Association between Long-Term Ambient PM2.5 Exposure and under-5 Mortality: A Scoping Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Source and Article Search
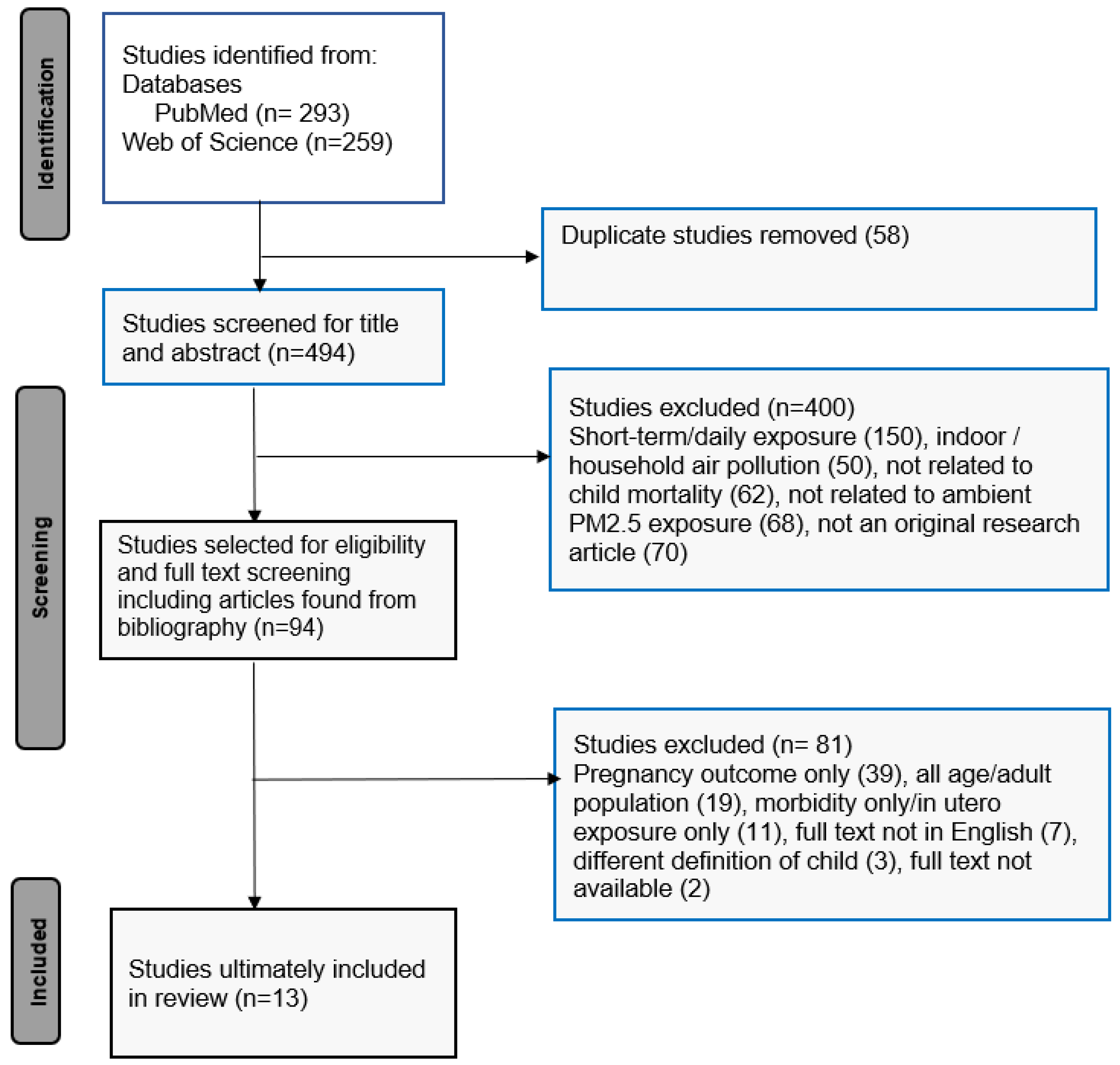
2.2. Selection of Articles
2.3. Data Extraction and Analysis
3. Results
3.1. Study Characteristics
3.2. Outcomes
3.3. Exposure Assessments
3.4. Adjusted Covariates
3.5. Effect Estimates
3.5.1. Post-Birth Exposure to PM2.5 and under-5 Mortality
3.5.2. Exposure to PM2.5 and Infant Mortality
4. Discussion
Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- United Nations Inter-agency Group for Child Mortality Estimation Levels & Trends in Child Mortality: Report 2017, Estimates Developed by the United Nations Inter-Agency Group for Child Mortality Estimation. United Nations Inter-Agency Group for Child Mortality Estimation (UN IGME). 2017. Available online: https://www.unicef.org/reports/levels-and-trends-child-mortality-report-2017 (accessed on 19 May 2020).
