Lifestyle Interventions with Mind-Body or Stress-Management Practices for Cancer Survivors: A Rapid Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Selection Criteria
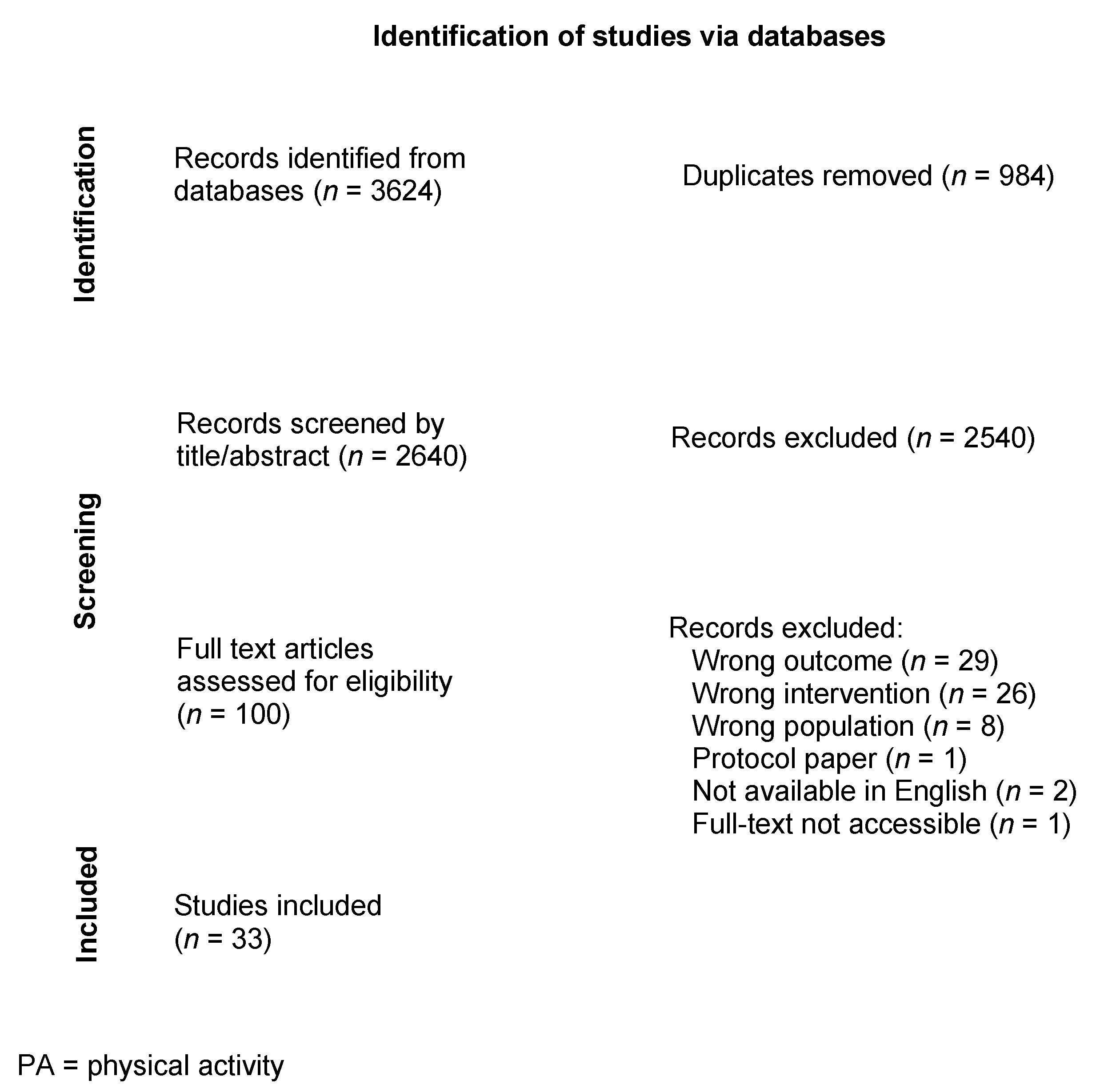
2.2. Search Strategy
2.3. Screening Process
2.4. Data Extraction
2.5. Study Quality Assessment
2.6. Data Synthesis
3. Results
3.1. Study Characteristics
3.2. Overview of Interventions
3.3. Outcomes of Interest
3.4. Significant Findings
3.5. Quality Assessment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.D.; Nogueira, L.; Devasia, T.; Mariotto, A.B.; Yabroff, K.R.; Jemal, A.; Kramer, J.; Siegel, R.L. Cancer treatment and survivorship statistics, 2022. CA Cancer J. Clin. 2022, 72, 409–436. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Deng, L.; Karr, M.A.; Wen, Y.; Wang, Q.; Perimbeti, S.; Shapiro, C.L.; Han, X. Chronic comorbid conditions among adult cancer survivors in the United States: Results from the National Health Interview Survey, 2002–2018. Cancer 2022, 128, 828–838. [Google Scholar] [CrossRef] [PubMed]
- Stull, V.B.; Snyder, D.C.; Demark-Wahnefried, W. Lifestyle interventions in cancer survivors: Designing programs that meet the needs of this vulnerable and growing population. J. Nutr. 2007, 137 (Suppl. S1), 243s–248s. [Google Scholar] [CrossRef]
- World Cancer Research Fund/American Institute for Cancer Research. Diet, nutrition, physical activity and cancer: A global perspective. In Continuous Update Project Expert Report; World Cancer Research Fund International: London, UK, 2018. [Google Scholar]
- Rock, C.L.; Doyle, C.; Demark-Wahnefried, W.; Meyerhardt, J.; Courneya, K.S.; Schwartz, A.L.; Bandera, E.V.; Hamilton, K.K.; Grant, B.; McCullough, M.; et al. Nutrition and physical activity guidelines for cancer survivors. CA Cancer J. Clin. 2012, 62, 243–274. [Google Scholar] [CrossRef] [PubMed]
- Kohler, L.N.; Garcia, D.O.; Harris, R.B.; Oren, E.; Roe, D.J.; Jacobs, E.T. Adherence to Diet and Physical Activity Cancer Prevention Guidelines and Cancer Outcomes: A Systematic Review. Cancer Epidemiol. Biomark. Prev. 2016, 25, 1018–1028. [Google Scholar] [CrossRef]
- Yi, J.C.; Syrjala, K.L. Anxiety and Depression in Cancer Survivors. Med. Clin. N. Am. 2017, 101, 1099–1113. [Google Scholar] [CrossRef] [PubMed]
- Torres, S.J.; Nowson, C.A. Relationship between stress, eating behavior, and obesity. Nutrition 2007, 23, 887–894. [Google Scholar] [CrossRef]
- Adam, T.C.; Epel, E.S. Stress, eating and the reward system. Physiol. Behav. 2007, 91, 449–458. [Google Scholar] [CrossRef]
- Stults-Kolehmainen, M.A.; Sinha, R. The effects of stress on physical activity and exercise. Sports Med. 2014, 44, 81–121. [Google Scholar] [CrossRef] [Green Version]
