Association between Sleep Duration and Grip Strength in U.S. Older Adults: An NHANES Analysis (2011–2014)
Abstract
:1. Introduction
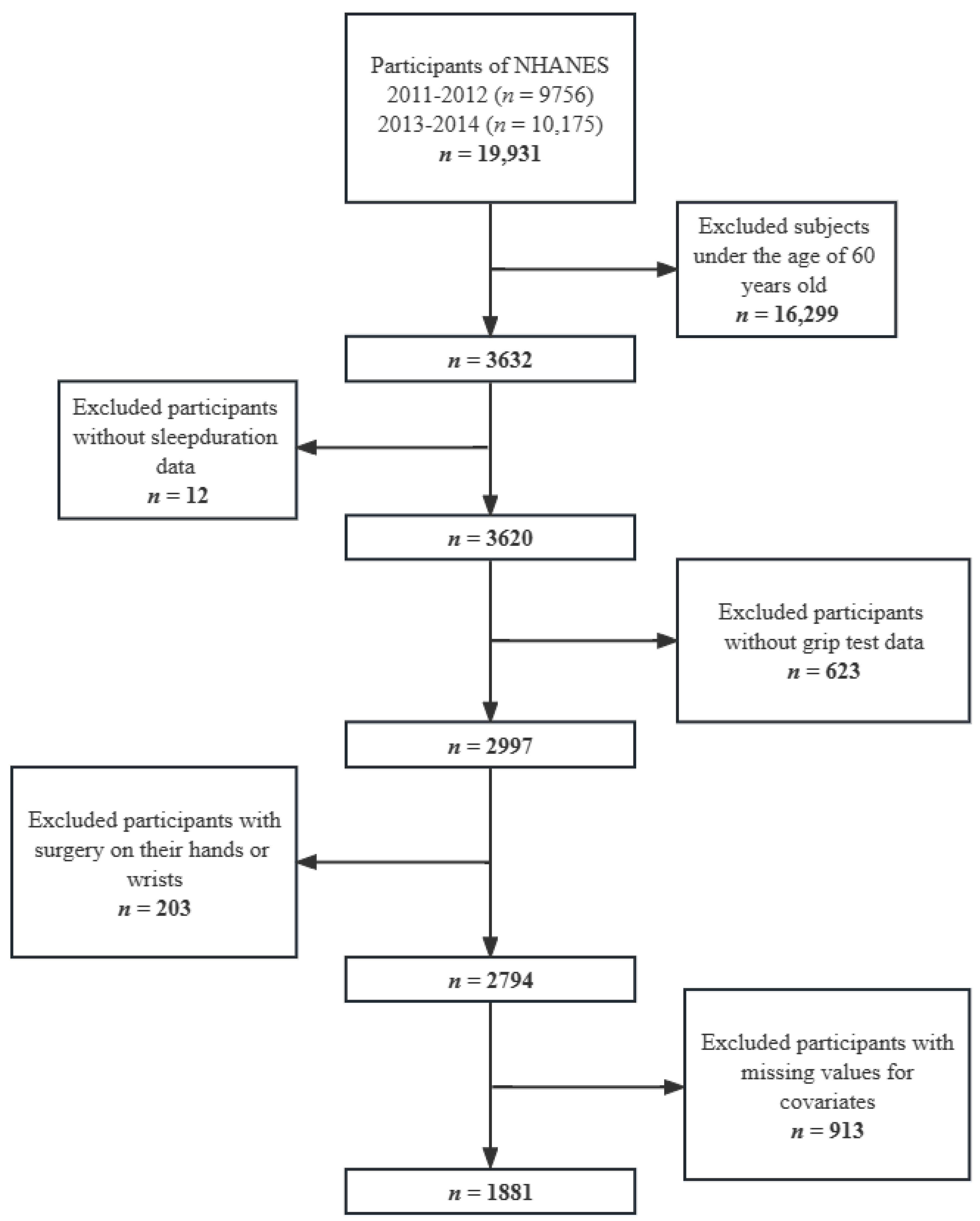
2. Materials and Methods
2.1. Data Source and Participants
2.2. Instruments
2.2.1. Grip Strength
2.2.2. Sleep Duration
2.2.3. Covariates
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hughes, V.A.; Frontera, W.R.; Wood, M.; Evans, W.J.; Dallal, G.E.; Roubenoff, R.; Fiatarone Singh, M.A. Longitudinal muscle strength changes in older adults: Influence of muscle mass, physical activity, and health. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, B209–B217. [Google Scholar] [CrossRef] [PubMed]
- Bohannon, R.W. Grip Strength: An Indispensable Biomarker For Older Adults. Clin. Interv. Aging 2019, 14, 1681–1691. [Google Scholar] [CrossRef] [Green Version]
- Luo, J.; Yao, W.; Zhang, T.; Ge, H.; Zhang, D. Exploring the bidirectional associations between handgrip strength and depression in middle and older Americans. J. Psychosom. Res. 2021, 152, 110678. [Google Scholar] [CrossRef] [PubMed]
- Veronese, N.; Stubbs, B.; Fontana, L.; Trevisan, C.; Bolzetta, F.; Rui, M.; Sartori, L.; Musacchio, E.; Zambon, S.; Maggi, S.; et al. A Comparison of Objective Physical Performance Tests and Future Mortality in the Elderly People. J. Gerontol. A Biol. Sci. Med. Sci. 2017, 72, 362–368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marques, A.; Gaspar de Matos, M.; Henriques-Neto, D.; Peralta, M.; Gouveia, É.R.; Tesler, R.; Martins, J.; Gomez-Baya, D. Grip Strength and Depression Symptoms among Middle-Age and Older Adults. Mayo Clin. Proc. 2020, 95, 2134–2143. [Google Scholar] [CrossRef]
- Alfaro-Acha, A.; Al Snih, S.; Raji, M.A.; Kuo, Y.F.; Markides, K.S.; Ottenbacher, K.J. Handgrip strength and cognitive decline in older Mexican Americans. J. Gerontol. A Biol. Sci. Med. Sci. 2006, 61, 859–865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leong, D.P.; Teo, K.K.; Rangarajan, S.; Lopez-Jaramillo, P.; Avezum, A., Jr.; Orlandini, A.; Seron, P.; Ahmed, S.H.; Rosengren, A.; Kelishadi, R.; et al. Prognostic value of grip strength: Findings from the Prospective Urban Rural Epidemiology (PURE) study. Lancet 2015, 386, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Cooper, R.; Kuh, D.; Cooper, C.; Gale, C.R.; Lawlor, D.A.; Matthews, F.; Hardy, R. Objective measures of physical capability and subsequent health: A systematic review. Age Ageing 2011, 40, 14–23. [Google Scholar] [CrossRef] [Green Version]
- Dyar, K.A.; Ciciliot, S.; Wright, L.E.; Biensø, R.S.; Tagliazucchi, G.M.; Patel, V.R.; Forcato, M.; Paz, M.I.P.; Gudiksen, A.; Solagna, F.; et al. Muscle insulin sensitivity and glucose metabolism are controlled by the intrinsic muscle clock. Mol. Metab. 2014, 3, 29–41. [Google Scholar] [CrossRef]
- Watson, E.J.; Coates, A.M.; Kohler, M.; Banks, S. Caffeine Consumption and Sleep Quality in Australian Adults. Nutrients 2016, 8, 479. [Google Scholar] [CrossRef]
- Balbo, M.; Leproult, R.; Van Cauter, E. Impact of sleep and its disturbances on hypothalamo-pituitary-adrenal axis activity. Int. J. Endocrinol. 2010, 2010, 759234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolkove, N.; Elkholy, O.; Baltzan, M.; Palayew, M. Sleep and aging: 1. Sleep disorders commonly found in older people. CMAJ 2007, 176, 1299–1304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shih, A.-C.; Chen, L.-H.; Tsai, C.-C.; Chen, J.-Y. Correlation between Sleep Quality and Frailty Status among Middle-Aged and Older Taiwanese People: A Community-Based, Cross-Sectional Study. Int. J. Environ. Res. Public Health 2020, 17, 9457. [Google Scholar] [CrossRef] [PubMed]
- Dam, T.-T.L.; Ewing, S.; Ancoli-Israel, S.; Ensrud, K.; Redline, S.; Stone, K. Association between sleep and physical function in older men: The osteoporotic fractures in men sleep study. J. Am. Geriatr. Soc. 2008, 56, 1665–1673. [Google Scholar] [CrossRef] [Green Version]
- Spira, A.P.; Covinsky, K.; Rebok, G.W.; Punjabi, N.M.; Stone, K.L.; Hillier, T.A.; Ensrud, K.E.; Yaffe, K. Poor sleep quality and functional decline in older women. J. Am. Geriatr. Soc. 2012, 60, 1092–1098. [Google Scholar] [CrossRef] [Green Version]
- Hamasaki, H. The association between handgrip strength and sleep duration in Japanese patients with type 2 diabetes. Diabetes Metab 2019, 45, 306–307. [Google Scholar] [CrossRef]