- Sharrow, D.; Hug, L.; You, D.; Alkema, L.; Black, R.; Cousens, S.; Croft, T.; Gaigbe-Togbe, V.; Gerland, P.; Guillot, M.; et al. Global, regional, and national trends in under-5 mortality between 1990 and 2019 with scenario-based projections until 2030, A systematic analysis by the UN Inter-agency Group for Child Mortality Estimation. Lancet Glob. Health 2022, 10, 195–206. [Google Scholar] [CrossRef]
- IGME U. Levels & Trends in Child Mortality: Report 2021, Estimates Developed by the United Nations Inter-Agency Group for Child Mortality Estimation. United Nations Inter-Agency Group for Child Mortality Estimation (UN IGME). 2021. Available online: https://www.unicef.org/reports/levels-and-trends-child-mortality-report-2021 (accessed on 5 July 2022).
- Feigin, V. GBD 2017 Causes of Death Collaborators. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017, A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1736–1788. [Google Scholar]
- World Health Organization. World Health Statistics 2022: Monitoring Health for the SDGs, Sustainable Development Goals; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- World Health Organization. Air Pollution and Child Health: Prescribing Clean Air. WHO/CED/PHE/18.01. 2018. Available online: https://www.who.int/publications/i/item/air-pollution-and-child-health (accessed on 2 December 2020).
- Bateson, T.F.; Schwartz, J. Children’s response to air pollutants. J. Toxicol. Environ. Health Part A 2007, 71, 238–243. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Li, C.; Tang, X. The impact of PM2. 5 on the host defense of respiratory system. Front. Cell Dev. Biol. 2020, 8, 91. [Google Scholar] [CrossRef]
- Odo, D.B.; Yang, I.A.; Dey, S.; Hammer, M.S.; van Donkelaar, A.; Martin, R.V.; Dong, G.H.; Yang, B.Y.; Hystad, P.; Knibbs, L.D. Ambient air pollution and acute respiratory infection in children aged under 5 years living in 35 developing countries. Environ. Int. 2022, 159, 107019. [Google Scholar] [CrossRef] [PubMed]
- Sherris, A.R.; Begum, B.A.; Baiocchi, M.; Goswami, D.; Hopke, P.K.; Brooks, W.A.; Luby, S.P. Associations between ambient fine particulate matter and child respiratory infection: The role of particulate matter source composition in Dhaka, Bangladesh. Environ. Pollut. 2021, 290, 118073. [Google Scholar] [CrossRef]
- Lavigne, É.; Talarico, R.; van Donkelaar, A.; Martin, R.V.; Stieb, D.M.; Crighton, E.; Weichenthal, S.; Smith-Doiron, M.; Burnett, R.T.; Chen, H. Fine particulate matter concentration and composition and the incidence of childhood asthma. Environ. Int. 2021, 152, 106486. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.N.; Cheng, F.J.; Tsai, M.T.; Tsai, C.M.; Chuang, P.C.; Cheng, C.Y. Fine particulate matter constituents associated with emergency room visits for pediatric asthma: A time-stratified case–crossover study in an urban area. BMC Public Health 2021, 21, 1593. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, J.; Chen, D.; Sun, P.; Ma, X. The association between air pollution and preterm birth and low birth weight in Guangdong, China. BMC Public Health 2019, 19, 3. [Google Scholar] [CrossRef]
