. Technometrics 1970, 12, 621–625. [Google Scholar] [CrossRef]
- Kyle, R.P.; Moodie, E.E.M.; Klein, M.B.; Abrahamowicz, M. Evaluating Flexible Modeling of Continuous Covariates in Inverse-Weighted Estimators. Am. J. Epidemiol. 2019, 188, 1181–1191. [Google Scholar] [CrossRef]
- Royston, P.; Altman, D.G. Regression using fractional polynomials of continuous covariates: Parsimonious parametric modelling. J. R. Stat. Soc. Ser. C 1994, 43, 429–453. [Google Scholar] [CrossRef]
- Abrahamowicz, M.; Mackenzie, T. Joint estimation of time-dependent and non-linear effects of continuous covariates on survival. Stat. Med. 2007, 26, 392–408. [Google Scholar] [CrossRef] [PubMed]
- Shadish, W.R.; Steiner, P.M. A Primer on Propensity Score Analysis. Newborn Infant Nurs. Rev. 2010, 10, 19–26. [Google Scholar] [CrossRef]
- National Academies of Sciences, Engineering, and Medicine. Reproducibility and Replicability in Science; National Academies Press: Washington, DC, USA, 2019. [Google Scholar]
- Ramspek, C.L.; Steyerberg, E.W.; Riley, R.D.; Rosendaal, F.R.; Dekkers, O.M.; Dekker, F.W.; van Diepen, M. Prediction or causality? A scoping review of their conflation within current observational research. Eur. J. Epidemiol. 2021, 36, 889–898. [Google Scholar] [CrossRef] [PubMed]
- Hernán, M.A.; Alonso, A.; Logan, R.; Grodstein, F.; Michels, K.B.; Stampfer, M.J.; Willett, W.C.; Manson, J.E.; Robins, J.M. Observational studies analyzed like randomized experiments: An application to postmenopausal hormone therapy and coronary heart disease. Epidemiology 2008, 19, 766. [Google Scholar] [CrossRef] [Green Version]
| Component | |
|---|---|
| Physical exercise | Supervised and structured exercise training at least twice a week [2] |
| Information | Advice on cardiovascular risk reduction [1] |
| Motivational techniques | Strategies to ensure patients adequately adhere to medications and implement lifestyle changes [3] |
| Education | Health education includes information on medication, exercise training, individual nutritional advice and support to stop smoking [3] |
| Psychological support and interventions | Psychological counselling, support of individual’s disease management and coping strategies, relaxation methods and individual behavior changes [3] |
| Social and vocational support | Support in social reintegration and reuptake of work [3] |
| Quality Aspect | Literature on Guidance | Assessment Procedure |
|---|---|---|
| Selection of variables … | ||
| …based on domain expertise and preceding studies (background knowledge) | [21,22] |
|
| …based on background knowledge with causal diagrams known as directed acyclic graphs (DAGs) | [7,8,22] |
|
| Assumption of … | ||
| …pretreatment covariates. | [7,22] |
|
| …conditional exchangeability. This means that conditional on the IVs, association is causation. | [7,22] |
|
| …positivity. This means each value of the confounders is observed for all treatment groups. | [7,22,23] |
|
| …consistency. This means that the mode of receiving, as opposed to the choice of treatment level per se, does not affect the outcome. | [7,22,23] |
|
| …linearity. This means that the relationship between a continuous IV and the outcome is linear. | [24] |
|
| Missing values | [20,25] |
|
| Highly influential points/outliers | [26] |
|
| Events-per-variable (EPV) | [13], p. 72; [27,28] |
|
| Dichotomization of continuous variables | [29] |
|
| Selection Method * | for Regression (n = 24) | for Propensity Score (n = 13) | ||
|---|---|---|---|---|
| n | % | n | % | |
| Background knowledge | 5 | 21 | 2 | 15 |
| Univariable screening | 8 | 33 | 3 | 23 |
| Stepwise selection | 4 | 17 | 0 | 0 |
| Shrinkage methods | 1 | 4 | 1 | 8 |
| Not described | 11 | 46 | 8 | 62 |
| Propensity Score Methods | Studies (n = 13) | |
|---|---|---|
| n | % | |
| Matching | 7 | 54 |
| Stratification | 4 | 31 |
| Multivariable analysis included: | ||