- Francesco, F.; Alice, N.; Filippo, G.; Daniela, R.; Enrico, B.; Emanuela, A. Stress Axis in the Cancer Patient: Clinical Aspects and Management. Endocrines 2021, 2, 502–513. [Google Scholar] [CrossRef]
- Carlson, L.E.; Toivonen, K.; Subnis, U. Integrative Approaches to Stress Management. Cancer J. 2019, 25, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Monti, D.A.; Sufian, M.; Peterson, C. Potential role of mind-body therapies in cancer survivorship. Cancer 2008, 112 (Suppl. S11), 2607–2616. [Google Scholar] [CrossRef] [PubMed]
- Subnis, U.B.; Starkweather, A.R.; McCain, N.L.; Brown, R.F. Psychosocial therapies for patients with cancer: A current review of interventions using psychoneuroimmunology-based outcome measures. Integr. Cancer Ther. 2014, 13, 85–104. [Google Scholar] [CrossRef] [PubMed]
- Duncan, M.; Moschopoulou, E.; Herrington, E.; Deane, J.; Roylance, R.; Jones, L.; Bourke, L.; Morgan, A.; Chalder, T.; Thaha, M.A.; et al. Review of systematic reviews of non-pharmacological interventions to improve quality of life in cancer survivors. BMJ Open 2017, 7, e015860. [Google Scholar] [CrossRef]
- Sierpina, V.; Moschopoulou, E.; Herrington, E.; Deane, J.; Roylance, R.; Jones, L.; Bourke, L.; Morgan, A.; Chalder, T.; Thaha, M.; et al. Nutrition, metabolism, and integrative approaches in cancer survivors. Semin Oncol. Nurs. 2015, 31, 42–52. [Google Scholar] [CrossRef]
- Rabin, C.; Pinto, B.; Fava, J. Randomized Trial of a Physical Activity and Meditation Intervention for Young Adult Cancer Survivors. J. Adolesc. Young Adult Oncol. 2016, 5, 41–47. [Google Scholar] [CrossRef]
- Adamsen, L.; Quist, M.; Andersen, C.; Møller, T.; Herrstedt, J.; Kronborg, D.; Baadsgaard, M.; Vistisen, K.; Midtgaard, J.; Christiansen, B.; et al. Effect of a multimodal high intensity exercise intervention in cancer patients undergoing chemotherapy: Randomised controlled trial. BMJ 2009, 339, b3410. [Google Scholar] [CrossRef]
- Garritty, C.; Gartlehner, G.; Nussbaumer-Streit, B.; King, V.J.; Hamel, C.; Kamel, C.; Affengruber, L.; Stevens, A. Cochrane Rapid Reviews Methods Group offers evidence-informed guidance to conduct rapid reviews. J. Clin. Epidemiol. 2021, 130, 13–22. [Google Scholar] [CrossRef]
- National Center for Complementary and Integrative Health. Mind and Body Practices. 2017. Available online: https://www.nccih.nih.gov/health/mind-and-body-practices (accessed on 11 February 2022).
- Scells, H.; Zuccon, G. Searchrefiner: A Query Visualisation and Understanding Tool for Systematic Reviews. In Proceedings of the 27th ACM International Conference on Information and Knowledge Management, Torino, Italy, 22–26 October 2018. [Google Scholar]
- National Heart, Lung, and Blood Institute. Study Quality Assessment Tools. 2021. Available online: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (accessed on 11 February 2022).