- Chen, Q.; Ren, Z.; Liu, Y.; Qiu, Y.; Yang, H.; Zhou, Y.; Wang, X.; Jiao, K.; Liao, J.; Ma, L. The association between preterm birth and ambient air pollution exposure in Shiyan, China, 2015–2017. Int. J. Environ. Res. Public Health 2021, 18, 4326. [Google Scholar] [CrossRef]
- Fong, K.C.; Kosheleva, A.; Kloog, I.; Koutrakis, P.; Laden, F.; Coull, B.A.; Schwartz, J.D. Fine particulate air pollution and birthweight: Differences in associations along the birthweight distribution. Epidemiology 2019, 30, 617. [Google Scholar] [CrossRef] [PubMed]
- Spears, D.; Dey, S.; Chowdhury, S.; Scovronick, N.; Vyas, S.; Apte, J. The association of early-life exposure to ambient PM 2.5 and later-childhood height-for-age in India: An observational study. Environ. Health 2019, 18, 62. [Google Scholar] [CrossRef]
- Ahmed, S.M.; Mishra, G.D.; Moss, K.M.; Yang, I.A.; Lycett, K.; Knibbs, L.D. Maternal and childhood ambient air pollution exposure and mental health symptoms and psychomotor development in children: An Australian population-based longitudinal study. Environ. Int. 2022, 158, 107003. [Google Scholar] [CrossRef]
- Wang, S.Y.; Cheng, Y.Y.; Guo, H.R.; Tseng, Y.C. Air pollution during pregnancy and childhood autism spectrum disorder in Taiwan. Int. J. Environ. Res. Public Health 2021, 18, 9784. [Google Scholar] [CrossRef]
- Morales-Ancajima, V.C.; Tapia, V.; Vu, B.N.; Liu, Y.; Alarcon-Yaquetto, D.E.; Gonzales, G.F. Increased outdoor PM2. 5 concentration is associated with moderate/severe anemia in children aged 6–59 months in Lima, Peru. J. Environ. Public Health 2019, 2019, 6127845. [Google Scholar] [CrossRef]
- Loomis, D.; Castillejos, M.; Gold, D.R.; McDonnell, W.; Borja-Aburto, V.H. Air pollution and infant mortality in Mexico City. Epidemiology 1999, 10, 118–123. Available online: http://www.jstor.org/stable/3703084 (accessed on 13 September 2021). [CrossRef] [PubMed]
- Heft-Neal, S.; Burney, J.; Bendavid, E.; Voss, K.K.; Burke, M. Dust pollution from the Sahara and African infant mortality. Nat. Sustain. 2020, 3, 863–871. [Google Scholar] [CrossRef]
- Son, J.Y.; Lee, H.J.; Koutrakis, P.; Bell, M.L. Pregnancy and lifetime exposure to fine particulate matter and infant mortality in Massachusetts, 2001–2007. Am. J. Epidemiol. 2017, 186, 1268–1276. [Google Scholar] [CrossRef]
- Carter, S.A.; Rahman, M.M.; Lin, J.C.; Shu, Y.H.; Chow, T.; Yu, X.; Martinez, M.P.; Eckel, S.P.; Chen, J.C.; Chen, Z.; et al. In utero exposure to near-roadway air pollution and autism spectrum disorder in children. Environ. Int. 2022, 158, 106898. [Google Scholar] [CrossRef] [PubMed]
- Pope, D.P.; Mishra, V.; Thompson, L.; Siddiqui, A.R.; Rehfuess, E.A.; Weber, M.; Bruce, N.G. Risk of low birth weight and stillbirth associated with indoor air pollution from solid fuel use in developing countries. Epidemiol. Rev. 2010, 32, 70–81. [Google Scholar] [CrossRef]
- Kleimola, L.B.; Patel, A.B.; Borkar, J.A.; Hibberd, P.L. Consequences of household air pollution on child survival: Evidence from demographic and health surveys in 47 countries. Int. J. Occup. Environ. Health 2015, 21, 294–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naz, S.; Page, A.; Agho, K.E. Household air pollution and under-five mortality in India (1992–2006). Environ. Health 2016, 15, 54. [Google Scholar] [CrossRef] [PubMed]