| as covariate | 5 | 38 |
| inverse probability weighting | 1 | 8 |
| inclusion not described | 1 | 8 |
| Studies (n = 24) | ||
|---|---|---|
| n | % | |
| Missing values reported | 10 | 42 |
| Missing value imputation applied | 4 | 17 |
| Highly influential points reported | 0 | 0 |
| Multicollinearity reported | 1 | 4 |
| Pair-wise interactions reported | 8 | 33 |
| Pair-wise interactions applied | 6 | 25 |
| Mixed Models applied | 2 | 8 |
| Reconstruction of EPV possible | 16 | 67 |
| Dichotomization of continuous variables applied | 12 | 50 |
| Study | Rauch 2014 [3] | Norris 2004 [34] | Kutner 2006 [35] | Milani 2007 [36] | Hansen 2009 [37] | Suaya 2009 [38] | Jünger 2010 [39] | Schwaab 2011 [40] | Martin 2012 [41] | Prince 2014 [42] | Doimo 2018 [43] | Sunamura 2018 [44] | Goel 2011 [45] | Marzolini 2013 [46] | Schlitt 2015 [47] | Pack 2013 [48] | Meurs 2015 [49] | Nielsen 2008 [50] | Alter 2009 [51] | Coll-Fernandez 2013 [52] | Lee 2016 [53] | Beauchamp 2013 [54] | Goel 2013 [55] | De Vries 2015 [56] |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Confounders selected based on background knowledge? | NC | NC | NC | NC | NC | NC | N | NC | NC | NC | NC | NC | N | N | N | Y | Y | N | N | N | N | Y | Y | Y |
| Was a DAG used? | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N |
| Only pretreatment covariates included? | Y | Y | NC | Y | N | Y | Y | Y | NC | NC | Y | Y | Y | Y | Y | Y | Y | NC | Y | Y | NC | Y | Y | Y |
| Exchangeability mentioned? | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N |
| Positivity mentioned? | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N |
| Consistency reported? | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N |
| Incorporation of non-linear functional forms? | Y | N | N | N | N | N | N | N | N | N | N | N | Y | N | N | N | N | N | N | N | N | N | N | N |
| Missing data reported? | NC | NC | NC | NC | NC | NC | Y | NC | Y | NC | NC | NC | Y | Y | NC | Y | Y | Y | Y | NC | NC | Y | NC | Y |
| Missing data imputation? | NC | NC | NC | NC | NC | NC | Y | NC | NN | NC | NC | NC | Y | Y | NC | NC | Y | NN | NC | NC | NC | NN | NC | NC |
| Highly influential points/outliers? | NC | NC | NC | NC | NC | NC | NC | NC | NC | NC | NC | NC | NC | NC | NC | NC | NC | NC | NC | NC | NC | NC | NC | NC |
| Multicollinearity? | NC | NC | NC | NC | NC | NC | NC | NC | NC | NC | NC | NC | NC | NC | NC | NC | NC | NC | NC | NC | NC | NC | NC | N |
| Events-per-variable (EPV) >15 | N | NC | NC | N | N | NC | Y | Y | Y | NC | NC | NC | Y | N | N | Y | Y | NC | N | N | NC | Y | N | Y |
| Dichotomization of continuous variables? | Y | Y | N | N | Y | Y | Y | NI | N | N | N | N | Y | NI | N | Y | Y | Y | N | Y | N | N | Y | Y |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akbari, N.; Heinze, G.; Rauch, G.; Sander, B.; Becher, H.; Dunkler, D. Causal Model Building in the Context of Cardiac Rehabilitation: A Systematic Review. Int. J. Environ. Res. Public Health 2023, 20, 3182. https://doi.org/10.3390/ijerph20043182
Akbari N, Heinze G, Rauch G, Sander B, Becher H, Dunkler D. Causal Model Building in the Context of Cardiac Rehabilitation: A Systematic Review. International Journal of Environmental Research and Public Health. 2023; 20(4):3182. https://doi.org/10.3390/ijerph20043182
Chicago/Turabian StyleAkbari, Nilufar, Georg Heinze, Geraldine Rauch, Ben Sander, Heiko Becher, and Daniela Dunkler. 2023. "Causal Model Building in the Context of Cardiac Rehabilitation: A Systematic Review" International Journal of Environmental Research and Public Health 20, no. 4: 3182. https://doi.org/10.3390/ijerph20043182
APA StyleAkbari, N., Heinze, G., Rauch, G., Sander, B., Becher, H., & Dunkler, D. (2023). Causal Model Building in the Context of Cardiac Rehabilitation: A Systematic Review. International Journal of Environmental Research and Public Health, 20(4), 3182. https://doi.org/10.3390/ijerph20043182