- Saxe, G.A.; Hébert, J.R.; Carmody, J.F.; Kabat-Zinn, J.; Rosenzweig, P.H.; Jarzobski, D.; Reed, G.W.; Blute, R.D. Can diet in conjunction with stress reduction affect the rate of increase in prostate specific antigen after biochemical recurrence of prostate cancer? J. Urol. 2001, 166, 2202–2207. [Google Scholar] [CrossRef]
- Andersen, B.L.; Farrar, W.B.; Golden-Kreutz, D.M.; Glaser, R.; Emery, C.F.; Crespin, T.R.; Shapiro, C.L.; Carson, W.E., III. Psychological, behavioral, and immune changes after a psychological intervention: A clinical trial. J. Clin. Oncol. 2004, 22, 3570–3580. [Google Scholar] [CrossRef]
- Rabin, C.; Pinto, B.; Dunsiger, S.; Nash, J.; Trask, P. Exercise and relaxation intervention for breast cancer survivors: Feasibility, acceptability and effects. Psychooncology 2009, 18, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Carmody, J.F.; Olendzki, B.C.; Merriam, P.A.; Liu, Q.; Qiao, Y.; Ma, Y. A novel measure of dietary change in a prostate cancer dietary program incorporating mindfulness training. J. Acad. Nutr. Diet 2012, 112, 1822–1827. [Google Scholar] [CrossRef]
- Hébert, J.R.; Hurley, T.G.; Harmon, B.E.; Heiney, S.; Hebert, C.J.; Steck, S.E. A diet, physical activity, and stress reduction intervention in men with rising prostate-specific antigen after treatment for prostate cancer. Cancer Epidemiol. 2012, 36, e128–e136. [Google Scholar] [CrossRef]
- Garrett, K.; Okuyama, S.; Jones, W.; Barnes, D.; Tran, Z.; Spencer, L.; Lewis, K.; Maroni, P.; Chesney, M.; Marcus, A. Bridging the transition from cancer patient to survivor: Pilot study results of the Cancer Survivor Telephone Education and Personal Support (C-STEPS) program. Patient Educ. Couns. 2013, 92, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, P.B.; Phillips, K.M.; Jim, H.S.L.; Small, B.J.; Faul, L.A.; Meade, C.D.; Thompson, L.; Williams, C.C.; Loftus, L.S.; Fishman, M.; et al. Effects of self-directed stress management training and home-based exercise on quality of life in cancer patients receiving chemotherapy: A randomized controlled trial. Psychooncology 2013, 22, 1229–1235. [Google Scholar] [CrossRef]
- Lucas, A.R.; Focht, B.C.; Cohn, D.E.; Buckworth, J.; Klatt, M.D. A Mindfulness-Based Lifestyle Intervention for Obese, Inactive Endometrial Cancer Survivors: A Feasibility Study. Integr. Cancer Ther. 2017, 16, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Schneeberger, D.; Golubíc, M.; Moore, H.C.; Weiss, K.; Abraham, J.; Montero, A.; Doyle, J.; Sumego, M.; Roizen, M. Lifestyle Medicine-Focused Shared Medical Appointments to Improve Risk Factors for Chronic Diseases and Quality of Life in Breast Cancer Survivors. J. Altern. Complement. Med. 2019, 25, 40–47. [Google Scholar] [CrossRef]
- Thomas, E.A.; Mijangos, J.L.; Hansen, P.A.; White, S.; Walker, D.; Reimers, C.; Beck, A.C.; Garland, E.L. Mindfulness-Oriented Recovery Enhancement Restructures Reward Processing and Promotes Interoceptive Awareness in Overweight Cancer Survivors: Mechanistic Results From a Stage 1 Randomized Controlled Trial. Integr. Cancer Ther. 2019, 18, 1534735419855138. [Google Scholar] [CrossRef]
- Midtgaard, J.; Rørth, M.; Stelter, R.; Tveterås, A.; Andersen, C.; Quist, M.; Møller, T.; Adamsen, L. The impact of a multidimensional exercise program on self-reported anxiety and depression in cancer patients undergoing chemotherapy: A phase II study. Palliat. Support. Care 2005, 3, 197–208. [Google Scholar] [CrossRef]
- Adamsen, L.; Quist, M.; Midtgaard, J.; Andersen, C.; Møller, T.; Knutsen, L.; Tveterås, A.; Rorth, M. The effect of a multidimensional exercise intervention on physical capacity, well-being and quality of life in cancer patients undergoing chemotherapy. Support. Care Cancer 2006, 14, 116–127. [Google Scholar] [CrossRef]
- Midtgaard, J.; Tveterå, A.; Rørth, M.; Stelter, R.; Adamsen, L. The impact of supervised exercise intervention on short-term postprogram leisure time physical activity level in cancer patients undergoing chemotherapy: 1- and 3-month follow-up on the body & cancer project. Palliat. Support. Care 2006, 4, 25–35. [Google Scholar] [PubMed]
- Quist, M.; Rorth, M.; Zacho, M.; Andersen, C.; Moeller, T.; Midtgaard, J.; Adamsen, L. High-intensity resistance and cardiovascular training improve physical capacity in cancer patients undergoing chemotherapy. Scand. J. Med. Sci. Sports 2006, 16, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Quist, M.; Rørth, M.; Langer, S.; Jones, L.W.; Laursen, J.H.; Pappot, H.; Christensen, K.B.; Adamsen, L. Safety and feasibility of a combined exercise intervention for inoperable lung cancer patients undergoing chemotherapy: A pilot study. Lung Cancer 2012, 75, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Minnella, E.M.; Awasthi, R.; Bousquet-Dion, G.; Ferreira, V.; Austin, B.; Audi, C.; Tanguay, S.; Aprikian, A.; Carli, F.; Kassouf, W. Multimodal Prehabilitation to Enhance Functional Capacity Following Radical Cystectomy: A Randomized Controlled Trial. Eur. Urol. Focus 2021, 7, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Fillion, L.; Gagnon, P.; Leblond, F.; Gélinas, C.; Savard, J.; Dupuis, R.; Duval, K.; Larochelle, M. A brief intervention for fatigue management in breast cancer survivors. Cancer Nurs. 2008, 31, 145–159. [Google Scholar] [CrossRef]