- DeFranco, E.; Hall, E.; Hossain, M.; Chen, A.; Haynes, E.N.; Jones, D.; Ren, S.; Lu, L.; Muglia, L. Air pollution and stillbirth risk: Exposure to airborne particulate matter during pregnancy is associated with fetal death. PLoS ONE 2015, 10, e0120594. [Google Scholar] [CrossRef]
- Huang, C.Y.; Liu, C.L.; Chen, M.H.; Tsao, P.N.; Chen, C.Y.; Chou, H.C.; Chen, P.C. Maternal exposure to air pollution and the risk of small for gestational age in offspring: A population-based study in Taiwan. Pediatr. Neonatol. 2020, 61, 231–237. [Google Scholar] [CrossRef]
- Zhang, B.; Liang, S.; Zhao, J.; Qian, Z.; Bassig, B.A.; Yang, R.; Zhang, Y.; Hu, K.; Xu, S.; Zheng, T.; et al. Maternal exposure to air pollutant PM2. 5 and PM10 during pregnancy and risk of congenital heart defects. J. Expo. Sci. Environ. Epidemiol. 2016, 26, 422–427. [Google Scholar] [CrossRef]
- UN Sustainable Development, Goal 3, Ensure Healthy Lives and Promote Well-Being at All Ages. Available online: https://www.un.org/sustainabledevelopment/health (accessed on 16 April 2021).
- Arksey, H.; O’Malley, L. Scoping studies: Towards a methodological framework. Int. J. Soc. Res. Methodol. 2005, 8, 19–32. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.; Horsley, T.; Weeks, L.; et al. PRISMA extension for scoping reviews (PRISMA-ScR): Checklist and explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef]
- Gard, L.A.; Peterson, J.; Miller, C.; Ghosh, N.; Youmans, Q.; Didwania, A.; Persell, S.D.; Jean-Jacques, M.; Ravenna, P.; O’Brien, M.J.; et al. Social determinants of health training in US primary care residency programs: A scoping review. Acad. Med. 2019, 94, 135–143. [Google Scholar] [CrossRef]
- Matamala-Gomez, M.; Maisto, M.; Montana, J.I.; Mavrodiev, P.A.; Baglio, F.; Rossetto, F.; Mantovani, F.; Riva, G.; Realdon, O. The role of engagement in tele neurorehabilitation: A systematic review. Front. Neurol. 2020, 11, 354. [Google Scholar] [CrossRef]
- Liao, J.; Liu, Y.; Steenland, K.; Pillarisetti, A.; Thompson, L.M.; Dey, S.; Balakrishnan, K.; Clasen, T. Child Survival and Early Lifetime Exposures to Ambient Fine Particulate Matter in India: A Retrospective Cohort Study. Environ. Health Perspect. 2022, 130, 017009. [Google Scholar] [CrossRef] [PubMed]
- Egondi, T.; Ettarh, R.; Kyobutungi, C.; Ng, N.; Rocklöv, J. Exposure to outdoor particles (PM2. 5) and associated child morbidity and mortality in socially deprived neighborhoods of Nairobi, Kenya. Atmosphere 2018, 9, 351. [Google Scholar] [CrossRef]
- Lien, W.-H.; Owili, P.O.; Muga, M.A.; Lin, T.-H. Ambient Particulate Matter Exposure and Under-Five and Maternal Deaths in Asia. Int. J. Environ. Res. Public Health 2019, 16, 3855. [Google Scholar] [CrossRef]
- Owili, P.O.; Lin, T.H.; Muga, M.A.; Lien, W.H. Impacts of discriminated PM2. 5 on global under-five and maternal mortality. Sci. Rep. 2020, 10, 17654. [Google Scholar] [CrossRef] [PubMed]
- Son, J.Y.; Bell, M.L.; Lee, J.T. Survival analysis of long-term exposure to different sizes of airborne particulate matter and risk of infant mortality using a birth cohort in Seoul, Korea. Environ. Health Perspect. 2011, 119, 725–730. [Google Scholar] [CrossRef] [PubMed]