- Huang, W.-C.; Lin, C.-Y.; Togo, F.; Lai, T.-F.; Liao, Y.; Park, J.-H.; Hsueh, M.-C.; Park, H. Association between objectively measured sleep duration and physical function in community-dwelling older adults. J. Clin. Sleep Med. 2021, 17, 515–520. [Google Scholar] [CrossRef]
- Goldman, S.E.; Stone, K.L.; Ancoli-Israel, S.; Blackwell, T.; Ewing, S.K.; Boudreau, R.; Cauley, J.A.; Hall, M.; Matthews, K.A.; Newman, A.B. Poor sleep is associated with poorer physical performance and greater functional limitations in older women. Sleep 2007, 30, 1317–1324. [Google Scholar] [CrossRef] [Green Version]
- Kauranen, K.; Vanharanta, H. Influences of aging, gender, and handedness on motor performance of upper and lower extremities. Percept. Mot. Skills 1996, 82, 515–525. [Google Scholar] [CrossRef]
- Vianna, L.C.; Oliveira, R.B.; Araújo, C.G.S. Age-related decline in handgrip strength differs according to gender. J. Strength Cond. Res. 2007, 21, 1310–1314. [Google Scholar] [CrossRef]
- Lam, N.W.; Goh, H.T.; Kamaruzzaman, S.B.; Chin, A.-V.; Poi, P.J.H.; Tan, M.P. Normative data for hand grip strength and key pinch strength, stratified by age and gender for a multiethnic Asian population. Singapore Med. J. 2016, 57, 578–584. [Google Scholar] [CrossRef] [Green Version]
- Leyk, D.; Gorges, W.; Ridder, D.; Wunderlich, M.; Rüther, T.; Sievert, A.; Essfeld, D. Hand-grip strength of young men, women and highly trained female athletes. Eur. J. Appl. Physiol. 2007, 99, 415–421. [Google Scholar] [CrossRef]
- Werle, S.; Goldhahn, J.; Drerup, S.; Simmen, B.R.; Sprott, H.; Herren, D.B. Age- and gender-specific normative data of grip and pinch strength in a healthy adult Swiss population. J. Hand Surg. Eur. Vol. 2009, 34, 76–84. [Google Scholar] [CrossRef]
- Centers for Disease, Control, and Prevention. National Health and Nutrition Examination Survey. Available online: https://www.cdc.gov/nchs/nhanes/index.htm (accessed on 10 August 2022).
- Centers for Disease, Control, and Prevention. National Health and Nutrition Examination Survey. Examination Data. Available online: https://wwwn.cdc.gov/Nchs/Nhanes/2011-2012/MGX_G.htm (accessed on 18 August 2022).