- Awasthi, R.; Minnella, E.M.; Ferreira, V.; Ramanakumar, A.V.; Scheede-Bergdahl, C.; Carli, F. Supervised exercise training with multimodal pre-habilitation leads to earlier functional recovery following colorectal cancer resection. Acta Anaesthesiol Scand 2019, 63, 461–467. [Google Scholar] [CrossRef]
- Barrett-Bernstein, M.; Carli, F.; Gamsa, A.; Scheede-Bergdahl, C.; Minnella, E.; Ramanakumar, A.V.; Tourian, L. Depression and functional status in colorectal cancer patients awaiting surgery: Impact of a multimodal prehabilitation program. Health Psychol. 2019, 38, 900–909. [Google Scholar] [CrossRef]
- Gillis, C.; Fenton, T.R.; Sajobi, T.T.; Minnella, E.M.; Awasthi, R.; Loiselle, S.-È.; Liberman, S.; Stein, B.; Charlebois, P.; Carli, F. Trimodal prehabilitation for colorectal surgery attenuates post-surgical losses in lean body mass: A pooled analysis of randomized controlled trials. Clin. Nutr. 2019, 38, 1053–1060. [Google Scholar] [CrossRef]
- Cheng, C.; Ho, R.T.H.; Guo, Y.; Zhu, M.; Yang, W.; Li, Y.; Liu, Z.; Zhuo, S.; Liang, Q.; Chen, Z.; et al. Development and Feasibility of a Mobile Health-Supported Comprehensive Intervention Model (CIMmH) for Improving the Quality of Life of Patients With Esophageal Cancer After Esophagectomy: Prospective, Single-Arm, Nonrandomized Pilot Study. J. Med. Internet Res. 2020, 22, e18946. [Google Scholar] [CrossRef]
- Lu, T.; Denehy, L.; Cao, Y.; Cong, Q.; Wu, E.; Granger, C.L.; Ni, J.; Edbrooke, L. A 12-Week Multi-Modal Exercise Program: Feasibility of Combined Exercise and Simplified 8-Style Tai Chi Following Lung Cancer Surgery. Integr. Cancer Ther. 2020, 19, 1534735420952887. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.C.; Zhao, Y.; Liu, X.; Cao, H.-P.; Wang, Y.-Q.; Yin, Y.-Y.; Xie, J. Multimodal Exercise Program: A Pilot Randomized Trial for Patients with Lung Cancer Receiving Surgical Treatment. Clin. J. Oncol. Nurs. 2021, 25, e26–e34. [Google Scholar] [CrossRef] [PubMed]
- Hwang, K.H.; Cho, O.; Yoo, Y. The Effect of Comprehensive Care Program for Ovarian Cancer Survivors. Clin. Nurs. Res. 2016, 25, 192–208. [Google Scholar] [CrossRef] [PubMed]
- Yun, Y.H.; Kim, Y.A.; Lee, M.K.; Sim, J.A.; Nam, B.-H.; Kim, S.; Lee, E.S.; Noh, D.-Y.; Lim, J.-Y.; Kim, S.; et al. A randomized controlled trial of physical activity, dietary habit, and distress management with the Leadership and Coaching for Health (LEACH) program for disease-free cancer survivors. BMC Cancer 2017, 17, 298. [Google Scholar] [CrossRef]
- Vardar Yağlı, N.; Şener, G.; Arıkan, H.; Sağlam, M.; Ince, D.I.; Savcı, S.; Kutukcu, E.Ç.; Altundağ, K.; Kaya, E.B.; Kutluk, T.; et al. Do yoga and aerobic exercise training have impact on functional capacity, fatigue, peripheral muscle strength, and quality of life in breast cancer survivors? Integr. Cancer Ther. 2015, 14, 125–132. [Google Scholar] [CrossRef]
- Felser, S.; Behrens, M.; Liese, J.; Strueder, D.F.; Rhode, K.; Junghanss, C.; Grosse-Thie, C. Feasibility and Effects of a Supervised Exercise Program Suitable for Independent Training at Home on Physical Function and Quality of Life in Head and Neck Cancer Patients: A Pilot Study. Integr. Cancer Ther. 2020, 19, 1534735420918935. [Google Scholar] [CrossRef]
- Fournié, C.; Verkindt, C.; Dalleau, G.; Bouscaren, N.; Mohr, C.; Zunic, P.; Cabrera, Q. Rehabilitation program combining physical exercise and heart rate variability biofeedback in hematologic patients: A feasibility study. Support. Care Cancer 2022, 30, 2009–2016. [Google Scholar] [CrossRef]
- Beumeler, L.F.E.; Waarsenburg, E.C.; Booij, S.H.; Scheurink, A.J.W.; Hoender, H.J.R. Evaluation of a lifestyle intervention program in primary care on physical and mental health and quality of life of cancer survivors: A pilot study. Eur. J. Integr. Med. 2018, 23, 1–5. [Google Scholar] [CrossRef]
- Lacey, J.; Lomax, A.J.; McNeil, C.; Marthick, M.; Levy, D.; Kao, S.; Nielsen, T.; Dhillon, H.M. A supportive care intervention for people with metastatic melanoma being treated with immunotherapy: A pilot study assessing feasibility, perceived benefit, and acceptability. Support. Care Cancer 2019, 27, 1497–1507. [Google Scholar] [CrossRef]
- Ruiz-Vozmediano, J.; Löhnchen, S.; Jurado, L.; Recio, R.; Rodríguez-Carrillo, A.; López, M.; Mustieles, V.; Expósito, M.; Arroyo-Morales, M.; Fernández, M.F. Influence of a Multidisciplinary Program of Diet, Exercise, and Mindfulness on the Quality of Life of Stage IIA-IIB Breast Cancer Survivors. Integr. Cancer Ther. 2020, 19, 1534735420924757. [Google Scholar] [CrossRef]