- Khadka, A.; Canning, D. Understanding the Pathways from Prenatal and Post-Birth PM2. 5 Exposure to Infant Death: An Observational Analysis Using US Vital Records (2011–2013). Int. J. Environ. Res. Public Health 2021, 19, 258. [Google Scholar] [CrossRef]
- Woodruff, T.J.; Parker, J.D.; Schoendorf, K.C. Fine particulate matter (PM2. 5) air pollution and selected causes of postneonatal infant mortality in California. Environ. Health Perspect. 2006, 114, 786–790. [Google Scholar] [CrossRef]
- Jung, E.M.; Kim, K.N.; Park, H.; Shin, H.H.; Kim, H.S.; Cho, S.J.; Kim, S.T.; Ha, E.H. Association between prenatal exposure to PM2. 5 and the increased risk of specified infant mortality in South Korea. Environ. Int. 2020, 144, 105997. [Google Scholar] [CrossRef]
- Heft-Neal, S.; Burney, J.; Bendavid, E.; Burke, M. Robust relationship between air quality and infant mortality in Africa. Nature 2018, 559, 254–258. [Google Scholar] [CrossRef]
- Goyal, N.; Karra, M.; Canning, D. Early-life exposure to ambient fine particulate air pollution and infant mortality: Pooled evidence from 43 low-and middle-income countries. Int. J. Epidemiol. 2019, 48, 1125–1141. [Google Scholar] [CrossRef]
- deSouza, P.N.; Dey, S.; Mwenda, K.M.; Kim, R.; Subramanian, S.V.; Kinney, P.L. Robust relationship between ambient air pollution and infant mortality in India. Sci. Total Environ. 2022, 815, 152755. [Google Scholar] [CrossRef] [PubMed]
- Bachwenkizi, J.; Liu, C.; Meng, X.; Zhang, L.; Wang, W.; van Donkelaar, A.; Martin, R.V.; Hammer, M.S.; Chen, R.; Kan, H. Fine particulate matter constituents and infant mortality in Africa: A multi country study. Environ. Int. 2021, 156, 106739. [Google Scholar] [CrossRef]
- Dagher, R.K.; Linares, D.E. A Critical Review on the Complex Interplay between Social Determinants of Health and Maternal and Infant Mortality. Children 2022, 9, 394. [Google Scholar] [CrossRef] [PubMed]
- Tarín-Carrasco, P.; Im, U.; Geels, C.; Palacios-Peña, L.; Jiménez-Guerrero, P. Reducing future air-pollution-related premature mortality over Europe by mitigating emissions from the energy sector: Assessing an 80% renewable energies scenario. Atmos. Chem. Phys. 2022, 22, 3945–3965. [Google Scholar] [CrossRef]
- Shi, L.; Zanobetti, A.; Kloog, I.; Coull, B.A.; Koutrakis, P.; Melly, S.J.; Schwartz, J.D. Low-concentration PM2. 5 and mortality: Estimating acute and chronic effects in a population-based study. Environ. Health Perspect. 2016, 124, 46–52. [Google Scholar] [CrossRef]
- Sohail, H.; Neupane, S. Prevalence of and factors associated with under-5 mortality in South Asia. Int. Health 2019, 11, 119–127. [Google Scholar] [CrossRef] [PubMed]
| Author and Year | Study Area, Country, and Study Period | Study Design and Sample Size | Health Outcome | Cause of Death | Adjusted Covariates | Statistical Model Used | Measures of Association and Selected Results of Percent Change in Excess Risk (95% CI) per 1 µg/m3 Increase in Particles (Unless Otherwise Stated) |
|---|---|---|---|---|---|---|---|
| Under-5 mortality | |||||||