- Wang, Y.-C.; Bohannon, R.W.; Li, X.; Sindhu, B.; Kapellusch, J. Hand-Grip Strength: Normative Reference Values and Equations for Individuals 18 to 85 Years of Age Residing in the United States. J. Orthop. Sports Phys. Ther. 2018, 48, 685–693. [Google Scholar] [CrossRef]
- Beydoun, H.A.; Beydoun, M.A.; Jeng, H.A.; Zonderman, A.B.; Eid, S.M. Bisphenol-A and Sleep Adequacy among Adults in the National Health and Nutrition Examination Surveys. Sleep 2016, 39, 467–476. [Google Scholar] [CrossRef] [Green Version]
- Luo, J.; Ge, H.; Sun, J.; Hao, K.; Yao, W.; Zhang, D. Associations of Dietary ω-3, ω-6 Fatty Acids Consumption with Sleep Disorders and Sleep Duration among Adults. Nutrients 2021, 13, 1475. [Google Scholar] [CrossRef]
- Chen, S.P.; Bhattacharya, J.; Pershing, S. Association of Vision Loss With Cognition in Older Adults. JAMA Ophthalmol. 2017, 135, 963–970. [Google Scholar] [CrossRef]
- Li, S.; Sun, W.; Zhang, D. Association of Zinc, Iron, Copper, and Selenium Intakes with Low Cognitive Performance in Older Adults: A Cross-Sectional Study from National Health and Nutrition Examination Survey (NHANES). J. Alzheimers Dis. 2019, 72, 1145–1157. [Google Scholar] [CrossRef]
- Alley, D.E.; Chang, V.W. The changing relationship of obesity and disability, 1988–2004. JAMA 2007, 298, 2020–2027. [Google Scholar] [CrossRef] [Green Version]
- McNutt, L.-A.; Wu, C.; Xue, X.; Hafner, J.P. Estimating the relative risk in cohort studies and clinical trials of common outcomes. Am. J. Epidemiol. 2003, 157, 940–943. [Google Scholar] [CrossRef]
- Zhang, J.; Yu, K.F. What’s the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA 1998, 280, 1690–1691. [Google Scholar] [CrossRef] [Green Version]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Centers for Disease, Control, and Prevention. National Health and Nutrition Examination Survey. Survey Methods and Analytic Guidelines. Available online: https://wwwn.cdc.gov/nchs/nhanes/analyticguidelines.aspx (accessed on 20 August 2022).
- Nakakubo, S.; Doi, T.; Tsutsumimoto, K.; Kurita, S.; Ishii, H.; Shimada, H. Sleep duration and progression to sarcopenia in Japanese community-dwelling older adults: A 4 year longitudinal study. J. Cachexia Sarcopenia Muscle 2021, 12, 1034–1041. [Google Scholar] [CrossRef]
- Wang, T.Y.; Wu, Y.; Wang, T.; Li, Y.; Zhang, D. A prospective study on the association of sleep duration with grip strength among middle-aged and older Chinese. Exp. Gerontol. 2018, 103, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Jia, L.; Zhang, W.; Han, P.; Kang, L.; Ma, Y.; Yu, H.; Zhai, T.; Chen, X.; Guo, Q. The association between sleep duration and physical performance in Chinese community-dwelling elderly. PLoS ONE 2017, 12, e0174832. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.-C.; Hsu, N.-W.; Chou, P. The Association Between Sleep Duration and Hand Grip Strength in Community-Dwelling Older Adults: The Yilan Study, Taiwan. Sleep 2017, 40, zsx021. [Google Scholar] [CrossRef] [PubMed]