- McDonnell, G.A.; Salley, C.G.; Barnett, M.; DeRosa, A.P.; Werk, R.S.; Hourani, A.; Hoekstra, A.B.; Ford, J.S. Anxiety Among Adolescent Survivors of Pediatric Cancer. J. Adolesc. Health 2017, 61, 409–423. [Google Scholar] [CrossRef] [PubMed]
- Crowder, S.L.; Buro, A.; Stern, M. Physical activity interventions in pediatric, adolescent, and young adult cancer survivors: A systematic review. Support. Care Cancer 2022, 30, 4635–4649. [Google Scholar] [CrossRef] [PubMed]
- Pugh, G.; Gravestock, H.L.; Hough, R.E.; King, W.M.; Wardle, J.; Fisher, A. Health Behavior Change Interventions for Teenage and Young Adult Cancer Survivors: A Systematic Review. J. Adolesc. Young Adult Oncol. 2016, 5, 91–105. [Google Scholar] [CrossRef]
- McClung, H.L.; Ptomey, L.T.; Shook, R.P.; Aggarwal, A.; Gorczyca, A.M.; Sazonov, E.S.; Becofsky, K.; Weiss, R.; Das, S.K. Dietary Intake and Physical Activity Assessment: Current Tools, Techniques, and Technologies for Use in Adult Populations. Am. J. Prev. Med. 2018, 55, e93–e104. [Google Scholar] [CrossRef] [PubMed]
- Carlsen, M.H.; Lillegaard, I.T.; Karlsen, A.; Blomhoff, R.; Drevon, C.A.; Andersen, L.F. Evaluation of energy and dietary intake estimates from a food frequency questionnaire using independent energy expenditure measurement and weighed food records. Nutr. J. 2010, 9, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Study | Study Design | Country | Sample Size | Participant Age (Range or Mean) | Participant Race/Ethnicity | Participant Cancer Type | Participant Time Since Treatment |
|---|---|---|---|---|---|---|---|
| Saxe, 2001 [25] | PPI | USA | 10 | 61–78 (mean 67) | NR | Prostate | ≥6 months post-treatment |
| Andersen, 2004 [26] | RCT | USA | 227 | 20–85 | 90% White | Breast | Pre-adjuvant therapy |
| Midtgaard, 2005 [35] | PPI | Denmark | 91 | 18–65 (mean 43) | NR | Non-specific | Undergoing cytostatic treatment |
| Adamsen, 2006 [36] | PPI | Denmark | 82 | 18–63 | NR | Non-specific | ≥1-month post-diagnosis, admitted for outpatient chemotherapy |
| Midtgaard, 2006 [37] | PPI | Denmark | 61 | 18–65 (mean 43) | NR | Non-specific | Undergoing cytostatic treatment |
| Quist, 2006 [38] | PPI | Denmark | 70 | 18–65 (mean 43) | NR | Non-specific | ≥1-month post-diagnosis, admitted for outpatient chemotherapy |
| Fillion, 2008 [41] | RCT | Canada | 87 | Mean 52 | NR | Breast | ≤2 years post-initial treatment |
| Adamsen, 2009 [20] | RCT | Denmark | 269 | 20–65 (mean 47) | NR | Non-specific | Undergoing adjuvant chemotherapy or treatment |
| Rabin, 2009 [27] | PPI | USA | 23 | 21+ (mean 53) | 96% non-Hispanic White, 4% Hispanic White | Breast | Post-treatment |
| Carmody, 2012 [28] | RCT | USA | 36 | 69 | 91% non-Hispanic White | Prostate | Post-primary treatment |
| Hébert, 2012 [29] | RCT | USA | 47 | 70–71 a | 70% White, 30% Black or African American | Prostate | Post-treatment |
| Quist, 2012 [39] | PPI | Denmark | 29 | 45–80 (mean 63) | NR | Lung | Undergoing chemotherapy |
| Garrett, 2013 [30] | PPI | USA | 46 | 22+ (mean 60) | 89% White | Non-specific | ≥1-year post-treatment |
| Jacobsen, 2013 [31] | RCT | USA | 286 | Mean 57–58 a | ≥90% White and ≥83% non-Hispanic per group | Non-specific | Scheduled to receive chemotherapy |
| Vardar Yağlı, 2015 [50] | RCT | Turkey | 52 (statistical analyses on 40) | 20–60 (mean 47–50 a) | NR | Breast | ≥3 years post-treatment |
| Hwang, 2016 [48] | PPI | South Korea | 40 | 30+ | NR | Ovarian | 6 months–3 years in remission |
| Rabin, 2016 [19] | RCT | USA | 35 | 18–39 (mean 34) | 74% White | Non-specific | Post-treatment |
| Lucas, 2017 [32] | PPI | USA | 17 | 45–70 (mean 61) | 88% White | Endometrial | Post-treatment |
| Yun, 2017 [49] | RCT | South Korea | 248 | 20+ (mean 51) | NR | Non-specific | <2 years post-treatment |
| Beumeler, 2018 [53] | PPI | Netherlands | 9 | 34–74 | NR | Non-specific | ≥6 months post-diagnosis, stable long-term |
| Awasthi, 2019 [42] | P | Canada | 150 | Mean 66–70 a | NR | Colorectal | Scheduled for colorectal resection of localized cancer |
| Barrett-Bernstein, 2019 [43] | P | Canada | 172 | 18+ (mean 67) | NR | Colorectal | Awaiting resection |
| Gillis, 2019 [44] | P | Canada | 139 | 18+ (mean 66–69 a) | NR | Colorectal | Awaiting resection |
| Lacey, 2019 [54] | PPI | Australia | 28 | 42–85 (median 66) | NR | Melanoma | Undergoing standard treatment with pembrolizumab |
| Schneeberger, 2019 [33] | PPI | USA | 21 | 41–73 (mean 56) | NR | Breast | Post-treatment |
| Thomas, 2019 [34] | RCT | USA | 51 | 29–76 (mean 58) | 96% White | Non-specific | Active or in remission |
| Cheng, 2020 [45] | PPI | China | 20 | 18–75 (mean 62) | NR | Esophageal | Scheduled for esophageal radical resection |
| Felser, 2020 [51] | PPI | Germany | 12 | 52–81 (mean 68) | NR | Head and neck | Post-treatment |
| Lu, 2020 [46] | PPI | China | 16 | 44–63 | NR | Lung | 6–12 weeks post-treatment |
| Ruiz-Vozmediano, 2020 [55] | RCT | Spain | 63 | 18+ (mean 48–51a) | NR | Breast | >1-year post-treatment |