| Liao et al., 2022 [36] | India, 2006–2016 | Cohort 259,627 live-birth children born 5 years preceding the survey from 175,865 women | Neonatal mortality, infant mortality, and child mortality | All-cause | Sex, birth month/year, birth order, location (hospital or not), age of mother, height, marital status, smoking, education level, passive/active smoking, location of house, area, cooking fuel, toilet facilities, wealth index, and monthly rainfall and temperature | Cox proportional hazards regression model | HR 0.13% [95% CI: 0.01%, 0.26%] for post-delivery lifetime exposure; 0.23% [95% CI: 0.09%, 0.38%] for in utero exposure |
| Egondi et al., 2018 [37] | Nairobi, Kenya, 2003–2013 | Cohort 21,641 children under age 5 | Under-5 mortality and morbidity | All-cause and respiratory | Sex, age, and socioeconomic status | Logistic and Poisson regression models | IRR Effect size was mentioned considering the exposure level binary (PM2.5 < 25 ≥ µg/m3): 22.00% [95%CI: 8.00%, 39.00%]; for all-cause: 12.00% [95%CI: −12.0%, 42%). |
| Lien et al., 2019 [38] | 45 countries in Asia by clustering in 5 regions, 2000–2015 | Ecological | Under-5 and maternal mortality | All-cause and respiratory | Country, year, total population, urban population, female population, employed population, HIV/AIDS-related death, TB death, undernourished population, and temperature | Generalized additive mixed-effects model | IRR 29.00% [95% (CI): 13.11%, 47.13%] for biomass PM2.5; 12.00% [95% (CI): 1.09%, 24.10%] for anthropogenic PM2.5. |
| Owili et al., 2020 [39] | Global, 2000–2015 | Ecological | Under-5 and maternal mortality | All-cause | Total number of undernourished, anemic pregnant women, tuberculosis cases, AIDS-related deaths, employed females, population in urban areas, year, country, and annual mean temperature | Generalized linear mixed-effects model | RR 8.90% [95% (CI): 4.11%, 13.91%] increase for biomass PM2.5 and 9.50% [95% (CI): −0.03%, 20.23%] for dust PM2.5 |
| Infant mortality | |||||||
| Son et al., 2011 [40] | Seoul, South Korea, 2004–2007 | Cohort 359,459 infants | Post-neonatal infant mortality | All-cause, respiratory, and SIDS | Sex, gestational length, season of birth, maternal age and educational level, and heat index | Cox proportional hazards model | HR 14.45% [95% (CI): 6.68%, 22.79%] increase in all-cause mortality for gestational exposure |
| Son et al., 2017 [22] | Massachusetts, USA, 2001–2007 | Cohort 465,682 infants | Post-neonatal infant mortality | All-cause, respiratory, and SIDS | Sex, birth weight, length of gestation, mother’s age, educational level, race/ethnicity, marital status, parity, and season of birth | Cox proportional hazards model | HR 112.24% [95% (CI): 71.72%, 162.32%] increase in all-cause infant mortality for post-birth lifetime exposure |
| Khadka et al., 2021 [41] | USA, 2011–2013 | Cohort 10,017,357 live births and 58,913 infant deaths | Infant mortality | All-cause | Individual level (age of parents, race, level of education, maternal smoking, marital status, and parity), county level (average temperature, precipitation, and unemployment), pregnancy covariates (method of payment for delivery, child sex, and multiple births), racial composition, poverty rate, median housing value, and number of physicians per 1000 persons | - | No effect size was mentioned. Prenatal PM2.5 exposure was positively associated with infant death in all trimesters; most significant relationship in third trimester; relationship between post-birth PM2.5 exposure and infant mortality was positive but less precisely estimated |