- Spira, A.P.; Kaufmann, C.N.; Kasper, J.D.; Ohayon, M.M.; Rebok, G.W.; Skidmore, E.; Parisi, J.M.; Reynolds, C.F. Association between insomnia symptoms and functional status in U.S. older adults. J. Gerontol. Ser. B Psychol. Sci. Soc. Sci. 2014, 69 (Suppl. S1), S35–S41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Auyeung, T.W.; Kwok, T.; Leung, J.; Lee, J.S.W.; Ohlsson, C.; Vandenput, L.; Wing, Y.K.; Woo, J. Sleep Duration and Disturbances Were Associated with Testosterone Level, Muscle Mass, and Muscle Strength—A Cross-Sectional Study in 1274 Older Men. J. Am. Med. Dir. Assoc. 2015, 16, 630.e631–630.e636. [Google Scholar] [CrossRef]
- Harfmann, B.D.; Schroder, E.A.; Esser, K.A. Circadian rhythms, the molecular clock, and skeletal muscle. J. Biol. Rhythms 2015, 30, 84–94. [Google Scholar] [CrossRef]
- Lane, J.M.; Liang, J.; Vlasac, I.; Anderson, S.G.; Bechtold, D.A.; Bowden, J.; Emsley, R.; Gill, S.; Little, M.A.; Luik, A.I.; et al. Genome-wide association analyses of sleep disturbance traits identify new loci and highlight shared genetics with neuropsychiatric and metabolic traits. Nat. Genet. 2017, 49, 274–281. [Google Scholar] [CrossRef]
- Patel, S.R.; Ayas, N.T.; Malhotra, M.R.; White, D.P.; Schernhammer, E.S.; Speizer, F.E.; Stampfer, M.J.; Hu, F.B. A prospective study of sleep duration and mortality risk in women. Sleep 2004, 27, 440–444. [Google Scholar] [CrossRef]

| Low Grip Strength | Normal Grip Strength | p Value | |
|---|---|---|---|
| Number of participants (%) a | 640 (34.02) | 1241 (65.98) | |
| Age, n (%) a | 0.996 | ||
| 60–70 years | 353 (55.16) | 687 (55.36) | |
| ≥70 years | 287 (44.84) | 554 (44.64) | |
| Gender, n (%) a | 0.642 | ||
| Male | 310 (48.44) | 620 (49.96) | |
| Female | 330 (51.56) | 621 (50.04) | |
| Body mass index, n (%) a | <0.05 | ||
| <25 kg/m2 | 172 (26.88) | 343 (27.64) | |
| 25 to <30 kg/m2 | 196 (30.63) | 464 (37.39) | |
| ≥30 kg/m2 | 272 (42.50) | 434 (34.97) | |
| Race, n (%) a | 0.226 | ||
| Mexican American | 56 (8.75) | 97 (7.82) | |
| Other Hispanic | 56 (8.75) | 120 (9.67) | |
| Non-Hispanic White | 369 (57.66) | 620 (49.96) | |
| Non-Hispanic Black | 120 (18.75) | 298 (24.01) | |
| Other races | 39 (6.09) | 106 (8.54) | |
| Marital status, n (%) a | 0.848 | ||
| Living with partner/married | 376 (58.75) | 754 (60.76) | |
| Widowed/separated/divorced/ never married | 264 (41.25) | 487 (39.24) | |
| Educational level, n (%) a | 0.919 | ||
| <high school | 132 (20.63) | 271 (21.84) | |