| Ma, 2021 [47] | 3-arm RCT | China | 101 | 37–72, (mean 55–58a) | NR | Lung | Scheduled for thoracoscopic surgery |
| Minnella, 2021 [40] | RCT | Canada | 70 | Mean 70 (intervention), 66 (control) | NR | Bladder | Scheduled for elective radical cystectomy |
| Fournié, 2022 [52] | PPI | France | 17 | 54.5 | NR | Hematological malignancies | ≤6 months post-treatment remission |
| Study | Diet | Physical Activity | Mind-Body | Length of Intervention |
|---|---|---|---|---|
| Diet & Mind-Body | ||||
| Saxe, 2001 [25] | Individual dietary counseling, instruction, and practice in preparing dishes | N/A | Mindfulness meditation training, yoga, social support | 12 weeks |
| Carmody, 2012 [28] | Dietary and cooking classes | N/A | Mindfulness training (e.g., sitting meditation, stretching, mindful awareness) | 11 weeks |
| Lucas, 2017 [32] | Dietary counseling | N/A | Mindfulness-based intervention emphasizing mindful yoga | 8 weeks (+6 weeks at home) |
| PA & Mind-Body | ||||
| Midtgaard, 2005 [35]; Adamsen, 2006 [36]; Midtgaard, 2006 [37]; Quist, 2006 [38]; Adamsen, 2009 [20]; Quist, 2012 [39] | N/A | Strength training, cardiovascular exercise | Relaxation, body awareness, massage | 6 weeks |
| Fillion, 2008 [41] | N/A | Supervised walking training, personalized walking program | Psycho-educative fatigue management sessions (e.g., relaxation, self-regulation) | 4 weeks |
| Rabin, 2009 [27] | N/A | Walking | Progressive muscle relaxation | 12 weeks |
| Jacobsen, 2013 [31] | N/A | Guidance and materials on paced breathing, progressive muscle relaxation with guided imagery, and coping self-statements to manage stress | Guidance and materials on regular exercise with an emphasis on walking | One-time meeting + materials with follow-up at 6-and 12-weeks post-intervention |
| Vardar Yağlı, 2015 [50] | N/A | Treadmill aerobic exercise | Yoga (asanas, pranayama, awareness meditation, and relaxation techniques) | 6 weeks |
| Hwang, 2016 [48] | N/A | Exercise instruction | Relaxation therapy instruction | 8 weeks |
| Rabin, 2016 [19] | N/A | Guidance on increasing moderate-intensity aerobic exercise | Meditation CD (sitting meditation, body scan, yoga stretches) | 12 weeks |
| Felser, 2020 [51] | N/A | Warm-up/mobilization, coordination exercises, resistance exercises, stretching | Relaxation exercises (e.g., progressive muscle relaxation) | 12 weeks |
| Lu, 2020 [46] | N/A | Brisk walking and resistance exercises with major limb movements | Tai chi adapted to 8 simple styles for strength, mobility, and range of motion | 12 weeks |
| Ma, 2021 [47] | N/A | Brisk walking on a treadmill before surgery and climbing stairs after surgery | Inspiratory muscle training and incentive deep-breathing exercise | 2 weeks |
| Fournié, 2022 [52] | N/A | Aerobic and resistance training at moderate intensity | Heart rate variability biofeedback | 12 weeks |
| Diet, PA, & Mind-Body | ||||
| Andersen, 2004 [26] | Sessions on health behaviors (diet, PA, smoking) | Sessions on health behaviors (diet, PA, smoking) | Sessions on stress and QOL | 18 weeks |
| Hébert, 2012 [29] | Food-related goal setting, cooking | Individualized PA goal setting | MBSR | 6 months |
| Garrett, 2013 [30] | Telephone counseling | Telephone counseling | Telephone counseling on uncertainty and stress management | 3 months |
| Yun, 2017 [49] | Health education workshop (PA, diet, and distress management) and telecoaching | Health education workshop (PA, diet, and distress management) and telecoaching | Health education workshop (PA, diet, and distress management) and telecoaching | 6 months (follow-up at 12 months) |
| Beumeler, 2018 [53] | Dietitian and group sessions | Physical therapy sessions | Mind-body therapy led by physical therapists | 12 months |
| Awasthi, 2019 [42]; Barrett-Bernstein, 2019 [43]; Gillis, 2019 [44] | Nutritional counseling with whey protein supplementation | Aerobic and resistance exercise | Anxiety reduction strategies (e.g., diaphragmatic breathing) | 4 weeks before surgery and 8 weeks after |
| Lacey, 2019 [54] | Tailored dietary changes | Exercise classes and activity monitoring | Complementary therapies (massage, reflexology yoga, qigong, mindfulness meditation, or acupuncture) | 8 weeks |
| Schneeberger, 2019 [33] | One dietitian-led educational visit and two culinary medicine visits in a teaching kitchen | PA instructions (i.e., on aerobic and strength exercises) incorporated into each visit | One visit on stress relief (e.g., guided imagery, mindful sitting and eating) and one visit on yoga poses and breathing | 14 weeks |
| Thomas, 2019 [34] | Nutritional counseling sessions | Group exercise sessions | Training in mindfulness, reappraisal, and savoring | 10 weeks |
| Cheng, 2020 [45] | Nutrition guidance for total and partial enteral nutrition and oral nutrition periods | Inspiratory muscle training, walking | MBCR courses, Baduanjin Qigong (defined as physical activity) | 12 weeks |