| Woodruff et al., 2006 [42] | California, USA, 1999–2001 | Matched case–control 3877 infants | Post-neonatal infant mortality | All-cause, respiratory, and SIDS | Maternal race, education, age, marital status, and parity; confounder: birth weight | Conditional logistic regression model | OR 7.00% [95% (CI): −7.33%, 23.55%] for all-cause mortality; 113.00% [95% (CI): −12.01%, 305.04%] for respiratory mortality |
| Jung et al., 2020 [43] | South Korea, 2010–2015 | Case–control 2,501,836 infants | Post-neonatal infant mortality | All-cause and respiratory | Maternal education, season of birth, birth weight (kg), gestational age (weeks), and region | Conditional logistic regression model | OR 2.58% [95% (CI): 0.70%, 4.50%] for infant mortality from gestational exposure 1.75% [95% (CI): 0.27%, 3.26%] and 1.92% [95% (CI): 0.0.42%, 3.45%] for exposure in 1st and 2nd trimesters, respectively |
| Heft-Neal et al., 2018 [44] | 30 countries in Sub-Saharan Africa, 2000–2015 | Cross-sectional | Infant mortality | All-cause | Child sex, birth order, age of mother, maternal education, type of cooking fuel, asset-based wealth index, temperature, and seasonal variability | Fixed-effects regression model | RR 0.87% [95% (CI): 0.40%, 1.33%] for infant mortality |
| Goyal et al., 2019 [45] | 43 low- and middle-income countries, 1998–2014 | Cross-sectional 534,476 children | Neonatal and post-neonatal mortality and infant mortality | All-cause and respiratory | Child-level (birth order, sex, multiple births, and birth interval), parent-level (age, smoking habit, and education of both parents), and household-level (location, cooking fuel, drinking water, improved sanitation, and wealth quantiles) characteristics | Multivariate logistic regression model | Odds Ratio (OR) 22.0% [95% (CI): 10.62%, 34.54%] increase in neonatal mortality and 13% [95% (CI): 3.90%, 22.89%] increase in infant mortality for anthropogenic PM2.5 |
| deSouza et al., 2021 [46] | India, 2015–2016 | Cross-sectional 259,627 children | Neonatal, post-neonatal, and infant mortality | All-cause | Child-level (sex and birth order), mother-level (age of marriage, education, age of giving birth, and smoking habit), and household-level (location, sanitation, fuel use, and safe drinking water access) covariates, including seasonality and long-term trend | Fixed-effects regression model | OR 0.16% [95% (CI): 0.03%, 0.30%] increase in neonatal mortality for exposure in 3rd trimester |
| Bachwenkizi et al., 2021 [47] | 15 countries in Africa, 2005–2015 | Cross-sectional 602,863 participants | Infant mortality | All-cause | Mother-level (age, education, and smoking habit), child-level (sex, birth order, vaccination record, and diarrhea), household-level (toilet facilities, drinking water, cooking fuel, and wealth index), and country-level (anemia, health expenditure, child stunting, temperature, and humidity) covariates | Multivariable logistic regression model | OR 3.00% [95% (CI): 0.54, 5.51%] for infant mortality |
| Characteristics | Frequency | |
|---|---|---|
| Study area | LMIC [38,39,45] | 3 |
| USA [22,41,42] | 3 | |
| South Korea [40,43] | 2 | |
| India [36,46] | 2 | |
| Africa [37,44,47] | 3 | |