| High school | 154 (24.06) | 306 (24.66) | |
| >high school | 354 (55.31) | 664 (53.51) | |
| Annual household income, n (%) a | 0.175 | ||
| <$20,000 | 157 (24.53) | 270 (21.76) | |
| ≥$20,000 | 483 (75.47) | 971 (78.24) | |
| Work activity, n (%) a | <0.01 | ||
| Vigorous | 50 (7.81) | 166 (13.38) | |
| Moderate | 131 (20.47) | 269 (21.68) | |
| Other | 459 (71.72) | 806 (64.95) | |
| Recreational activity, n (%) a | 0.304 | ||
| Vigorous | 54 (8.44) | 144 (11.60) | |
| Moderate | 211 (32.97) | 440 (35.46) | |
| Other | 375 (58.59) | 657 (52.94) | |
| Sleep duration, n (%) a | <0.05 | ||
| <5 h | 24 (3.75) | 53 (4.27) | |
| 5 to <7 h | 176 (27.50) | 364 (29.33) | |
| 7 to <9 h | 357 (55.78) | 716 (57.70) | |
| ≥9 h | 83 (12.97) | 108 (8.70) | |
| Low CERAD Test Performance, n (%) a | 163 (25.47) | 267 (21.51) | 0.153 |
| Low Animal Fluency Test Performance, n (%) a | 168 (26.25) | 315 (25.38) | 0.140 |
| Low Digit Symbol Test Performance, n (%) a | 154 (24.06) | 266 (21.43) | 0.061 |
| Cognitive impairment, n (%) a | 294 (45.94) | 537 (43.27) | 0.102 |
| Functional limitations, n (%) a | 223 (34.84) | 226 (18.21) | <0.01 |
| ADL limitations, n (%) a | 20 (3.13) | 26 (2.10) | 0.685 |
| Smoke at least 100 cigarettes in life, n (%) a | 315 (49.22) | 613 (49.40) | 0.394 |
| Had at least 12 alcohol drink a year, n (%) a | 450 (70.31) | 876 (70.59) | 0.531 |
| Hypertension, n (%) a | 514 (80.31) | 970 (78.16) | 0.362 |
| Diabetes, n (%) a | 229 (35.78) | 337 (27.16) | <0.01 |
| Ever told you had a cancer, n (%) a | 136 (21.25) | 249 (20.06) | 0.931 |
| Ever told you had a stroke, n (%) a | 55 (8.59) | 64 (5.16) | <0.05 |
| Ever told you had arthritis, n (%) a | 363 (56.72) | 530 (42.71) | <0.01 |
| Ever told you had coronary heart disease, n (%) a | 68 (10.63) | 107 (8.62) | 0.158 |
| Depressive symptoms, n (%) a | 65 (10.16) | 81 (6.53) | <0.05 |
| Caffeine intake (mg/d), median (IQR) b | 109.75 (164.50) | 108.00 (177.50) | 0.676 |
| Total energy intake (kcal/d), median (IQR) b | 1715.25 (866.5) | 1765.00 (811.50) | <0.05 |
| Total testosterone level (ng/dL), median (IQR) b | 44.64 (327.65) | 61.92 (355.01) | 0.213 |
| Sleep Duration (h/Day) | Cases/Participants (Prevalence Values) | Model 1 a | Model 2 b |
|---|---|---|---|
| IRR (95% CI) | IRR (95% CI) | ||
| <5 | 24/77 (31.17%) | 1.10 (0.68–1.75) | 1.08 (0.68–1.72) |
| 5–<7 | 176/540 (32.59%) | 1.07 (0.81–1.41) | 1.03 (0.78–1.39) |
| 7–<9 | 357/1073 (33.27%) | 1.00 (reference) | 1.00 (reference) |
| ≥9 | 83/191 (43.46%) | 1.52 (1.22–1.90) ** | 1.38 (1.12–1.69) ** |
| Sleep Duration (h/Day) | Cases/Participants (Prevalence Values) | Model 1 a | Model 2 b |
|---|---|---|---|
| IRR (95% CI) | IRR (95% CI) | ||
| Males | |||
| <5 | 8/29 (27.59%) | 1.28 (0.60–2.74) | 1.50 (0.73–3.08) |