| Ruiz-Vozmediano, 2020 [55] | Workshops on healthy eating | Classes with circuits and stretching, as well as dance classes | MBSR sessions focused on meditation and yoga | 6 months |
| Minnella, 2021 [40] | Dietitian-created food-based intervention with personalized energy and macronutrient requirements | Individualized, home-based, moderate-intensity aerobic and resistance training | Psychosocial counseling session with training on relaxation and imagery techniques, including passive breathing exercise, meditation skills, and guided imagery | Not reported |
| Study | Diet | PA | Psychological or QOL |
|---|---|---|---|
| Diet & Mind-Body | |||
| Saxe, 2001 [25] | 7-Day Dietary Recall; no statistical tests conducted | Ancillary questions on the 7-Day Dietary Recall; no statistical tests conducted | None |
| Carmody, 2012 [28] | 24-h recall; decreased animal:vegetable protein ratio (p = 0.01) | N/A | Reported elsewhere |
| Lucas, 2017 [32] | FHQ; intervention completers analysis showed small to moderate effect size for change in fruit and vegetable intake (d = 0.23) | Accelerometer, PAQ, SPPB; intervention completers analysis showed moderate effect for MVPA (d = 0.45) | FFMQ, MAAS, SWLS, SF-36; Intervention completers analysis showed improved mindfulness (p = 0.0039) |
| PA & Mind-Body | |||
| Midtgaard, 2005 [35] | N/A | VO₂ max; improved (p < 0.001) | HADS; anxiety (p = 0.001) and depression (p = 0.042) reduced |
| Adamsen, 2006 [36] | N/A | 1RM and VO₂ max; improved muscular strength (p < 0.001), physical fitness (p < 0.001) and PA levels (p < 0.001) | EORTC QLQ-C30, SF-36; improved physical functioning (p < 0.001) and role functioning (p < 0.001) |
| Midtgaard, 2006 [37] | N/A | Interview and quantitative assessment for leisure time PA, developed by Saltin and Grimby (1968); PA significantly improved at 1 and 3 months post-intervention (p < 0.0001) | HADS; depression was significantly reduced (p = 0.0016) |
| Quist, 2006 [38] | N/A | 1RM and VO₂ max; increased muscular strength, aerobic fitness, and VO₂ max (p < 0.001) | None |
| Fillion, 2008 [41] | N/A | VO₂ submax, accelerometer; no significant differences | POMS, SF-12; improved emotional distress (d = 0.33) and quality of life (d = 0.34) |
| Adamsen, 2009 [20] | Questionnaire by Aadahl and Jørgensen (2003), 1RM and VO₂ max; maximum oxygen consumption and muscular strength improved (p < 0.0001) | EORTC QLQ-C30, SF-36; improved fatigue (d = 0.33), vitality (d = 0.55) physical functioning (d = 0.37), role physical (d = 0.37), role emotional (d = 0.32), and mental health (d = 0.28) scores | |
| Rabin, 2009 [27] | N/A | PAR, accelerometer; increased moderate- intensity activity (p < 0.0001) and energy expenditure (p < 0.001) | POMS; reduced tension/anxiety and overall mood disturbance (p < 0.05) |
| Quist, 2012 [39] | N/A | VO₂ max, 6MWT, 1RM; improved VO₂ max (p = 0.014), 6MWT distance (p = 0.006), muscular strength (p < 0.05) | FACT-L; emotional well-being improved (p = 0.03, d = 0.38) |
| Jacobsen, 2013 [31] | N/A | GSLTPAQ; improved (p = 0.022) | SF-36, CES-D, 21-item BAI, SRC; decreased depression (p = 0.019), anxiety (p = 0.049), and stress (p = 0.013) |
| Vardar Yağlı, 2015 [50] | N/A | 6MWT, hand-held dynamometer; improved 6MWT distance and peripheral muscular strength (p < 0.05) | EORTC QOL-C30; QOL improved (p < 0.05) |
| Hwang, 2016 [48] | N/A | 12-min walk, chair-stand test; improved walking distance (p = 0.005) and muscular strength (p = 0.001) | FACT-G; physical, social/familial, emotional, and functional well-being subscales (p ≤ 004) |
| Rabin, 2016 [19] | N/A | PAR, one-mile walk test, accelerometer; moderate-intensity PA (p = 0.002) and cardiovascular fitness (p = 0.008) | POMS; no significant change in overall mood disturbance |
| Felser, 2020 [51] | N/A | 6MWT, SPPB, ROM, stand and reach; improved head rotation (p = 0.042), walking distance (p = 0.010), and SPPB score (p = 0.031) | EORTC QLQ-30; overall difference not significant; positive changes in physical function (p = 0.008), cognitive function (p = 0.015), and social function scales (p = 0.031) |
| Lu, 2020 [46] | N/A | 6MWT, SPPB, electronic pedometer, FEV; improved FEV (p = 0.02) | SAS, SDS, EORTC QLQ-C30; improved emotional functioning (p = 0.012), SAS (p = 0.002), and SDS (p = 0.02) scores |
| Ma, 2021 [47] | N/A | 6MWT; improved exercise capacity (p = 0.028) | HADS; depression reduced (p = 0.002) |
| Fournié, 2022 [52] | N/A | 6MWT, 50-foot walk test, grip force test, single limb stance test, toe touch and back scratch test; improved 6MWT and 50-foot walk test (p < 0.001), muscular strength (p ≤ 0.01), flexibility (p ≤ 0.01) | MFI-20; no significant differences |
| Diet, PA, & Mind-Body | |||
| Andersen, 2004 [26] | FHQ; improved dietary patterns (p = 0.03) | PAR; no significant differences | POMS, IES; Total Mood Disturbance (p = 0.04) and anxious moods (p = 0.04) decreased |