| Study design | Cohort [22,36,37,40,41] | 5 |
| Cross-sectional [44,45,46,47] | 4 | |
| Case–control [42,43] | 2 | |
| Ecological [38,39] | 2 | |
| Outcomes | Under-5 mortality [36,37,38,39] | 4 |
| Post-neonatal mortality [22,40,42,43] | 4 | |
| Neonatal/post-neonatal/infant mortality [45,46] | 2 | |
| Infant mortality [41,44,47] | 3 | |
| Cause of death | All-cause mortality [22,36,37,38,39,40,41,42,43,44,45,46,47] | 13 |
| Respiratory mortality [22,37,40,42] | 4 | |
| Sudden infant death syndrome [22,40,42] | 3 |
| Author and Year | Exposure Assessment | Source of PM2.5 and Constituents | Exposure Window | |
|---|---|---|---|---|
| Post-Birth/Lifetime Exposure | In Utero/Prenatal and Post-Birth Exposure | |||
| Lien et al., 2019 [38] | Aerosol optical depth retrieved from MODIS | PM2.5 (biomass burning, anthropogenic pollutant, mineral dust, biomass/dust mixture, anthropogenic/dust mixture, and biomass/anthropogenic mixture) | ■ | |
| Owili et al., 2020 [39] | Aerosol optical depth retrieved from MODIS | PM2.5 (anthropogenic, biomass, and dust) | ■ | |
| Egondi et al., 2018 [37] | Ground-based monitoring station data | PM2.5 | ■ | |
| Goyal et al., 2019 [45] | High-resolution calibrated satellite data | Dust and sea salt from other carbonaceous and manmade PM | ○ | |
| Son et al., 2017 [22] | From PM2.5 prediction models based on satellite imagery | Ambient PM2.5 | ■ | |
| Son et al., 2011 [40] | Ground-based monitoring station data | PM2.5 among other pollutants | ○ | |
| Jung et al., 2020 [43] | CMAQ model | Ambient PM2.5 | ○ | |
| Woodruff et al., 2006 [42] | Ground-based monitoring station data | Ambient PM2.5 | ■ | |
| Liao et al., 2022 [36] | Combination of monitoring station data, satellite-based AOD data, and random forest model | Ambient PM2.5 | ○ | |
| Heft-Neal et al., 2018 [44] | Data from satellite-based estimates of PM2.5 | Ambient PM2.5 | ○ | |
| Khadka et al., 2021 [41] | Combination of ground-based monitoring and CMAQ model | Ambient PM2.5 | ○ | |
| deSouza et al., 2021 [46] | Satellite-derived PM2.5 concentrations | Ambient PM2.5 | ○ | |
| Bachwenkizi et al., 2021 [47] | Satellite-derived PM2.5 for mass and chemical transport model (GEOS-Chem) | Ambient PM2.5, including constituents such as organic matter (OM), black carbon (BC), sulfate, nitrate (NO3, NH4), and soil dust (DUST). | ■ | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anita, W.M.; Ueda, K.; Uttajug, A.; Seposo, X.T.; Takano, H. Association between Long-Term Ambient PM2.5 Exposure and under-5 Mortality: A Scoping Review. Int. J. Environ. Res. Public Health 2023, 20, 3270. https://doi.org/10.3390/ijerph20043270
Anita WM, Ueda K, Uttajug A, Seposo XT, Takano H. Association between Long-Term Ambient PM2.5 Exposure and under-5 Mortality: A Scoping Review. International Journal of Environmental Research and Public Health. 2023; 20(4):3270. https://doi.org/10.3390/ijerph20043270
Chicago/Turabian StyleAnita, Wahida Musarrat, Kayo Ueda, Athicha Uttajug, Xerxes Tesoro Seposo, and Hirohisa Takano. 2023. "Association between Long-Term Ambient PM2.5 Exposure and under-5 Mortality: A Scoping Review" International Journal of Environmental Research and Public Health 20, no. 4: 3270. https://doi.org/10.3390/ijerph20043270