| 5–<7 | 88/258 (34.11%) | 1.17 (0.80–1.73) | 1.13 (0.76–1.69) |
| 7–<9 | 172/543 (31.68%) | 1.00 (reference) | 1.00 (reference) |
| ≥9 | 42/100 (42.00%) | 1.49 (1.05–2.11) ** | 1.40 (1.04–1.89) * |
| Females | |||
| <5 | 16/48 (33.33%) | 0.97 (0.59–1.61) | 0.93 (0.57–1.51) |
| 5–<7 | 88/282 (31.21%) | 0.98 (0.71–1.37) | 0.94 (0.68–1.29) |
| 7–<9 | 185/530 (34.91%) | 1.00 (reference) | 1.00 (reference) |
| ≥9 | 41/91 (45.05%) | 1.56 (1.18–2.06) ** | 1.35 (1.02–1.79) * |
| 60–70 years | |||
| <5 | 18/53 (33.96%) | 1.65 (0.68–3.99) | 1.55 (0.95–2.51) |
| 5–<7 | 103/325 (31.69%) | 1.06 (0.59–1.90) | 1.00 (0.70–1.44) |
| 7–<9 | 197/586 (33.62%) | 1.00 (reference) | 1.00 (reference) |
| ≥9 | 35/76 (46.05%) | 3.19 (1.86–5.46) ** | 1.76 (1.40–2.22) ** |
| ≥70 years | |||
| <5 | 6/24 (25.00%) | 0.65 (0.20–2.13) | 0.71 (0.26–1.96) |
| 5–<7 | 73/215 (33.95%) | 1.17 (0.74–1.86) | 1.04 (0.75–1.44) |
| 7–<9 | 160/487 (32.85%) | 1.00 (reference) | 1.00 (reference) |
| ≥9 | 48/115 (41.74%) | 1.38 (0.80–2.38) | 1.08 (0.79–1.48) |
| <25 kg/m2 | |||
| <5 | 7/18 (38.89%) | 1.45 (0.60–3.49) | 1.44 (0.68–3.02) |
| 5–<7 | 46/142 (32.39%) | 1.11 (0.72–1.72) | 1.12 (0.74–1.69) |
| 7–<9 | 92/309 (29.77%) | 1.00 (reference) | 1.00 (reference) |
| ≥9 | 27/46 (58.70%) | 2.32 (1.67–3.22) ** | 2.30 (1.64–3.22) ** |
| 25–30 kg/m2 | |||
| <5 | 6/17 (35.29%) | 1.03 (0.34–3.17) | 1.19 (0.34–4.24) |
| 5–<7 | 43/173 (24.86%) | 0.93 (0.53–1.63) | 0.91 (0.50–1.66) |
| 7–<9 | 122/397 (30.73%) | 1.00 (reference) | 1.00 (reference) |
| ≥9 | 25/73 (34.25%) | 1.38 (0.91–2.10) | 1.32 (0.87–2.01) |
| ≥30 kg/m2 | |||
| <5 | 11/42 (26.19%) | 0.88 (0.46–1.67) | 0.80 (0.45–1.44) |
| 5–<7 | 87/225 (38.67%) | 1.04 (0.77–1.40) | 1.04(0.78–1.37) |
| 7–<9 | 143/367 (38.96%) | 1.00 (reference) | 1.00 (reference) |
| ≥9 | 31/72 (43.06%) | 1.23 (0.95–1.58) | 1.03 (0.84–1.27) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Zhang, T.; Luo, J.; Chen, S.; Zhang, D. Association between Sleep Duration and Grip Strength in U.S. Older Adults: An NHANES Analysis (2011–2014). Int. J. Environ. Res. Public Health 2023, 20, 3416. https://doi.org/10.3390/ijerph20043416
Liu J, Zhang T, Luo J, Chen S, Zhang D. Association between Sleep Duration and Grip Strength in U.S. Older Adults: An NHANES Analysis (2011–2014). International Journal of Environmental Research and Public Health. 2023; 20(4):3416. https://doi.org/10.3390/ijerph20043416
Chicago/Turabian StyleLiu, Jin, Tianhao Zhang, Jia Luo, Shumin Chen, and Dongfeng Zhang. 2023. "Association between Sleep Duration and Grip Strength in U.S. Older Adults: An NHANES Analysis (2011–2014)" International Journal of Environmental Research and Public Health 20, no. 4: 3416. https://doi.org/10.3390/ijerph20043416