| Hébert, 2012 [29] | 24-h recall; decreased saturated fatty acid (p < 0.0001), total energy (p = 0.01), and total fat (p = 0.02) intake | CHAMPS; increased PA at 3 (p = 0.009) and 6 months (p = 0.08) | None |
| Garrett, 2013 [30] | Two questions from the Block FFQ; fruit and vegetable consumption increased (p = 0.02) | Standardized questions from the BRFSS; PA increased (p = 0.006, p = 0.01) | IES; cancer-specific distress decreased (p < 0.001) |
| Yun, 2017 [49] | Validated questions based on the “Rules for National Cancer Prevention: Dietary Practice Guideline”; no difference in vegetable intake | Survey responses about the time, length, and intensity of PA; no difference in METS | HADS, EORTC QLQ-C30; anxiety decreased at 3 months (p = 0.025) but not 12 months |
| Beumeler, 2018 [53] | None | Grip strength, bicep curl, steep ramp; bicep curl increased (p = 0.043) | DASS-21, SF-36, VAS-F: anxiety (p = 0.011), stress (p = 0.017), and role limitations due to physical health (p = 0.034) and emotional problems (p = 0.016) decreased |
| Awasthi, 2019 [42] | None | 6MWT and muscular strength; improved functional capacity and muscular strength (p < 0.01) | HADS, SF-36; mental and physical QOL improved (p < 0.0001) |
| Barrett-Bernstein, 2019 [43] | None | 6MWT, CHAMPS; improved 6-min walking distance (p = 0.006) | SF-36, HADS; no statistical comparisons |
| Gillis, 2019 [44] | None | 6MWT; no statistical comparisons | None |
| Lacey, 2019 [54] | None | Steps, 1RM row and leg press, exercise hours, VO₂ max; no statistical comparisons | FACT-G, HADS, ESAS; no statistical comparisons |
| Schneeberger, 2019 [33] | Block Dietary Fat Screener and Block Dietary Fruit, Vegetable, Fiber, and Screener; reduced weekly fat consumption (p < 0.01) | None | PSS-4, CES-D, PROMIS; no significant differences |
| Thomas, 2019 [34] | N/A | None | DEBQ, MAIA; significant time × treatment interaction for eating in response to external cures (p = 0.01), improvements in noticing body sensations (p = 0.01), attention regulation (p = 0.02), self-regulation (p = 0.006), and body listening (p = 0.001) |
| Cheng, 2020 [45] | None | 6MWT; no significant changes | EORTC QLQ-C30, PHQ-9, GAD-7, and PSS-10; depression increased 1-month after surgery (p < 0.001); most QOL dimensions returned to preoperative level at 3-month follow-up |
| Ruiz-Vozmediano, 2020 [55] | FFQ, 24 h recall, 14-item Mediterranean diet adherence questionnaire; improved Mediterranean diet adherence (p = 0.02) | None | EORTC QLQ-C30; improved physical functioning (p = 0.027) and role functioning (p = 0.028) |
| Minnella, 2021 [40] | None | 6MWT, CHAMPS; 6MWT difference between groups at 4 weeks (p = 0.014) but no differences in reported PA | SF-36; no significant differences |
| Study | Category | Score |
|---|---|---|
| Controlled Interventions | ||
| Andersen, 2004 [26] | G | 11.5 |
| Fillion, 2008 [41] | G | 12 |
| Adamsen, 2009 [20] | G | 12 |
| Carmody, 2012 [28] | F | 10 |
| Hébert, 2012 [29] | F | 9 |
| Jacobsen, 2013 [31] | F | 9 |
| Vardar Yağlı, 2015 [50] | F | 8.5 |
| Rabin, 2016 [19] | F | 8 |
| Yun, 2017 [49] | F | 10 |
| Awasthi, 2019 [42] | F * | 5 |
| Barrett-Bernstein, 2019 [43] | F | 7 |
| Gillis, 2019 [44] | G | 11 |
| Thomas, 2019 [34] | G | 11.5 |
| Ruiz-Vozmediano, 2020 [55] | G | 12 |
| Ma, 2021 [47] | G | 11.5 |
| Minnella, 2021 [40] | G | 12.5 |
| Pre-post Interventions | ||
| Saxe, 2001 [25] | F | 7 |
| Midtgaard, 2005 [35] | G | 8 |
| Adamsen, 2006 [36] | F | 7.5 |
| Midtgaard, 2006 [37] | G | 9 |
| Quist, 2006 [38] | F | 7.5 |
| Rabin, 2009 [27] | G | 8 |
| Quist, 2012 [39] | F | 7 |
| Garrett, 2013 [30] | F | 7 |
| Hwang, 2016 | G | 10 |
| Lucas, 2017 [32] | F | 7 |
| Beumeler, 2018 [53] | G | 8 |
| Lacey, 2019 [54] | F | 6 |
| Schneeberger, 2019 [33] | G | 8.5 |
| Cheng, 2020 [45] | G | 8 |
| Felser, 2020 [51] | G | 8 |
| Lu, 2020 [46] | G | 8 |
| Fournié, 2022 [52] | G | 8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buro, A.W.; Crowder, S.L.; Rozen, E.; Stern, M.; Carson, T.L. Lifestyle Interventions with Mind-Body or Stress-Management Practices for Cancer Survivors: A Rapid Review. Int. J. Environ. Res. Public Health 2023, 20, 3355. https://doi.org/10.3390/ijerph20043355
Buro AW, Crowder SL, Rozen E, Stern M, Carson TL. Lifestyle Interventions with Mind-Body or Stress-Management Practices for Cancer Survivors: A Rapid Review. International Journal of Environmental Research and Public Health. 2023; 20(4):3355. https://doi.org/10.3390/ijerph20043355
Chicago/Turabian StyleBuro, Acadia W., Sylvia L. Crowder, Emily Rozen, Marilyn Stern, and Tiffany L. Carson. 2023. "Lifestyle Interventions with Mind-Body or Stress-Management Practices for Cancer Survivors: A Rapid Review" International Journal of Environmental Research and Public Health 20, no. 4: 3355. https://doi.org/10.3390/ijerph20043355





